Abstract
Purpose
The goals of immediate postmastectomy breast reconstruction are to minimize deformity and optimize quality of life as perceived by patients. We prospectively evaluated patient-reported outcomes (PROs) in women undergoing immediate implant-based or autologous reconstruction.
Methods
Women undergoing immediate postmastectomy reconstruction for invasive cancer and/or carcinoma in situ were enrolled at 11 sites. Women underwent implant-based or autologous tissue reconstruction. Patients completed the BREAST-Q, a condition-specific PRO measure for breast surgery patients, and Patient-Reported Outcomes Measurement Information System–29, a generic PRO measure, before and 1 year after surgery. Mean changes in PRO scores were summarized. Mixed-effects regression models were used to compare PRO scores across procedure types.
Results
In total, 1,632 patients (n = 1,139 implant, n = 493 autologous) were included; 1,183 (72.5%) responded to 1-year questionnaires. After analysis was controlled for baseline values, patients who underwent autologous reconstruction had greater satisfaction with their breasts than those who underwent implant-based reconstruction (difference, 6.3; P < .001), greater sexual well-being (difference, 4.5; P = .003), and greater psychosocial well-being (difference, 3.7; P = .02) at 1 year. Patients in the autologous reconstruction group had improved satisfaction with breasts (difference, 8.0; P = .002) and psychosocial well-being (difference, 4.6; P = .047) compared with preoperative baseline. Physical well-being of the chest was not fully restored in either the implant group (difference, −3.8; P = .001) or autologous group (−2.2; P = .04), nor was physical well-being of the abdomen in patients who underwent autologous reconstruction (−13.4; P < .001). Anxiety and depression were mitigated at 1 year in both groups. Compared with their baseline reports, patients who underwent implant reconstruction had decreased fatigue (difference, −1.4; P = .035), whereas patients who underwent autologous reconstruction had increased pain interference (difference, 2.0; P = .006).
Conclusion
At 1 year after mastectomy, patients who underwent autologous reconstruction were more satisfied with their breasts and had greater psychosocial and sexual well-being than those who underwent implant reconstruction. Although satisfaction with breasts was equal to or greater than baseline levels, physical well-being was not fully restored. This information can help patients better understand expected outcomes and may guide innovations to improve outcomes.
INTRODUCTION
Breast cancer affects one in eight women during their lifetimes.1 Although most women survive breast cancer, many must contend with the long-term effects of surgery on body image and quality of life (QOL). After years of declining use of mastectomy in favor of breast-conserving surgery, rates of mastectomy are now increasing in tandem with the increasing use of bilateral mastectomy.2-6 This trend is likely driven by fear of recurrence,7 more-sophisticated imaging modalities,8,9 and advances in reconstructive techniques.10,11 During the 1980s, less than 20% of patients who underwent mastectomy received immediate reconstruction, but rates of reconstruction have steadily increased since then.12-14
Breast reconstruction can help restore body image and alleviate distress associated with mastectomy.15,16 Although the literature to compare the options for breast reconstruction is substantial,17-22 few studies have evaluated patient perceptions of outcomes. Such information is important to help new patients understand the expected results of reconstruction and make informed decisions. Previous efforts to assess patient-reported outcomes (PROs) have relied on generic measures or ad hoc surveys with limited evidence of reliability, validity, or ability to detect clinically meaningful change.23,24 The development and use of the BREAST-Q—a validated PRO instrument designed specifically for patients who undergo breast surgery—have helped address this gap in knowledge.25-28
Another limitation of the existing literature is the absence of baseline assessment of body image and QOL. Women who undergo immediate breast reconstruction may begin the process at different levels of satisfaction with their breasts and QOL. To meaningfully compare the outcomes of reconstruction, baseline status must be taken into account. In addition, the majority of studies to assess the outcomes of reconstruction have been single-center experiences. A multicenter trial may help elucidate whether choice of procedure predicts outcomes and also may allow procedure type to be distinguished from surgeon and institution factors.
The Mastectomy Reconstruction Outcomes Consortium (MROC) is a 5-year, prospective, multicenter study designed to address these knowledge gaps in breast-reconstruction outcomes research. The objective of this substudy was to prospectively evaluate and compare satisfaction and QOL 1 year after mastectomy and immediate reconstruction within and between autologous breast reconstruction and implant-based breast reconstruction. This information can directly support shared medical decision making and guide innovation in the care of patients with breast cancer.
METHODS
Study Population
Patients were recruited as part of the MROC study. This project involved 57 plastic surgeons at 11 academic and private practice sites across the United States and Canada. Nine of 11 centers were academic institutions; two were private practices. Appropriate institutional review board or research ethics board approval was obtained from all sites.
Women were eligible to participate in the MROC study if they were age 18 years or older and undergoing first-time, immediate or delayed, bilateral or unilateral postmastectomy breast reconstruction for cancer treatment or prophylaxis. Women undergoing reconstruction after previous failed attempts were excluded because of potential confounding effects. Choice of reconstructive procedure was based on patient and surgeon preference and was not randomly assigned. Patients were excluded if they did not complete a baseline (ie, preoperative) questionnaire.
In this MROC substudy, only patients with a cancer diagnosis (ie, not patients undergoing prophylactic mastectomy) and those undergoing immediate reconstruction were included. Patients who experienced reconstructive failure (flap loss or removal of tissue expander [TE] or implant) were excluded, because this was a small (n = 25) and heterogeneous group. Patients who changed reconstructive methods after initial immediate reconstruction were excluded, as were patients who had not completed removal of their TE and exchange for implant at the time of the 1-year questionnaire or who had their exchange procedure less than 3 months before the survey. Patients who had a mixed approach to reconstruction (bilateral reconstruction with unilateral implant and unilateral flap) also were excluded. Because of the small sample size, patients undergoing superior gluteal artery perforator, inferior gluteal artery perforator, or latissimus dorsi flap reconstruction were excluded.
Patient Recruitment
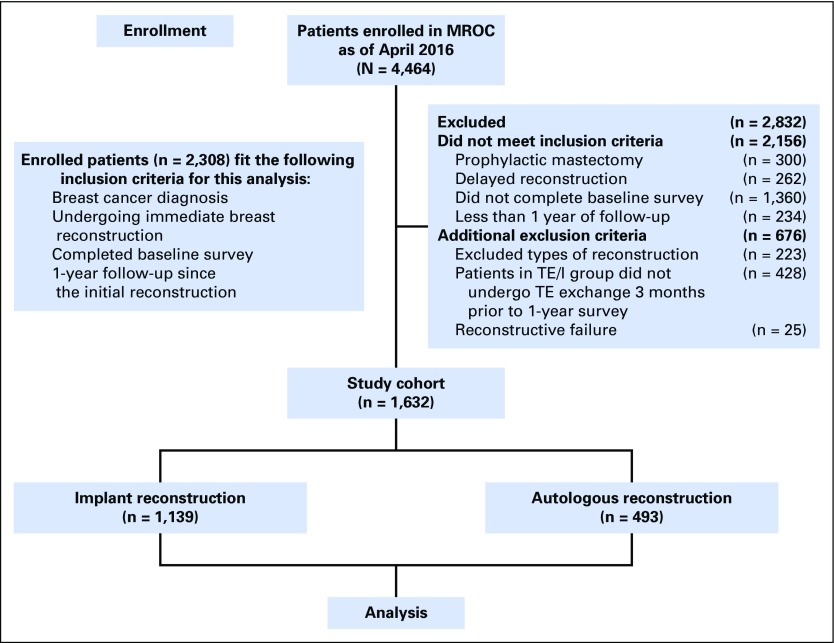
Patients were screened for eligibility by research staff and approached in person before reconstruction. Written informed consent was obtained from all participants. Enrollment took place between February 2012 and July 2015 (Fig 1).
Fig 1.
Study flow diagram. MROC, Mastectomy Reconstruction Outcomes Consortium; TE, tissue expander; TE/I, TE/I, tissue expander implant.
Questionnaire Administration
Patients completed the BREAST-Q and Patient-Reported Outcomes Measurement Information System (PROMIS) –29 before (up to 30 days before surgery) and 1 year after surgery. Patients were encouraged to complete the questionnaires electronically. If they were unable to do this, a paper/pencil version was provided either in clinic or by mail.
Dependent Variables
The primary outcomes of interest were BREAST-Q and PROMIS-29 scores. The BREAST-Q (reconstruction module) is a condition-specific PRO instrument that measures breast-related QOL and satisfaction in patients undergoing breast reconstruction. The BREAST-Q is a Rasch-developed measure; details of development and validation have been published elsewhere.29,30 We focused on the following five BREAST-Q domains: satisfaction with breasts, psychosocial well-being, sexual well-being, physical well-being (chest and upper body), and physical well-being (abdomen). Each domain score was obtained by transforming the scale item responses with the Q-score software program. The transformed scores range from 0 to 100, and higher scores indicated greater satisfaction or QOL.
PROMIS-29 scores were collected with the PROMIS Profile–29 (version 1), a self-administered survey system for evaluation of patient-reported symptoms and QOL. The details of the development and validation of this system have been published elsewhere.31 We used a short profile form that consisted of seven primary domains: depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and satisfaction with participation in social roles. Each domain score was obtained by transforming the original survey item responses with a prespecified algorithm. A higher domain score indicated that more of the concept was measured. For negatively worded concepts, such as depression, a higher score indicated worse function; for positively worded concepts, such as physical function, a higher score indicated better function.
Primary Predictor and Covariates
The primary independent variable was procedure type (autologous v implant reconstruction). Demographic variables included age, race, ethnicity, education level, annual household income, marital status, and employment status. Clinical characteristics included body mass index (BMI), laterality, lymph node procedures, comorbidities (defined by Charlson comorbidity score32), and radiation and chemotherapy. Lymph node biopsy was defined as none, sentinel node biopsy only, and axillary lymph node dissection. Radiation therapy was defined as none, radiation before reconstruction, and radiation during or after reconstruction. Chemotherapy was defined as treatment received during or after reconstruction and none.
Sample Size Calculation and Statistical Methods
Full descriptions of sample size calculation and statistical methodology are provided in the Appendix (online only).
RESULTS
In total, 2,308 patients in the MROC study had a breast cancer diagnosis, underwent immediate reconstruction, and completed a baseline survey (Fig 1). Of these, 676 were excluded according to the criteria listed in the Methods; 1,632 patients remained eligible. Of these eligible patients, 1,139 (69.8%) had implant-based reconstruction, and 493 (30.2%) had autologous reconstruction (Table 1). Of the included patients, 1,183 completed a 1-year questionnaire (72.5% response rate). A comparison of responders and nonresponders is provided in the Appendix (Tables A1 and A2, online only). Clinical and demographic characteristics are listed in Table 2.
Table 1.
Patients by Procedure Type
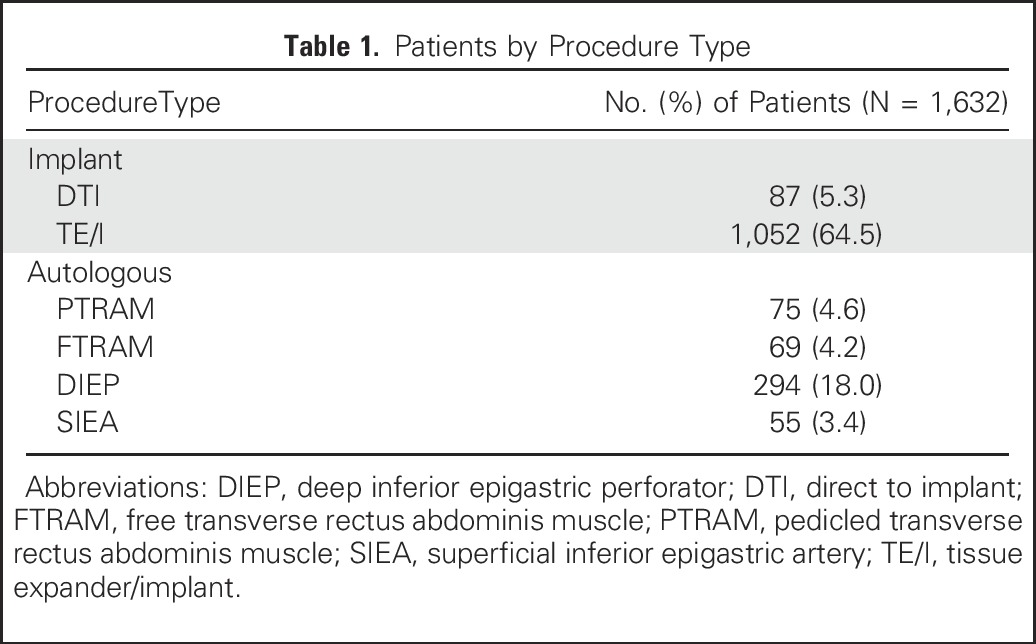
Table 2.
Clinical and Demographic Characteristics of the Study Cohort by Procedure Type
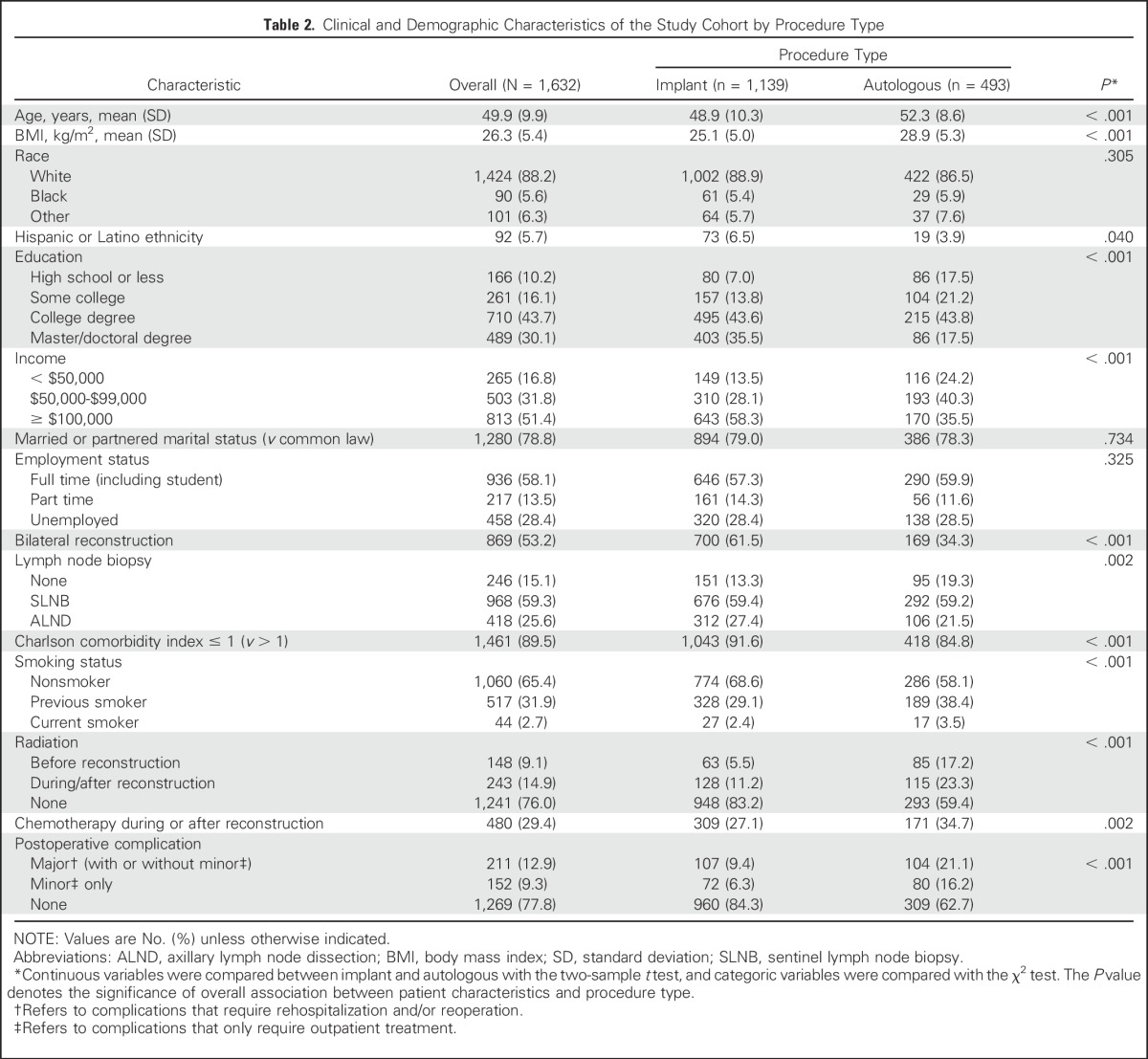
Mean PRO scores at baseline and 1 year, as well as the mean difference in PRO scores before and after surgery, are listed in Table 3. At 1 year postreconstruction, patient satisfaction with breasts and psychosocial well-being were equal to (for implant) or better than (for autologous) baseline levels. Sexual well-being was restored among patients who underwent autologous but not implant reconstruction (mean difference pre- to postsurgery, −5.2; P = .005). Physical well-being of the chest was not fully restored in either group (implant, −3.8 [P = .001]; autologous, −2.2 [P = .038]), nor was physical well-being of the abdomen for patients who underwent autologous reconstruction (−13.4; P < .001). For patients in both implant and autologous groups, anxiety and depression were improved at 1 year. Compared with baseline measures, patients in the autologous group reported significantly increased levels of pain interference (P = .006), and patients in the implant group reported decreased fatigue (P = .035).
Table 3.
Summary of Patient-Reported Outcomes at Baseline and Year 1 Postreconstruction in Patients After Implant or Autologous Reconstruction
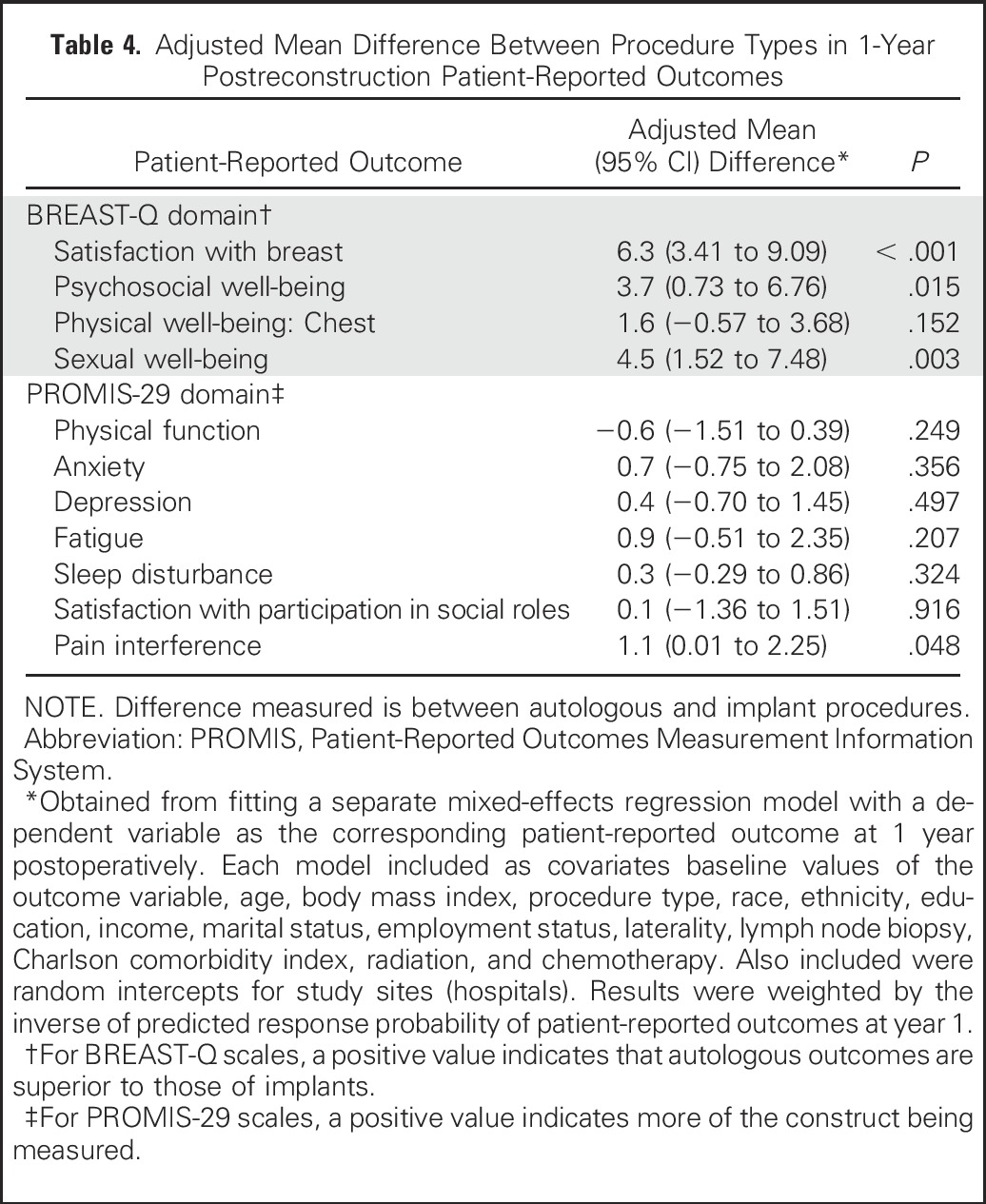
Table 4 lists the results of a mixed-effects regression model for each PRO domain score. When analysis was controlled for baseline values of the outcome measure and other covariates, autologous reconstruction was associated with higher levels of breast satisfaction than implant reconstruction (estimated mean difference in score between autologous and implant, 6.3; P < .001) and higher psychosocial and sexual well-being (mean differences, 3.7 and 4.5, respectively). Physical well-being of the chest did not differ significantly between patients in the implant and autologous groups. Within the implant procedural subgroups (two-stage TE/implant and direct to implant), when analysis was controlled for baseline outcome and other covariates, no significant differences emerged between these two subgroups in postoperative year-1satisfaction or QOL, as measured by the BREAST-Q or PROMIS-29 domains (results based on mixed-effects regression model, not shown). Similarly, within the autologous tissue reconstruction subgroups (pedicled transverse rectus abdominis muscle, free transverse rectus abdominis muscle, deep inferior epigastric perforator, and superficial inferior epigastric artery), no significant differences in 1-year PRO scores were noted across the four procedure subgroups (results based on mixed-effects regression model, not shown).
Table 4.
Adjusted Mean Difference Between Procedure Types in 1-Year Postreconstruction Patient-Reported Outcomes
DISCUSSION
Women who undergo mastectomies for breast cancer treatment make difficult decisions about their preferred method of reconstruction with only limited information about the expected results and the comparative effectiveness of different techniques. The key finding of this study is that women who are candidates for and who choose immediate abdominally based autologous reconstruction report greater satisfaction with their breasts and greater psychosocial and sexual well-being 1 year after mastectomy surgery compared with women who choose implants. In the United States, an increasing number of breast reconstructions are performed with implants14,33,34; however, this study raises the question of whether patient health care value is being maximized by this approach. The reasons for the greater use of implants are multifactorial but reflect patient preferences for a shorter operation,35 quicker recovery, and avoidance of donor site morbidity.28 In addition, some surgeons who perform breast reconstruction do not even offer autologous tissue options, or they limit it to those who are perfect candidates.34 Moreover, implant reconstructions often are linked with bilateral mastectomies, because the combination of the two restores symmetry and alleviates the anxiety of a possible future contralateral breast cancer.26,36 However, proponents of autologous tissue reconstruction suggest that, by replacing like with like, a more natural-looking and -feeling breast can be achieved. Our results support this contention. In addition, the superiority of autologous reconstruction in this regard is supported by several cross-sectional studies that use the BREAST-Q,25,37 which have demonstrated more durable results with autologous tissue as the reconstructive breast ages. Health care value is defined as the relationship between cost and quality across the full cycle of care for the medical condition of a patient, so health policy changes should be aligned to steer patients, providers, and the medical system in the direction of the greatest value.38 In this study, we report outcomes at 1 year; longer-term follow up of this patient cohort will shed more light on differences between reconstructive methods over time.
This study also provides new insights about how women feel and function both before and after surgery. An important finding is that patients who underwent implant reconstruction reported satisfaction with their breasts at 1 year that was similar to baseline. In addition, patients with autologous reconstruction reported being more satisfied with their breasts than they were before surgery. This improvement may relate to the fact that patients who are eligible for flap reconstruction often have higher BMIs and large breasts. In such patients, reconstruction often is accompanied by a contralateral symmetrical breast reduction, and this might contribute to patient happiness about the size and shape of their breasts overall. Future research to explore potential reasons for this finding would be worthwhile.
A concerning finding was that physical well-being at 1 year did not return to baseline levels for women in either group. On both the BREAST-Q and PROMIS-29, patients reported worse physical well-being of the chest and worse physical function at 1 year. For example, in the BREAST-Q, they described pain and tightness at 1 year, and patients in the implant group reported more symptoms than those in the autologous group. These results likely relate to that the significant nerve disruption39-41 from surgery and that requirement of elevation of the pectoralis, and often serratus, muscle for implant reconstruction.42 In addition, patients who underwent autologous reconstruction reported abdominal discomfort and weakness, likely related to dissection of the rectus fascia muscle and motor nerves.
This study reveals an important unmet need in reconstructive breast surgery. Specifically, although current techniques may restore how a woman looks, they do little to address how she feels physically. As women increasingly choose mastectomy instead of breast-conserving therapy, it is especially important to share information about the PROs of the various options—specifically, the physical morbidities associated with mastectomy and reconstruction—during preoperative counseling. Much work remains to understand and improve physical well-being after mastectomy surgery. This analysis, for example, was underpowered to determine whether different approaches to harvest the abdominal flap may result in less abdominal morbidity. For patients who undergo implant reconstruction, new techniques, such as prepectoral placement of implants (which minimizes muscles dissection and stretching) may be beneficial, but rigorous PRO data are still lacking.43,44
It is important to consider not only the statistical significance of our findings but also the extent to which these differences are clinically meaningful. For the BREAST-Q and PROMIS-29 domain scores, there are no widely accepted minimally important differences (MIDs). However, for each of the T-score scale domains of the PROMIS-29 used in this study, differences of two, five, and eight points can be considered small, medium, and large effect sizes, respectively, as defined by Cohen.45-47 There were no MIDs in QOL between patients in the autologous and implant groups, as measured by any of the PROMIS-29 domains. However, for both procedure types, within-group improvement in anxiety during the 1-year period was larger than the medium effect size. Similarly, the distribution-based MID can be applied to the BREAST-Q. Because the standard deviation of BREAST-Q domain scores is approximately 20 at baseline for the satisfaction with breast and sexual well-being domains, the MID can be defined as four points for a between-group difference and 10 points for a change over time for these domains. For the psychosocial well-being domain, in which the standard deviation is approximately 18 points, the MID is 3.6 for a between-group difference and nine for a change after surgery. On the basis of these definitions, autologous procedures show a statistical difference and MID compared with implant procedures in terms of satisfaction with breast, psychosocial well-being, and sexual well-being. An additional benefit of the BREAST-Q is that it was developed with Rasch psychometric methods, which improve the ability to interpret the clinical impact of differences across procedures. This is because Rasch scales have an empiric item order and provide accurate interval-level data (ie, one unit on the scale represents the same magnitude measured across the whole scale). In this study, patients who underwent implant reconstruction reported mean satisfaction with breasts of 64. With Rasch-derived clinical meaning tables, this can be interpreted as moderate satisfaction with how bras fit. In comparison, the mean score for women who underwent autologous reconstruction was 68, which means that they were very satisfied with how bras fit and with how their reconstructed breasts feel to the touch.
The strengths of this study are its multicenter design, the collection of preoperative PRO data to determine baseline statuses of patients, the high response rate,48,49 and the use of a breast-specific PRO measure (the BREAST-Q) calibrated to detect differences in outcomes across reconstructive procedures on important patient-centered concerns. To our knowledge, this is the largest prospective study of PROs after immediate breast reconstruction to date. This study also has limitations. As previously noted, the relatively small number of patients in some procedure groups required collapse across categories or exclusion of groups from analysis. We also did not include patients whose reconstruction had failed because of the small number (n = 25) and heterogeneous clinical outcomes. The generalizability of our study results, therefore, is limited to patients who successfully completed reconstruction. Future studies should build on these findings and achieve greater patient numbers to allow for meaningful comparisons of procedural subtypes. This population also had relatively limited ethnic and racial diversity, and the participating sites were largely academic, high-volume centers. For instance, more than 50% of patients underwent bilateral mastectomies, which is higher the national average50,51 and may reflect patterns of practice in more urban, academic practices. These factors also may limit the generalizability of our findings.
Our study had a nonrandomized clinical trial design—choice of reconstructive method was based on patient characteristics and preferences. Observed difference in outcomes between procedure types, therefore, may be influenced by certain patient and surgeon preferences. Although analysis was adjusted for variables known to be predictive of outcomes,52,53 such as radiation and laterality, it is possible that additional demographic and clinical variables not measured in this study may influence outcomes and thus introduce bias. Another consideration is that patients who had two-stage implant reconstruction generally would have had relatively recent surgery at the time of the 1-year outcome assessment, whereas patients who had autologous reconstruction would have had a year to recover; this may bias the finding that physical well-being of the chest and upper body was superior in patients in the autologous reconstruction group. This study did not include a control population of patients who underwent mastectomy without reconstruction or breast-conserving therapy; thus, interpretation of findings is limited to a comparison of the outcomes of postmastectomy reconstruction techniques.
In summary, the results of this prospective, multicenter study suggest that women who are candidates for and who choose immediate abdominal-based autologous reconstruction are more satisfied with their breasts 1 year after surgery and experience better breast-related QOL than women who undergo implant-based reconstruction. This study also provides evidence that immediate reconstruction restores the look and feel of a woman’s breasts, as evidenced by patient-reported satisfaction with their breasts that was equal to or greater than preoperative levels. Reconstruction does not, however, undo the physical morbidity of mastectomy surgery and, in the case of implant surgery, may even contribute to symptoms of pain and tightness. Improvement in physical well-being after mastectomy surgery is an important area for future research and innovation. The findings from this study may inform the advancement of reconstruction techniques and also may be shared with patients to improve the understanding of expected outcomes and enhance the ability to make informed decisions.
ACKNOWLEDGMENT
We thank the Mastectomy Reconstruction Outcomes Consortium site principal investigators for their contributions: Yoon S. Chun (Brigham and Women’s Hospital), Richard Greco (Georgia Institute of Plastic Surgery), Troy A. Pittman (Georgetown University), Mark W. Clemens (MD Anderson Cancer Center), John Kim (Northwestern University), Daniel Sherick (Saint Joseph Mercy Hospital), Ed Buchel (University of Manitoba), and Nancy Van Laeken (University of British Columbia). We thank all of the patients who participated in the Mastectomy Reconstruction Outcomes Consortium study.
Appendix
Sample size was considered in the original National Institutes of Health proposal on the basis of the total number of reconstruction procedures performed at the participating sites in 2009; an approximately 80% 2-year response rate was assumed. Statistical power to detect the difference in outcomes between implant- and natural tissue–based procedures on the basis of the projected sample size also was considered. With the projection of 75% of patients to undergo an implant-based procedure patients versus 25% to undergo a natural tissue–based procedure, and given a within-surgeon-site correlation of 0.002, we expected to have 83% power to detect a between-procedure difference of 0.135 standard deviation by using a .01-level two-sided test.
Statistical Analysis
Participants were categorized into two groups: (1) autologous tissue reconstruction with tissue transfer from the lower abdomen (pedicled transverse rectus abdominis muscle, free transverse rectus abdominis muscle, superficial inferior epigastric artery, and deep inferior epigastric perforator flaps) and (2) implant reconstruction (direct to implant and two-stage tissue expander/implant).
Patient characteristics between the two procedure groups (autologous v implant) were analyzed with the two-sample t test for continuous variables and the χ2 test for categoric variables. Mean patient-reported outcome (PRO) scores at baseline and at 1 year after surgery, as well as the mean difference in PRO scores before and after surgery, were summarized separately for patients who underwent implant and autologous procedures. For each PRO domain measure, 1-year outcome scores were modeled with a series of mixed-effects regression models. Each model included procedure type and baseline values of the corresponding outcome variable as well as patient demographic and clinical characteristics. Each model also included centers (hospitals) as random intercepts to account for between-center variability. The parameter estimate of the procedure type from the model provided the adjusted expected outcome difference between procedures at 1 year. PRO scores at 1 year were missing for approximately 30% of patients. Baseline characteristics of the responders and nonresponders were compared, and, to reduce potential bias from missing PROs at 1 year, analyses were weighted by the inverse of the probability of response. Specifically, the probability of response was estimated on the basis of data from all eligible study participants, by using a separate logistic regression model for each outcome measure, in which the dependent variable was an indicator of nonmissing response status and the predictors included baseline patient characteristics as well as baseline values of the outcome variable. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC), and statistical significance was set at .05.
Table A1.
Clinical and Demographic Characteristics of Patients by Survey Response Status at 1 Year Postoperation
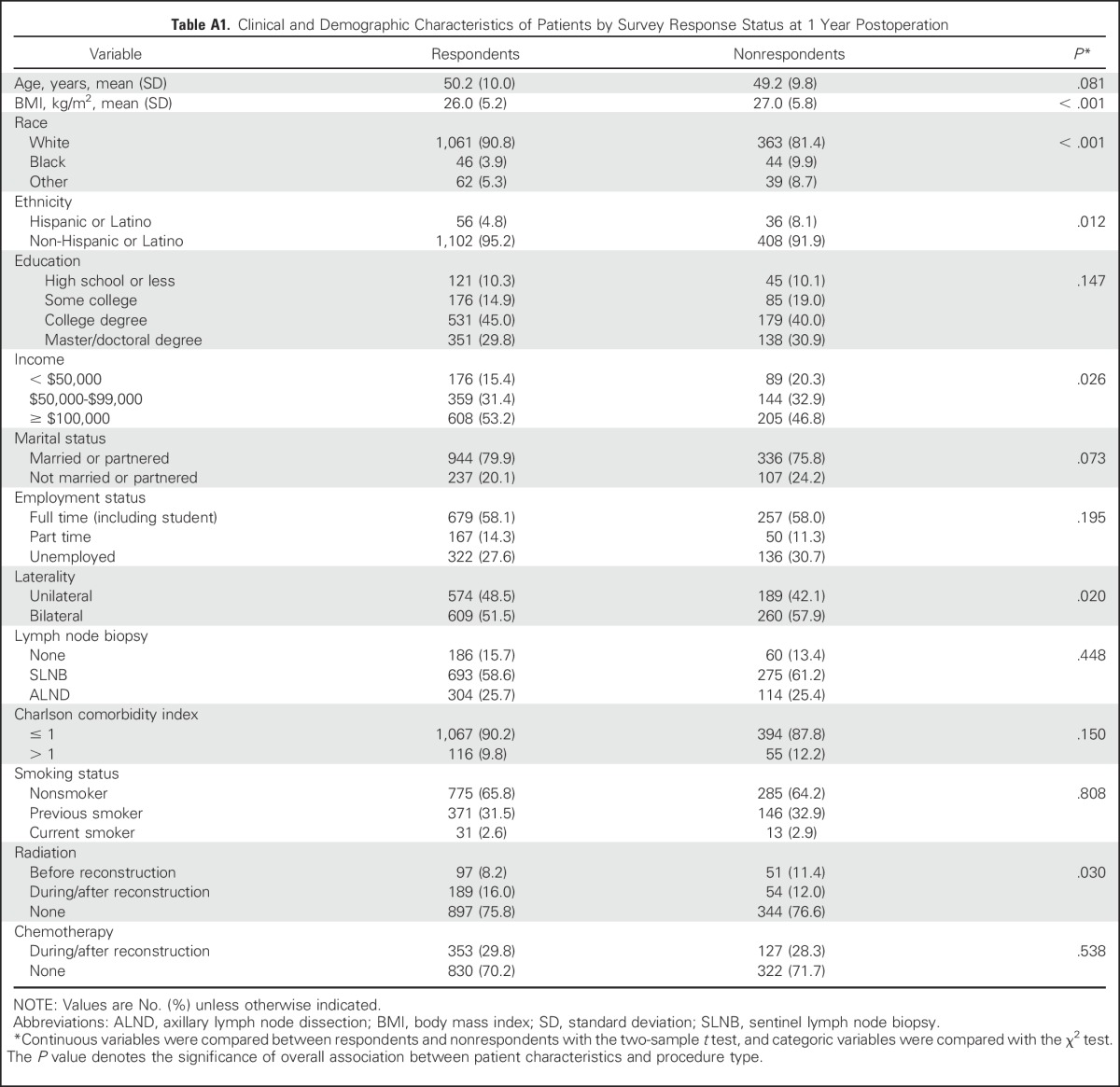
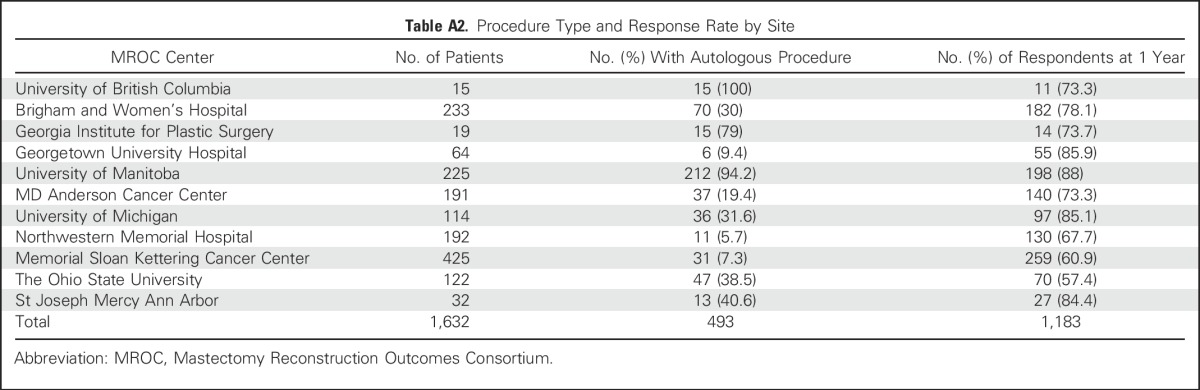
Table A2.
Procedure Type and Response Rate by Site
Footnotes
Supported by National Cancer Institute Grant No. R01 CA152192 and in part by National Cancer Institute Support Grant No. P30 CA008748 to A.L.P. and E.M.
The BREAST-Q is owned by Memorial Sloan Kettering Cancer Center and the University of British Columbia. A.L.P. is a co-developer of the BREAST-Q and receives royalties when it is used in for-profit industry-sponsored clinical trials.
Clinical trial information: NCT01723423.
See accompanying Oncology Grand Rounds on page 2467
Listen to the podcast by Dr Lee at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Andrea L. Pusic, Neil Fine, Hyungjin M. Kim, Claudia Albornoz, Edwin G. Wilkins
Collection and assembly of data: Andrea L. Pusic, Edward Buchel, Gayle M. Gordillo, Jennifer B. Hamill, Edwin G. Wilkins
Data analysis and interpretation: Andrea L. Pusic, Evan Matros, Hyungjin M. Kim, Ji Qi, Anne F. Klassen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andrea L. Pusic
Patents, Royalties, Other Intellectual Property: I am co-developer of BREAST-Q and receive royalty payments when it is used in industry-sponsored trials.
Evan Matros
No relationship to disclose
Neil Fine
No relationship to disclose
Edward Buchel
No relationship to disclose
Gayle M. Gordillo
Stock or Other Ownership: Med Compliance IQ
Consulting or Advisory Role: Gerson Lehrman Group
Research Funding: Smith & Nephew, GWR Medical, Natreon, Carotech BHD, Osata International
Patents, Royalties, Other Intellectual Property: Software patent for wound image analysis that is owned by Ohio State University and licensed to Med Compliance IQ, Inc
Travel, Accommodations, Expenses: NACCME, Peter Sheehan Diabetes Care Foundation
Jennifer B. Hamill
No relationship to disclose
Hyungjin M. Kim
No relationship to disclose
Ji Qi
No relationship to disclose
Claudia Albornoz
No relationship to disclose
Anne F. Klassen
Patents, Royalties, Other Intellectual Property: Royalties for Breast-Q, Face-Q patient-reported outcome instruments
Edwin G. Wilkins
No relationship to disclose
REFERENCES
- 1. Surveillance, Epidemiology, and End Results (SEER): Cancer Statistics Review 1975–2009 (Vintage 2009 Populations). Bethesda, MD, National Cancer Institute, 2012. [Google Scholar]
- 2.Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 3.Portschy PR, Tuttle TM. Rise of mastectomy. J Surg Oncol. 2013;107:563–564. doi: 10.1002/jso.23340. [DOI] [PubMed] [Google Scholar]
- 4.Stucky CC, Gray RJ, Wasif N, et al. Increase in contralateral prophylactic mastectomy: Echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol. 2010;17:330–337. doi: 10.1245/s10434-010-1259-x. [DOI] [PubMed] [Google Scholar]
- 5.Tracy MS, Rosenberg SM, Dominici L, et al. Contralateral prophylactic mastectomy in women with breast cancer: Trends, predictors, and areas for future research. Breast Cancer Res Treat. 2013;140:447–452. doi: 10.1007/s10549-013-2643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early-stage breast cancer. Ann Surg Oncol. 2013;20:1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 7.Fisher CS, Martin-Dunlap T, Ruppel MB, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19:3246–3250. doi: 10.1245/s10434-012-2525-x. [DOI] [PubMed] [Google Scholar]
- 8.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI on breast cancer treatment. Ann Surg Oncol. 2012;19:536–540. doi: 10.1245/s10434-011-1932-8. [DOI] [PubMed] [Google Scholar]
- 10.Franceschini G, Martin Sanchez A, Di Leone A, et al. New trends in breast cancer surgery: A therapeutic approach increasingly efficacy and respectful of the patient. G Chir. 2015;36:145–152. doi: 10.11138/gchir/2015.36.4.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassileth L, Kohanzadeh S, Amersi F. One-stage immediate breast reconstruction with implants: A new option for immediate reconstruction. Ann Plast Surg. 2012;69:134–138. doi: 10.1097/SAP.0b013e3182250c60. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S, Pappas L, Neumayer L, et al. An analysis of immediate postmastectomy breast reconstruction frequency using the Surveillance, Epidemiology, and End Results database. Breast J. 2011;17:352–358. doi: 10.1111/j.1524-4741.2011.01105.x. [DOI] [PubMed] [Google Scholar]
- 13.Alderman AK, Wei Y, Birkmeyer JD. Use of breast reconstruction after mastectomy following the Women’s Health and Cancer Rights Act. JAMA. 2006;295:387–388. doi: 10.1001/jama.295.4.387. [DOI] [PubMed] [Google Scholar]
- 14.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalfe KA, Zhong T, Narod SA, et al. A prospective study of mastectomy patients with and without delayed breast reconstruction: Long-term psychosocial functioning in the breast cancer survivorship period. J Surg Oncol. 2015;111:258–264. doi: 10.1002/jso.23829. [DOI] [PubMed] [Google Scholar]
- 16.Zhong T, McCarthy C, Min S, et al. Patient satisfaction and health-related quality of life after autologous tissue breast reconstruction: A prospective analysis of early postoperative outcomes. Cancer. 2012;118:1701–1709. doi: 10.1002/cncr.26417. [DOI] [PubMed] [Google Scholar]
- 17.Tanos G, Prousskaia E, Chow W, et al. Locally advanced breast cancer: Autologous versus implant-based reconstruction Plast Reconstr Surg Glob Open 4e622, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voineskos SH, Frank SG, Cordeiro PG. Breast reconstruction following conservative mastectomies: Predictors of complications and outcomes. Gland Surg. 2015;4:484–496. doi: 10.3978/j.issn.2227-684X.2015.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila AA, Mioton LM, Chow G, et al. Immediate two-stage tissue expander breast reconstruction compared with one-stage permanent implant breast reconstruction: A multi-institutional comparison of short-term complications. J Plast Surg Hand Surg. 2013;47:344–349. doi: 10.3109/2000656X.2013.767202. [DOI] [PubMed] [Google Scholar]
- 20.Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg. 2006;117:1–11. doi: 10.1097/01.prs.0000194899.01875.d6. [DOI] [PubMed] [Google Scholar]
- 21.Selber JC, Fosnot J, Nelson J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg. 2010;126:1438–1453. doi: 10.1097/PRS.0b013e3181ea42ed. [DOI] [PubMed] [Google Scholar]
- 22.Selber JC, Nelson J, Fosnot J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part I. Unilateral reconstruction. Plast Reconstr Surg. 2010;126:1142–1153. doi: 10.1097/PRS.0b013e3181f02520. [DOI] [PubMed] [Google Scholar]
- 23.Velanovich V. Experience with a generic quality of life instrument in a general surgical practice. Int J Surg Investig. 2000;1:447–452. [PubMed] [Google Scholar]
- 24.Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 2004;10:223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 25.Atisha DM, Rushing CN, Samsa GP, et al. A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22:361–369. doi: 10.1245/s10434-014-4246-9. [DOI] [PubMed] [Google Scholar]
- 26.Hwang ES, Locklear TD, Rushing CN, et al. Patient-reported outcomes after choice for contralateral prophylactic mastectomy. J Clin Oncol. 2016;34:1518–1527. doi: 10.1200/JCO.2015.61.5427. [DOI] [PubMed] [Google Scholar]
- 27.Jeevan R, Cromwell DA, Browne JP, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthet Surg. 2014;67:1333–1344. doi: 10.1016/j.bjps.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Macadam SA, Zhong T, Weichman K, et al. Quality of life and patient-reported outcomes in breast cancer survivors: A multicenter comparison of four abdominally based autologous reconstruction methods. Plast Reconstr Surg. 2016;137:758–771. doi: 10.1097/01.prs.0000479932.11170.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cano SJ, Klassen AF, Scott AM, et al. The BREAST-Q: Further validation in independent clinical samples. Plast Reconstr Surg. 2012;129:293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 30.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: The BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 31.Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24:2333–2344. doi: 10.1007/s11136-015-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 33.Albornoz CR, Cordeiro PG, Pusic AL, et al. Diminishing relative contraindications for immediate breast reconstruction: A multicenter study. J Am Coll Surg. 2014;219:788–795. doi: 10.1016/j.jamcollsurg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Farhangkhoee H, Matros E, Disa J. Trends and concepts in post-mastectomy breast reconstruction. J Surg Oncol. 2016;113:891–894. doi: 10.1002/jso.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulkarni AR, Sears ED, Atisha DM, et al. Use of autologous and microsurgical breast reconstruction by US plastic surgeons. Plast Reconstr Surg. 2013;132:534–541. doi: 10.1097/PRS.0b013e31829ae03e. [DOI] [PubMed] [Google Scholar]
- 36.Hawley ST, Jagsi R, Morrow M, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149:582–589. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderman AK, Chung KC. Discussion. Sensibility following innervated free TRAM flap for breast reconstruction: Part II. Innervation improves patient-rated quality of life. Plast Reconstr Surg. 2009;124:1426–1428. doi: 10.1097/PRS.0b013e3181baba54. [DOI] [PubMed] [Google Scholar]
- 38.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 39.Alves Nogueira Fabro E, Bergmann A, do Amaral E Silva B, et al. Post-mastectomy pain syndrome: Incidence and risks. Breast. 2012;21:321–325. doi: 10.1016/j.breast.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Couceiro TC, Valença MM, Raposo MC, et al. Prevalence of post-mastectomy pain syndrome and associated risk factors: A cross-sectional cohort study. Pain Manag Nurs. 2014;15:731–737. doi: 10.1016/j.pmn.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz KH, Stout NL, Andrews K, et al. Prospective evaluation of physical rehabilitation needs in breast cancer survivors: A call to action. Cancer. 2012;118:2187–2190. doi: 10.1002/cncr.27471. [DOI] [PubMed] [Google Scholar]
- 42.McNeely ML, Binkley JM, Pusic AL, et al. A prospective model of care for breast cancer rehabilitation: Postoperative and postreconstructive issues. Cancer. 2012;118:2226–2236. doi: 10.1002/cncr.27468. [DOI] [PubMed] [Google Scholar]
- 43.Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705. doi: 10.1097/PRS.0000000000002227. [DOI] [PubMed] [Google Scholar]
- 44.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: A new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. doi: 10.1016/j.bjps.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: A review of the literature 2009-2015. J Plast Reconstr Aesthet Surg. 2016;69:149–162. doi: 10.1016/j.bjps.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawilowsky S. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8:467–474. [Google Scholar]
- 47.Yost KJ, Eton DT, Garcia SF, et al. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System–cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64:507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groves RM. Nonresponse rates and nonresponse bias in household surveys. Public Opin Q. 2006;70:646–675. [Google Scholar]
- 49.Johnson TP, Wislar JS. Response rates and nonresponse errors in surveys. JAMA. 2012;307:1805–1806. doi: 10.1001/jama.2012.3532. [DOI] [PubMed] [Google Scholar]
- 50. doi: 10.1097/SLA.0000000000001698. Wong SM, Freedman RA, Sagara Y, et al: Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg [epub ahead of print on March 8, 2016] [DOI] [PubMed] [Google Scholar]
- 51.Yao K, Stewart AK, Winchester DJ, et al. Trends in contralateral prophylactic mastectomy for unilateral cancer: A report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol. 2010;17:2554–2562. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 52.Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: A multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol. 2014;21:2159–2164. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]
- 53.Momoh AO, Cohen WA, Kidwell KM, et al. Tradeoffs associated with contralateral prophylactic mastectomy in women choosing breast reconstruction: Results of a prospective multicenter cohort Ann Surg, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]