Abstract
HIV/AIDS is one of the leading causes of death among reproductive-age women throughout the world, and substance abuse plays a major role in HIV infection. We conducted a systematic review, in accordance with the 2015 Preferred Items for Reporting Systematic Reviews and Meta-analysis tool, to assess HIV risk-reduction intervention studies among reproductive-age women who abuse substances. We initially identified 6,506 articles during our search and, after screening titles and abstracts, examining articles in greater detail, and finally excluding those rated methodologically weak, a total of 10 studies were included in this review. Studies that incorporated behavioral skills training into the intervention and were based on theoretical model(s) were the most effective in general at decreasing sex and drug risk behaviors. Additional HIV risk-reduction intervention research with improved methodological designs is warranted to determine the most efficacious HIV risk-reduction intervention for reproductive-age women who abuse substances.
According to the World Health Organization (WHO, 2013), throughout the world, HIV/AIDS is one of the leading causes of death among women of reproductive age (15 to 49 years old). Substance abuse plays a major role in HIV infection; approximately 29% of HIV-infected women contracted the virus through injection drug use, and another 15% contracted HIV through sexual contact with an HIV-infected drug user (Ramsey, Bell, & Engler-Field, 2010). Individuals who abuse injection and non-injection drugs and alcohol engage in HIV risk behaviors including unprotected sex and multiple sex partners (Colfax & Shoptaw, 2005; Des Jarlais et al., 2007; Strathdee & Stockman, 2010; Wagner, Bloom, Hathazi, Sanders, & Lankenau, 2013; WHO, 2015). Women who inject drugs have been disproportionately affected by HIV; namely, in 2013, HIV prevalence was 13% among women who inject drugs and 9% among men who inject drugs (Joint United Nations Programme on HIV/AIDS, 2014).
In addition to the high risks associated with sharing syringes and injection paraphernalia (Cook & Clark, 2005), intravenous drug users (IDUs) play an additional, critical role in transmitting HIV to non-IDUs through sex-risk behaviors including unprotected sex (Cook & Clark, 2005; Noor, Ross, Lai, & Risser, 2014; Strathdee & Stockman, 2010), multiple sex partners (Noor et al., 2014), and sexual intercourse with other IDUs (Noor et al., 2014; Strathdee & Stockman, 2010). Moreover, consuming alcohol and smoking, ingesting, or inhaling drugs such as alcohol, crack cocaine, methamphetamine, and amyl nitrite (poppers) are also associated with increased risk for HIV infection by reducing users’ inhibitions to engage in risky sexual behavior, impairing judgment, and enhancing libido (Colfax & Shoptaw, 2005; Des Jarlais et al., 2007; Walley, Krupitsky, & Cheng, 2008). Exchange of sex for drugs or money, another risk factor for HIV infection, is common among those who abuse injection and non-injection drugs (Baker, Heather, Wodak, & Lewin, 2001; Draus & Carlson, 2009; El-Bassel et al., 1997; Inciardi, 1995; Meader et al., 2013; Westreich, Rosenberg, Schwartz, & Swamy, 2013). Of note, the risk of becoming infected with HIV via needle sharing varies in different parts of the world. For example, in Russia, 54% of new HIV infections in 2013 occurred among people who inject drugs (European Center for Disease Prevention and Control, 2016). HIV prevalence among people who inject drugs is also high in South-West Asia (29%) and Eastern and South-Eastern Europe (23%; United Nations Office on Drugs and Crime, 2015). On the contrary, in sub-Saharan African, HIV transmission via injection drug use is only 0.2%. In the United States in 2015, 6% of new HIV infections were attributed to injection drug use, and 3% were attributed to male-to-male sexual contact plus injection drug use (Centers for Disease Control and Prevention, 2017b).
The interplay of high HIV incidence and prevalence among women of reproductive age and the increased risk of HIV infection among substance abusers suggests that there is a critical public health need to develop HIV-prevention interventions for women of reproductive age who abuse substances. For this article, substance abuse refers to nonmedical use of prescription drugs, use or abuse of injection or non-injection illicit drugs (e.g., cocaine, heroin), or alcohol abuse.
HIV risk-reduction intervention studies from around the world conducted 10 or more years ago have demonstrated effectiveness of interventions aimed at reducing HIV risk behaviors among women of reproductive age who abuse substances (Neaigus et al., 1990; Rhoades, Creson, Elk, Schmitz, & Grabowski, 1998; Stein et al., 2005). These interventions’ results have provided the foundation for the development of more recent HIV intervention studies. In addition, recent and older reviews have also assessed the efficacy of HIV prevention interventions among drug users (Meader, Li, Des Jarlais, & Pilling, 2010; Semaan et al., 2002). However, these studies neither addressed both sex and drug risk behaviors nor stratified findings by gender (Des Jarlais & Semaan, 2008; Meader et al., 2010; Semaan et al., 2002). In addition, the Centers for Disease Control and Prevention (CDC) maintain a publicly accessible Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention (CDC, 2017a). This ongoing systematic review is conducted annually. The review synthesizes evidence-based interventions that show evidence of efficacy in changing drug-injection or sex behaviors that directly impact HIV-transmission risk. However, studies that focus on substance abuse treatment only are explicitly excluded despite that substance abuse has been shown to be associated with increased risk for HIV infection (Colfax & Shoptaw, 2005; Des Jarlais et al., 2007; Walley et al., 2008). Moreover, many of the studies have not been stratified by gender and, because the studies are not summarized, there are no suggestions of research gaps.
To our knowledge, no existing recent (within the past 10 years) systematic reviews have evaluated the efficacy of HIV risk-reduction interventions among women of reproductive age who abuse substances, therefore limiting researchers’ ability to expand knowledge on HIV prevention interventions for this population. The purpose of this systematic review was to assess HIV risk-reduction interventions among women of reproductive age (~15–44 years old) throughout the world who abuse substances, so that evidence-based recommendations can be made for future intervention studies and for clinical practitioners who work with patients at risk for HIV infection and suffering from substance use disorders.
METHODS
The present systematic review was conducted in accordance with the 2015 Preferred Items for Reporting Systematic Reviews and Meta-analysis (PRISMA-P) tool (Moher et al., 2015; Shamseer et al., 2015). PRISMA-P, an expansion of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines, was created by an international group of experts to improve the transparency, accuracy, completeness, and frequency of documented systematic review and meta-analysis protocols (Moher et al., 2015; Shamseer et al., 2015). PRISMA-P has been used by authors preparing systematic review protocols for publication as well as journal editors and peer reviewers for assessing the adequacy of review protocols for publication (Shamseer et al., 2015).
The PRISMA-P checklist provides a list of recommended items to address in a systematic review for each section of a manuscript. The checklist is based on elements from the PROSPERO register, the PRISMA checklist, SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist, and Standard 2.6 from the Institute of Medicine’s Standards for Systematic Reviews. The checklist includes, for example, an assessment of whether the introduction section contains: (a) a rationale for the review in the context of what is already known, and (b) an explicit statement of the question(s) that the review will address. With respect to the methods section, the checklist includes, for example, an assessment of the inclusion criteria and characteristics of the search strategy. Detailed explanations and evidence based rationales for each checklist item can be found in the article by Shamseer et al. (2015). PRISMA and PRISMA-P have been used in other systematic reviews of HIV/AIDS-related topics (Genberg et al., 2016; Takah, Kennedy, & Johnman, 2016).
INCLUSION CRITERIA
This review includes HIV risk-reduction intervention studies that: (a) were conducted and published between January 1, 2005, and June 30, 2015, (b) were published in peer-reviewed journals, and (c) included a sample of women of reproductive age (~15–44 years old) who abuse substances, specifically, any type of nonmedical use of prescription drugs, illicit drug (e.g., marijuana, heroin) or alcohol (liquor, wine) use. If our population of interest (reproductive-age women who abuse substances) was included in a broader study (i.e., men and women who abuse alcohol) and results were stratified fittingly, we included the study in this review. We did not limit our search based on type of intervention study design (e.g., four-arm randomized controlled trial vs. clinical trial with no control group). However, we excluded observational studies; and, HIV risk-reduction studies were only included if they measured an HIV biological outcome (i.e., HIV test) or an outcome related to HIV risk behavior, for example, sex without a condom or sharing needles. No restriction was placed on the country where studies were conducted due to the scarcity of research on the topic and specific population of interest.
LITERATURE SEARCH
The search strategy, including specific databases and search terms, was identified and agreed upon by four of the authors after consultation with a librarian with expertise in health database searches. The initial searches were conducted independently by two reviewers in the following seven electronic databases: PubMed, MED-LINE, Embase, CINAHL, PsychINFO, ASSIA, and Web of Science. The searches combined HIV/AIDS-related terms with terms related to the HIV risk-reduction intervention, sex, and substance abuse. The specific terms used are presented in Table 1. The literature search was conducted between May 25, 2016 and June 30, 2016.
TABLE 1.
Search Terms
| Query |
|---|
| HIV/AIDS: HIV OR “human immunodeficiency virus” OR “human immune deficiency virus” OR “Acquired Immunodeficiency Syndrome” OR AIDS OR “HTLV-III” OR “Human T Cell Lymphotropic Virus Type III” OR “Human T Lymphotropic Virus Type III” OR “Human T-Cell Leukemia Virus Type III” OR “Human T Cell Leukemia Virus Type III” OR “Human T-Cell Lymphotropic Virus Type III” OR “Human T-Lymphotropic Virus Type III” OR “Immunodeficiency Virus, Human” OR “Immunodeficiency Viruses, Human” OR “LAV-HTLV-III” OR “Lymphadenopathy-Associated Virus” OR “Lymphadenopathy Associated Virus” OR “Lymphadenopathy- Associated Viruses” OR “Virus, Lymphadenopathy-Associated” OR “Viruses, Lymphadenopathy-Associated” OR “Virus, Human Immunodeficiency” OR “Viruses, Human Immunodeficiency” OR “Immunologic Deficiency Syndrome, Acquired” OR “acquired immune deficiency syndrome” OR “Acquired Immuno-Deficiency Syndrome” OR “Acquired Immuno Deficiency Syndrome” OR “Acquired Immuno-Deficiency Syndromes” OR “Immuno- Deficiency Syndrome, Acquired” OR “Immuno-Deficiency Syndromes, Acquired” OR “Syndrome, Acquired Immuno-Deficiency” OR “Syndromes, Acquired Immuno-Deficiency” OR “Immunodeficiency Syndrome, Acquired” OR “Immunodeficiency Syndromes, Acquired” OR “Syndrome, Acquired Immunodeficiency” OR “Syndromes, Acquired Immunodeficiency” |
| Intervention: intervention OR interventions OR experiment OR experiments OR “Randomized Controlled Trials as Topic” OR “Trials, Randomized Clinical” OR quasi-experiment OR quasi-experiments OR “Non-Randomized Controlled Trials as Topic” OR “Non Randomized Controlled Trials as Topic” OR “Trial, Nonrandomized Clinical” OR “Trials, Nonrandomized Clinical” OR “Quasi-Experimental Studies” OR “quasi experimental study” OR “quasiexperimental study” OR “Quasi Experimental Studies” OR “Quasi-Experimental Study” OR “Studies, Quasi-Experimental” OR “Study, Quasi-Experimental” OR “Nonrandomized Controlled Trials as Topic” OR “Trial, Non-Randomized Clinical” OR “Trials, Non-Randomized Clinical” OR “Randomized Controlled Trials” OR “Randomized Controlled Trial” OR RCT OR “experimental study” OR “experimental studies” OR “clinical trials” OR “randomized controlled trial (topic)” OR “clinical trial” OR “Intervention Trials” OR “intervention trial” OR “experimental design” |
| Sex: women OR woman OR female OR females OR girl OR girls |
| Substance abuse: “Alcohol Drinking” OR “Drinking, Alcohol” OR “Alcohol Consumption” OR “Consumption, Alcohol” OR alcoholic OR alcoholics OR “drugs of abuse” OR “street drug” OR “Street Drugs” OR “Drugs, Street” OR “Recreational Drugs” OR “Drugs, Recreational” OR “Illicit Drugs” OR “illicit drug” OR “Drugs, Illicit” OR “drug, illicit” OR “Abuse Drugs” OR “drug abuse” OR “Prescription Drug Misuse” OR “Drug Misuse, Prescription” OR “Misuse, Prescription Drug” OR “Prescription Drug Misuses” OR “NMUPD” OR “Non-Medical Use of Prescription Drugs” OR “Non Medical Use of Prescription Drugs” OR Cocaine OR Heroin OR Diamorphine OR Diacetylmorphine OR Diagesil OR Diamorf OR “Min-I-Jet Morphine Sulphate” OR “Min I Jet Morphine Sulphate” OR “crystal meth” OR "Amphetamine-Related Disorders" OR “Amphetamine Related Disorders” OR “Disorder, Amphetamine-Related” OR “Disorders, Amphetamine-Related” OR “Amphetamine Abuse” OR “Abuse, Amphetamine” OR “Amphetamine Addiction” OR “Addiction, Amphetamine” OR “Amphetamine Dependence” OR “Dependence, Amphetamine” OR Methamphetamine OR “N-Methylamphetamine” OR “N Methylamphetamine” OR Metamfetamine OR Methylamphetamine OR Deoxyephedrine OR Desoxyephedrine OR Desoxyn OR Madrine OR Cannabis OR Cannabi OR Marihuana OR Marihuanas OR Marijuana OR Marijuanas OR Ganja* OR Hashish OR Hashishs OR Hemp OR Hemps OR Bhang OR Bhangs OR Phencyclidine OR “1-(1-Phenylcyclohexyl)piperidine” OR “Angel Dust” OR “Dust, Angel” OR “GP-121” OR “GP 121” OR “GP121” OR Serylan OR Sernyl OR “CL-395” OR “CL 395” OR “CL395” OR inhalant OR inhalants “Glue Sniffing” OR “Glue Sniffings” OR “Glue Abuse” OR “Abuse, Glue” OR “Abuses, Glue” OR “Glue Abuses” OR Hallucinogens OR Hallucinogen OR Psychedelic OR Psychedelics OR “Hypnotics and Sedatives” OR “Sedatives and Hypnotics” OR “Central Nervous System Stimulants” OR “Central Stimulants” OR “Stimulants, Central” OR Analeptic OR Analeptics OR “Opioid-Related Disorders” OR “Disorder, Opioid-Related” OR “Opiate Dependence” OR “Dependence, Opiate” OR “Opiate Addiction” OR “Addiction, Opiate” OR “Narcotic Abuse” OR “Abuse, Narcotic” OR “Abuses, Narcotic” OR “Narcotic Abuses” OR “Narcotic Dependence” OR “Dependence, Narcotic” OR “Narcotic Addiction” OR “Addiction, Narcotic” OR alcoholism OR “alcohol addiction” OR “addiction, alcohol” OR liquor OR liquors OR “illegal drug” OR “illegal drugs” OR “drug misuse” OR PCP OR “drug users” OR “Drug User” OR “User, Drug” OR “Users, Drug” OR “Drug Abusers” OR “Abuser, Drug” OR “Abusers, Drug” OR “Drug Abuser” OR “Drug Addicts” OR “1 (1 phenylcyclohexyl) piperidine” OR “ci 395” OR “ci395” OR “cn 25, 253 2” OR “cn 25253 2” OR “cn 25253-2” OR “cn25, 253 2” OR “cn25253 2” OR “cn25253-2” OR “nsc 40902” OR nsc40902 OR phencyclidin OR phencyclohexylpiperidine OR phenycyclidine OR phenylcyclidine OR sernylan OR syclan OR “abuse, drug” OR “chronic drug overuser” OR “drug problem” OR “needle sharing” OR “Narcotic Addict” OR “narcotic depression” OR “addict, narcotic” OR “narcotism” OR “psychoactive agent” OR “psychoactive drug” OR “psychoactive drugs” OR “psychodynamic agent” OR “psychopharmaceutic agent” OR psychopharmacon OR psychotropic OR psychotropics OR hallucinogenic OR hallucinogenics OR “mind expander” OR psychedelia OR psychodelic OR psychodelics OR psychodysleptic OR psychomimetic OR “psychotic drug” OR psychotomimetic OR psychotomimetics OR “addiction, opium” OR “opiate addict” OR “opioid dependence” OR “opium addict” OR “opium addiction” OR “opium alkaloid addiction” OR “substance abuse” OR “substance abuser” OR “substance abusers” OR “Intravenous Drug” OR “Intravenous Drugs” OR “IV drug” OR “IV drugs” OR “drug addiction” OR “Alcohol Dependence” OR “Alcohol Abuse” OR “Problem Drinking” OR “Drug Dependency” OR “Dependency (Drug)” OR Methedrine OR “Narcotic Drugs” OR “prescription drug abuse” OR “prescription drug addiction” OR Sedative OR Sedatives OR Hypnotic OR Hypnotics |
After the four authors determined the inclusion criteria and defined the search terms, two reviewers then independently screened abstracts of the articles identified in the initial search to determine whether they met inclusion criteria for this systematic review. For all relevant or ambivalent articles, the full text was reviewed. The reviewers obtained the opinion of a third or fourth independent reviewer for ambiguous articles that did not clearly meet the inclusion criteria. Every article that met the inclusion criteria was identified and the full text of each article was searched for additional relevant studies. Every article was assigned a numerical code for organizational identification purposes. Reviewers were not blind to the authors, funding, or any other characteristics of the studies reviewed.
DATA EXTRACTION AND ASSESSMENT OF METHODOLOGICAL QUALITY
With respect to data extraction and quality assessment, the two reviewers followed five major steps. First, the reviewers collaborated to create a data extraction and quality assessment evidence tool in Microsoft Excel. Second, the two reviewers independently extracted data and determined quality assessment ratings for each article. Third, the two reviewers compared their extracted data and quality assessment ratings to assess inter-rater reliability as has been done in previously published systematic reviews (Berg, Ross, & Tikkanen, 2011; Lloyd & Operario, 2012). Next, a third or fourth reviewer provided input regarding the disagreement. Differences in opinion during the data extraction and quality assessment ratings process were less than 10% and were resolved through consensus via the last step: the two reviewers made the final determination to include or exclude articles together.
With respect to data extraction, the two reviewers extracted data regarding the study design, participants, setting, intervention, control groups, data collection methods, outcome variables, main findings, and study limitations. The quality of each study was rated by two of the reviewers using the Quality Assessment Tool for Quantitative Studies, developed by the Effective Public Health Practice Project (Thomas, Ciliska, Dobbins, & Micucci, 2004). In accordance with the tool’s guidelines, studies were assessed on six components: selection bias, study design, confounders, blinding, data collection, and withdrawals/drop-outs. To assess selection bias, the reviewers determined (1) whether the individuals selected to participate in the study were likely to be representative of the target population and (2) the percentage of selected individuals who agreed to participate. Based on scores for these two items, reviewers referred to the Quality Assessment Tool for Quantitative Studies and its corresponding Dictionary (Thomas et al., 2004) to determine the score for the selection bias category. With respect to blinding, most data was self-reported, hence it was not relevant to bind the assessors or interviewers. It was also assumed not possible to blind participants to the research question given the nature of behavioral interventions. However, as per the guidelines, studies were assigned a weak rating if blinding was not mentioned and a moderate rating if researches stated that they did not blind assessors/interviewers and participants. Each of the remaining four components (study design, confounders, data collection, and withdrawals/drop-outs) was likewise reviewed, and, as per the guidelines, based on the ratings of these components, each study received an overall global rating of strong, moderate, or weak.
For a study to receive a strong rating, four of the six quality assessment criteria had to be rated as strong, with no weak ratings. Studies received moderate ratings if fewer than four criteria were rated strong and one criterion was rated weak; weak ratings were earned for studies in which two or more criteria were rated weak. Only strong and moderate studies were included in this review. After assessing quality, extracted data from each of the final nine studies were entered into a separate table of study characteristics including the quality assessment ratings.
RESULTS
ARTICLES IDENTIFIED DURING LITERATURE SEARCH
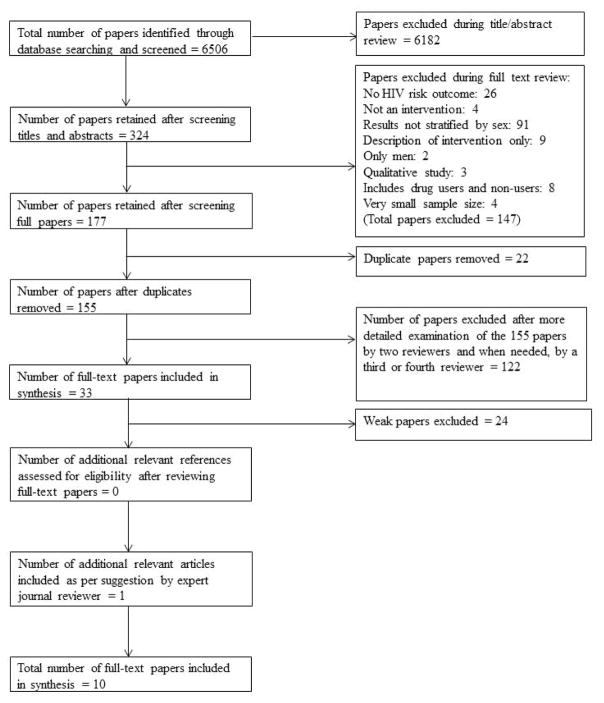
The flowchart in Figure 1 describes the articles examined and excluded in our search. A total of 6,506 articles were identified through our initial database search. After screening titles and abstracts, the list was narrowed down to 324 articles and after (a) excluding articles that clearly did not meet inclusion criteria and (b) removing articles that reported findings from the same intervention, 155 total articles remained. The 155 articles were examined in greater detail by two of the reviewers to determine whether they met inclusion criteria. A third or fourth reviewer was asked for her opinion if the first two reviewers agree that an article was too ambiguous. We determined that 33 articles met inclusion criteria. After rating the quality of the 33 studies, 24 were excluded because they were rated as weak using the procedure described above. One additional article was included in the analysis as suggested by an expert reviewer during the journal review process. As shown in Table 2, 9 included HIV risk-reduction studies received an overall methodological quality rating of moderate and one was rated strong. These HIV risk-reduction studies received moderate or weak ratings with respect to selection bias and blinding (assessor/interviewer and participants) and strong ratings for study design. Additionally, most studies received strong ratings for confounders, data collection methods, and withdrawals and dropouts.
FIGURE 1.
Search Strategy. The systematic review was conducted in accordance with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) tool (Genberg et al., 2016; Moher et al., 2015; Shamseer et al., 2015; Takah et al., 2016). The literature search was conducted between May 25, 2016 and June 30, 2016. All peer-reviewed published HIV risk-reduction intervention studies that were conducted and published between January 1, 2005, and June 30, 2015 and included women of reproductive age (~15–44 years old) who abuse substances were included in this review.
TABLE 2.
Quality Assessment of the 10 Studies Included in Systematic Review
| Reference | Selection Bias | Study Design | Confounders | Blinding | Data Collection Methods | Withdrawals and Dropouts | Overall Rating of the Article |
|---|---|---|---|---|---|---|---|
| Barry et al., 2008 | 2 | 1 | 1 | 3 | 1 | 1 | Moderate |
| Strathdee et al., 2013 | 2 | 1 | 1 | 2 | 3 | 1 | Moderate |
| Tross et al., 2008 | 2 | 1 | 3 | 2 | 1 | 2 | Moderate |
| Knudsen et al., 2014 | 2 | 1 | 1 | 3 | 2 | 1 | Moderate |
| Hien et al., 2010 | 2 | 1 | 1 | 2 | 1 | 2 | Moderate |
| Wechsberg et al., 2006 | 3 | 1 | 2 | 2 | 1 | 1 | Moderate |
| Wechsberget al., 2013 | 2 | 1 | 1 | 2 | 2 | 1 | Moderate |
| Woody et al., 2014 | 2 | 1 | 1 | 2 | 1 | 2 | Moderate |
| Koblin et al., 2010 | 2 | 1 | 1 | 2 | 3 | 1 | Moderate |
| El-Bassel et al., 2014 | 2 | 1 | 1 | 2 | 1 | 1 | Strong |
Note. Based on Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project. 1: strong, 2: moderate, 3: weak.
SAMPLE RECRUITMENT AND DEMOGRAPHICS OF STUDY PARTICIPANTS
A summary of the sample, location, intervention, outcome variables, and main findings for the 10 HIV risk-reduction studies is presented in Table 3 (Barry, Weinstock, & Petry, 2008; El-Bassel et al., 2014; Hien et al., 2010; Knudsen, Staton-Tindall, Oser, Havens, & Leukefeld, 2014; Koblin et al., 2010; Strathdee et al., 2013; Tross, Campbell, & Cohen, 2008; Wechsberg, Luseno, Lam, Parry, & Morojele, 2006; Wechsberg et al., 2013; Woody et al., 2014). Studies that met the inclusion criteria for this systematic review enrolled a total of 3,796 participants at baseline. Study populations varied by race/ethnicity, country of residence, country of birth, and socioeconomic status. Two studies excluded pregnant women from participating (Koblin et al., 2010; Tross et al., 2008), and the other eight did not clarify whether pregnant women were included in the sample. Seven studies were conducted in the United States. Two were conducted in South Africa (Wechsberg et al., 2006, 2013) and one in Mexico (Strathdee et al., 2013). Most studies examined samples of only female participants; however, one study examined both male and female participants, but was included in our review because findings were stratified by sex (Woody et al., 2014). Recruitment occurred within drug treatment centers (i.e., methadone maintenance clinics), correctional facilities, community supervision settings, via outreach work in locations such as bars, brothels, and shooting galleries, and within communities such as at beauty parlors and corner shops. Inclusion criteria for participation included self-reported intravenous drug abuse, non-injection drug abuse, or nonspecified injection behavior for substance abuse. In three studies, participants’ drug of choice was cocaine (Barry et al., 2008; Tross et al., 2008; Wechsberg et al., 2006). In five studies, participants reported using a variety of drugs including marijuana, cocaine, crack, heroin, oxycodone, benzodiazepines, other opiates, and/or alcohol (El-Bassel et al., 2014; Knudsen et al., 2014; Koblin et al., 2010; Wechsberg et al., 2013; Woody et al., 2014). Two studies did not report the specific type of drug(s) used by participants (Barry et al., 2008; Hien et al., 2010).
TABLE 3.
Details of 10 Studies That Met Inclusion Criteria for this Systematic Review
| Reference | Location | Sample Size | Intervention Group | Comparison Group/Control Condition | Outcome Variables | Main (Significant) Findings |
|---|---|---|---|---|---|---|
| Barry et al., 2008 | Hartford, CT, U.S. | 123 women (38.2% African American, 38.2% Hispanic, 23.6% White) | 3 months of standard methadone treatment plus weekly individual or group counseling with 3 months of contingency management (CM) intervention. | Standard methadone treatment and individual or group counseling. Not time and attention matched. | Sex risk behaviors, drug use risk behaviors | CM intervention group showed greater reductions in risky drug use behaviors than standard care participants at 6-month follow up (p = .016). No changes in sex risk behavior (p > .05). |
| Strathdee et al., 2013 | Tijuana and Ciudad Juarez, Mexico | 584 women living in Mexico | One 60-minute session of interactive injection risk/didactic sexual risk intervention (Group B), interactive sexual risk/didactic injection risk intervention (Group C), or interactive injection risk/interactive sexual risk intervention (Group D) | Didactic injection risk intervention and didactic sexual risk intervention (Group A). Time and attention matched. | Presence of HIV and STD (Treponema pallidum, Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis) infection | At follow-up, in Tijuana, HIV/STD incidence in Groups C and D was 62% and 63% lower than Group A (95% CI [0.16, 0.89]). In Cd. Juarez, HIV/STI incidence was 56% lower for women in Group C than in Group A (95% CI [0.19, 0.99]). For Cd. Juarez, the interactive injection risk intervention was associated with declines in receptive needle sharing (p = .04) and injection risk index score (p = .01). |
| Tross et al., 2008 | 12 treatment programs across 9 states in the U.S. (West: 2; Midwest: 2; Northeast: 4; Southeast: 4) | 515 women (56.8% White, 23.2% Black/African American, 10.4% Hispanic/Latina, 9.6% mixed or other) | Five 90-minute group sessions designed to build cognitive, affective, and behavioral skills for safer sexual decision making and behavior: Safe Sex Skill Building (SSSB) | HIV/STD Health Education (HE): Single 60-minute group session consisting of discussion of HIV definitions, transmission, testing and counseling, treatment, and prevention. Not time and attention matched. | Sex risk behaviors | Decrease in unprotected sexual occasions at 3-month follow-up for both groups (p < .05). No difference between groups from baseline to 3-month follow-up (p > .05). At 6-month follow-up, participants in the SSSB participants in SSSB condition had 29% fewer unprotected sexual occasions than those in the HE condition at 6-month follow-up (p < .0001, women). |
| Knudsen et al., 2014 | Four correctional facilities in CT, DE, KY, and RI, U.S. | 270 women (71.4% White, 21.7% African American, 6.9% other) | Viewed 17-minute video, Drug Abuse and HIV: Reaching Those at Risk, then five group sessions and one individual booster session 30 days post prison release: Reducing Risky Relationships for HIV (RRR-HIV). | Not time and attention matched. Watched same video as intervention group but received no further intervention. | Sex risk behaviors | Participants in the RRR-HIV group had fewer unprotected vaginal, anal, and/or oral sexual encounters occasions at follow-up (p = .02). |
| Hien et al., 2010 | Six geographically diverse community- based substance abuse treatment program in the U.S. | 346 women (46.2% White, 33.5% African American, 6.1% Latina, 14.2% other) | Two 90-minute sessions per week for 6 weeks (12 total sessions) of cognitive behavioral intervention for women with PTSD and substance use disorder: Seeking Safely. | Time and attention matched: Women’s Health Education. | Sex risk behaviors | Seeking Safely was more effective in reducing sex risk behaviors for women with higher levels of unprotected sex compared with a Women’s Health Education curriculum (p < .05). |
| Wechsberg et al., 2006 | Pretoria, South Africa | 93 Black or South African women | Women-Focused Intervention plus HIV/AIDS issues facing women in South Africa: two private one-hour sessions held within 2 weeks, each lasting a little longer than one hour. Women also received a risk-reduction and toiletry kit and referrals to resources. | Adapted version of the revised NIDA Standard Intervention, two private one-hour sessions. Time and attention matched. | Sex risk behaviors, STI symptoms | Proportion of women who reported always using male condoms in the past month with boyfriends increased in the Women-Focused group (23% to 33%; p < .01) and remained stable in standard group (36%). Proportion of women using a male condom with a boyfriend during last sexual encounter increased (28% to 55%) in Woman- Focused group and to a lesser extent in standard group (44% to 48%). Both groups showed a decrease (Woman-Focused: 65% to 54%; Standard: 58% to 53%) in the proportion of participants who reported using substances during sex work (p > .05 for both groups). Participants in the Woman- Focused group reported fewer STI symptoms at follow-up than women in the standard group (mean = 0.64 vs. 1.07, effect size [d] = −0.43). |
| Wechsberg et al., 2013 | Cape Town, South Africa | 720 women (45% Black African and 55% Coloured) | Women’s Health CoOp (WHC) intervention: four-module intervention conducted over two sessions lasting about 1 hour each. | Nutrition control group and an HIV Counseling and Testing (HCT)- only control. Not time and attention matched. | Biologically confirmed abstinence from drug use, sexual risk behaviors and victimization, drug use during sex. | At 12-month follow up, 26.9% of participants were abstinent in the WHC arm compared to 16.9% in the nutrition arm and 20% in the HCT only control arm (OR = 1.54, 95% CI [1.07, 2.22]; Cohen’s d: 0.238). Proportion of women in the WHC arm reporting not being impaired during their last sexual encounter was lower than the other two arms combined (OR: 1.32, 95% CI [1.02, 1.84]; Cohen’s d: 0.153). Protection during sex with main partner increased for all three groups at 12-month follow-up (HCT: 23.4% to 32.5, p = .022; nutrition: 22.0% to 41.0%, p < .001; WHC: 28.5 to 42.9, p < .001). |
| Woody et al., 2014 | Baltimore, MD, U.S. | 495 men, 236 women (13% Hispanic, 60.9% White, 9.9% Black or African American, 6% American Indian/Alaskan Native, 0.5% Asian, 0.1% Native Hawaiian/Pacific Islander, 9.2% other, 0.4% unknown) | 24 weeks of methadone treatment and four or more blood draws to test for drug use. | Buprenorphine- naloxone treatment. Not time and attention matched. | Sex risk composite score (more than one sexual partner and/or sex without a condom) | Sex risk composite score was reduced with no differences between groups (p > .05). Follow-up assessments showed reductions from baseline in the mean number of times heroin, speedball, or other opioids were injected in the past 30 days, as well as the overall number of injection events (p < .0008). Reductions were also noted in the percentage who shared needles, did not clean shared needles with bleach, shared cookers, engaged in front/back loading, and the needle risk composite score (p < .0001). There were no significant differences between groups on any of these outcomes. The only significant group difference on injecting risk was for the mean number of times amphetamines were injected (BUP group: rose from 0.05 at baseline to 1.9 at 24 weeks; MET: decreased from 0.29 to 0.22; p < .05). |
| Koblin et al., 2010 | New York City, NY, U.S. | 311 women (65% Black, 23.8% Latina, 10% mixed/white/other) | Standard HIV vaccine education: (application vignettes, standardized flipcharts, and a 7-minute video consisting of testimonials by women who participated in HIV vaccine trials) and enhanced HIV risk reduction (3 counseling sessions). | Standardized risk reduction counseling. Not time and attention matched. | Sex risk behaviors | Percent of women reporting unprotected vaginal sex declined (p < .0001). No differences were found by study arm. |
| El-Bassel et al., 2014 | New York City, NY, U.S. | 306 women (68% Black, 15% Latino, 17% other) | Two intervention groups: Traditional WORTH: Four face-to-face sessions once per week, 90–120 minutes each. Intervention core components: HIV/STI knowledge, risk reduction problem-solving and negotiation skills, condom use intentions, outcome expectancies, self-efficacy, partner abuse risk assessment, safety planning, social support, identification of service needs and linkage to services, risk reduction goal setting. Multimedia WORTH: weekly face-to-face meetings with core components translated to interactive computerized games, video enhancements, and interactive visual tools plus a computerized web- based case management service tool. | Wellness Promotion Arm: Delivered in group setting. Core components: maintaining a healthy diet, promoting fitness, addressing tobacco use risk, stress-reduction, personal health goals. Attention matched. | Sex risk behaviors, presence of HIV and STD (chlamydia, gonorrhea, and trichomoniasis) infection. | At 12-month follow-up, women in the two intervention groups were more likely to report consistent condom use during sex with primary partners than women in control group (OR = 2.36; 95% CI [1.28, 4.37]). Both intervention conditions had significant effects in lowering the number of unprotected sex acts (multimedia WORTH: IRR = 0.72 and 95% CI [0.55, 0.93]; traditional worth: IRR = 0.72 and 95% CI [0.55, 0.95]), increasing the proportion of protected sex acts (multimedia WORTH: b = 0.11 and 95% CI [0.02, 0.20]; traditional WORTH: b = 0.10 and 95% CI [0.01, 0.19]), and increasing consistent condom use (multimedia WORTH: OR = 2.38 and 95% CI [1.16, 4.87]; traditional WORTH: OR = 2.34 and 95% CI [1.16, 4.75]). Of 244 women, two HIV seroconverted (one in each intervention group). Forty-seven new STD cases were found at the 12-month follow-up with no differences between groups. |
CHARACTERISTICS OF INTERVENTIONS
All 10 HIV risk-reduction studies included in this review were identified as randomized controlled trials, with most (n = 7) comprised of two study arms; two studies had three arms (El-Bassel et al., 2014; Wechsberg et al., 2013), and another had four arms (Strathdee et al., 2013). HIV risk-reduction interventions were delivered via group sessions, individual sessions, or a combination of group and individual sessions. One study included an independently viewed multimedia intervention group that consisted of interactive visual tools and activities (El-Bassel et al., 2014). Sessions focused on improving sex-risk behaviors, drug risk behaviors, or a combination of the two types of risk behaviors. Intervention types included behavioral skill-building (i.e., condom use practice, role-playing) with education and daily methadone dosing only or in conjunction with individual and/or group counseling. Four HIV risk-reduction intervention studies included time/attention matched control groups (El-Bassel et al., 2014; Hien et al., 2010; Strathdee et al., 2013; Wechsberg et al., 2006). More than half (70%) of the studies were developed according to a specific theoretical basis such as Social Cognitive Theory and Feminist Theory (El-Bassel et al., 2014; Hien et al., 2010; Koblin et al., 2010; Strathdee et al., 2013; Tross et al., 2008; Wechsberg et al., 2006, 2013). Three studies used evidence-based interventions (El-Bassel et al., 2014; Tross et al., 2008; Wechsberg et al., 2013). Four studies addressed intervention fidelity including training of the interviewers or interventionists or quality control throughout the duration of the intervention period (El-Bassel et al., 2014; Knudsen et al., 2014; Koblin et al., 2010; Tross et al., 2008).
OUTCOME VARIABLES MEASURED
Most HIV risk-reduction studies measured changes in sex-risk behaviors (n = 9), and half measured changes in drug risk behaviors (n = 5). However, some of the studies also measured other outcome variables including incidence of HIV and infection (El-Bassel et al., 2014; Strathdee et al., 2013), STD symptoms (Wechsberg et al., 2006), any drug use (El-Bassel et al., 2014; Wechsberg et al., 2006), and HIV testing history (Woody et al., 2014). Three studies measured biological outcomes: abstinence from drug (methamphetamine, cocaine, opiates, THC, methaqualone) use (Wechsberg et al., 2013) and incidence of HIV and STD’s (El-Bassel et al., 2014; Strathdee et al., 2013). Study outcomes were assessed at time points ranging from one month (Wechsberg et al., 2006) to twelve months after baseline (El-Bassel et al., 2014; Hien et al., 2010; Koblin et al., 2010; Strathdee et al., 2013; Wechsberg et al., 2013).
MAJOR FINDINGS: SEX-RELATED HIV RISK BEHAVIORS
Among the HIV risk-reduction studies that measured changes in sexual risk behaviors, there were mixed results. Barry et al. (2008) showed no changes in unprotected sexual occasions; however, that study entailed a standard methadone treatment plus contingency management (intervention group) versus a standard methadone only treatment without any sex-risk (i.e., condom education) component incorporated into the intervention. Among the five studies (El-Bassel et al., 2014; Hien et al., 2010; Hien et al., 2010; Knudsen et al., 2014; Tross et al., 2008; Wechsberg et al., 2006) that reported greater improvements in sex-risk behaviors for those in the intervention group versus the control group, the respective control groups received an HIV and STD knowledge-based intervention or a health promotion intervention (i.e., nutrition and exercise counseling) whereas the intervention group received behavioral skills training specific to HIV prevention. Among the three studies (Koblin et al., 2010; Wechsberg et al., 2013; Woody et al., 2014) that reported decreases in sex-risk behaviors for both the intervention and control groups, two studies did not provide any behavioral skills training (Koblin et al., 2010; Woody et al., 2014). In addition, control groups for all three studies did not receive time or attention matched interventions.
MAJOR FINDINGS: DRUG-RELATED HIV RISK BEHAVIORS
Among the three HIV risk-reduction studies that demonstrated favorable changes with respect to drug risk behaviors that were greater for the intervention group compared to the control group (Barry et al., 2008; Wechsberg et al., 2006, 2013), the respective control groups received drug therapy only (Barry et al., 2008), education only (Wechsberg et al., 2006), or HIV testing and counseling only (Wechsberg et al., 2013); whereas, the intervention groups received additional behavioral skills training. For the study that demonstrated similar favorable changes for the intervention and control groups (Woody et al., 2014), both the intervention group (methadone treatment) and the control group (Buprenorphine-naloxone) received only drug therapy without any behavioral skills component.
DISCUSSION
The results presented in this systematic review suggest that behavioral interventions have the potential to be efficacious at improving sex- and drug-related HIV risk behaviors among women of reproductive age who abuse substances. However, consistent evidence during the last 10 years does not exist and studies of strong methodological quality are lacking. As such, it is difficult to make specific recommendations with respect to the most efficacious HIV risk-reduction interventions for reproductive-age women who abuse substances. For example, although all 10 HIV risk-reduction studies in this systematic review had a strong study design, all were also moderate or weak with respect to selection bias, indicating results may not be highly generalizable to the larger population of reproductive-age women who abuse substances. Additionally, because all studies had moderate or weak ratings with respect to blinding, reporting bias may have played a role. Although it is not usually possible to completely blind participants to the nature of a behavioral intervention study, future studies should describe any efforts taken to blind participants. In addition, no study mentioned blinding of the individuals who conducted statistical analyses, so we suggest future studies take this into consideration to decrease the chance of bias.
Our findings suggest that behavioral interventions have the potential to be effective at decreasing sex-related risk behaviors among reproductive-age women who abuse substances. Studies that reported greater improvements in sex-risk behaviors among those in the intervention group compared with the control group were characterized by: (1) having an intervention that included behavioral skills training (e.g., condom negotiation skills) in addition to HIV and STD education (El-Bassel et al., 2014; Hien et al., 2010; Knudsen et al., 2014; Tross et al., 2008; Wechsberg et al., 2006) and (2) having an intervention based on a theoretical concept (e.g., Social Cognitive Theory). As such, our findings suggest that future HIV risk-reduction studies with reproductive-age women who abuse substances should include behavioral skills training (i.e., condom use skills) and an overall intervention that is grounded in theory to most effectively prevent sex-risk behaviors in this population. One efficacious study included a multimedia intervention that was comprised of independently viewed interactive computerized games, video enhancements, visual tools, and interactive skill-building activities (El-Bassel et al., 2014). Accordingly, self-paced multi-media interventions may be worth further exploration in future studies as it may be a cost-effective HIV risk reduction intervention strategy.
Clinical practitioners should consider incorporating behavioral skills training into HIV and STD counseling sessions for clients/patients. Using the included studies as a template for intervention strategies may help prevent the spread of HIV and other STDs to sex and drug partners and unborn children of reproductive-age women who abuse substances (Baker et al., 2001; Colfax & Shoptaw, 2005; Cook & Clark, 2005; Des Jarlais et al., 2007; Draus & Carlson, 2009; El-Bassel et al., 1997; Inciardi, 1995; Noor et al., 2014; Wagner et al., 2013; Walley et al., 2008).
Given that only four (Barry et al., 2008; Wechsberg et al., 2006, 2013; Woody et al., 2014) of the HIV risk-reduction studies measured changes in drug risk behaviors (i.e., needle sharing), our findings suggest that the current body of literature is lacking HIV prevention intervention research that incorporates drug risk-reduction behaviors for reproductive-age women who abuse substances. More research is warranted to prevent the spread of HIV via drug risk behaviors. Regardless, among the three studies that demonstrated favorable changes with respect to drug-related HIV risk behaviors that were greater for the intervention group compared to the control group (Barry et al., 2008; Wechsberg et al., 2006, 2013), the intervention groups received behavioral skills training in addition to drug therapy (Barry et al., 2008), education (Wechsberg et al., 2006), and HIV testing and counseling (Woody et al., 2014). Accordingly, future HIV risk-reduction programs for reproductive-age women who abuse substances should incorporate behavioral skills training aimed at decreasing or controlling substance abuse risk behaviors (e.g., needle sharing) into HIV related drug risk-reduction intervention. Findings of this systematic review should be interpreted with consideration of the specific context. Specifically, because seven of the 10 studies were conducted in the United States, findings may not be generalizable to persons who live in other countries. Importantly, however, of the estimated 12 million people who inject drugs worldwide, nearly half of injection drug users live in China, Russia, and the U.S. (European Center for Disease Prevention and Control, 2015). As such, the recent (previous 10 years) peer-reviewed literature is lacking HIV risk reduction intervention studies among women who abuse substances and live in China or Russia. There is a need for published studies in these countries.
This review highlighted a number of gaps in the existing literature. First, although it is not clear whether any pregnant women were included in any of the studies, two studies explicitly excluded pregnant women from participating (Koblin et al., 2010; Tross et al., 2008), and no study specifically targeted pregnant women who abuse substances. This is a concerning gap considering that both sex- and drug-related HIV risk behaviors are common among pregnant women who abuse substances (Baker et al., 2001; Ramsey, Bell, & Engler-Field, 2010), and if infected with HIV, pregnant women can infect their child via vertical transmission. This finding is in accordance with another systematic review that revealed that pregnant women tend to be underrepresented in HIV/AIDS research (Westreich et al., 2013). Second, results from our study suggest that no studies during the last 10 years have been conducted with women 15 to 17 years old who abuse substances, which is concerning since HIV is one of the leading causes of death among women as young as 15 through age 49 throughout the world (World Health Organization, 2013). Intervening with women before they turn 18 years old may be an effective way to prevent the start or the early onset of HIV risk behaviors. Third, the longest follow-up period was 12 months (El-Bassel et al., 2014; Hien et al., 2010; Koblin, 2010; Strathdee et al., 2013; Wechsberg et al., 2013); consequently, we do not know if any intervention effects were sustained beyond this point. Fourth, because only two studies tested for HIV and STD infection through biological tests, we have determined that the current body of literature is lacking biological outcome measures. Future HIV risk-reduction studies should assess HIV and STD diagnosis to gain a better understanding of intervention effectiveness. Fifth, although more than half (1.4 million out of 2.1 million) of new HIV infections in 2015 occurred in Africa (Global AIDS Response Progress Reporting, 2016), only two of the studies in this review examined participants in (South) Africa (Wechsberg et al., 2006, 2013). Although HIV risk-reduction interventions may occur in other regions, the Western, Central, and North African regions are lacking any published HIV research for reproductive-age women who abuse substances. Last, we suggest authors provide more details about the interventions, so that they can be replicated in appropriate settings. For example, Koblin et al. (2010) did not explain how long the counseling sessions lasted, and so it is difficult for interested researchers to replicate their work.
There are several limitations in this systematic review that should be noted. Because only two studies tested participants for HIV or STDs (El-Bassel et al., 2014; Strathdee et al., 2013), most outcomes were based on self-reported information, increasing the chance for recall bias and social desirability bias. We excluded studies that only assessed changes in alcohol drinking behaviors or non-intravenous drug use without a measure of HIV risk behavior because of our inclusion/exclusion criteria; however, alcohol abuse or non-intravenous drug abuse may itself lead to increased HIV risk behavior (Colfax & Shoptaw, 2005; Cook & Clark, 2005; Des Jarlais et al., 2007; Draus & Carlson, 2009; El-Bassel, 1997; Inciardi, 1995; Noor et al., 2014; Strathdee & Stockman, 2010; Walley et al., 2008). We also excluded studies that investigated changes in HIV medication adherence, which some researchers assert is an HIV risk behavior as HIV transmission risk is decreased when an HIV-infected individual’s viral load is suppressed (Cohen et al., 2011; Fideli et al., 2001; Quinn et al., 2000; Rodger et al., 2016; Tovanabutra et al., 2002). In addition, this review examined a broad scope of research, including studies measuring different outcomes along the HIV risk behavior continuum and studies conducted in different countries, which limited our ability to combine results for a meta-analysis. Furthermore, because participants in the 10 studies used a variety of drugs and combinations of drugs and/or alcohol, it was not possible to determine which types of interventions were most effective for specific types of drug users (e.g., cocaine only users vs. combination cocaine, heroin, and alcohol abusers). Similarly, due to the inadequate amount of studies published with the target population, we were unable to identify interventions that were more effective for specific racial or ethnic groups. Additionally, only four studies (El-Bassel et al., 2014; Knudsen et al., 2014; Koblin et al., 2010; Toss et al., 2008) addressed intervention fidelity, and therefore, it is not known whether intervention protocols were carried out as intended by the research team. Further, we only included studies in peer-reviewed journals although we recognize that findings from HIV risk-reduction interventions among reproductive-age women may be published elsewhere (e.g., dissertations). Finally, we excluded studies conducted more than 10 years ago. However, this was deliberate so that researchers could review the most recent findings, which could in turn serve as a basis for the development of more effective interventions with this high-risk population.
Future systematic reviews of HIV risk-reduction interventions among reproductive-age women who abuse substances should consider reviewing HIV-protective factors. In addition to the well-known protective factors such as wearing condoms and being in a monogamous sexual relationship, studies have also shown that other factors such as social norms, acculturation, socioeconomic issues (food insecurity), and interpersonal issues (e.g., higher power of male sex partner) may play a role, namely they may increase risk of HIV infection among substance abusers (Ebrahim, Davis, & Tomaka, 2016; Gelpí-Acosta et al., 2016; Villar-Loubet et al., 2016). However, these protective factors were not addressed in the present study.
CONCLUSIONS
This article provided a review of the most current literature with respect to HIV risk-reduction intervention studies among reproductive-age women who abused substances, providing researchers with a summary of current literature with which they may expand current knowledge with respect to HIV prevention interventions among women who abuse substances. Accordingly, recent HIV risk-reduction intervention studies among reproductive-age women indicate that interventions that incorporate behavioral skills components and are theory driven may be most effective and should be further explored in future studies. Additional HIV risk-reduction research, especially that which focuses on reducing drug risk behaviors and with improved methodological design, is needed so that strong recommendations can be made with respect to the most efficacious evidence-based HIV risk-reduction interventions for women of reproductive age who abuse substances.
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse (award #K99DA041494) and the National Institute on Minority Health and Health Disparities (award #P20MD002288) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute on Minority Health and Health Disparities, or the National Institutes of Health. We acknowledge Mr. Arnaldo Gonzalez for his editing support.
Contributor Information
Jessica Weissman, Center for Research on U.S. Latino HIV/AIDS and Drug Abuse, Florida International University, Miami, Florida., Robert Stempel College of Public Health & Social Work, Florida International University.
Mariano Kanamori, Center for Research on U.S. Latino HIV/AIDS and Drug Abuse, Florida International University, Miami, Florida.
Jessy G. Dévieux, Robert Stempel College of Public Health & Social Work, Florida International University.
Mary Jo Trepka, Center for Research on U.S. Latino HIV/AIDS and Drug Abuse, Florida International University, Miami, Florida., Robert Stempel College of Public Health & Social Work, Florida International University.
Mario De La Rosa, Center for Research on U.S. Latino HIV/AIDS and Drug Abuse, Florida International University, Miami, Florida., Robert Stempel College of Public Health & Social Work, Florida International University.
References
- Baker A, Heather N, Wodak A, Lewin T. Heroin use and HIV risk-taking behaviour among women injecting drug users. Drug and Alcohol Review. 2001;20:205–211. [Google Scholar]
- Barry D, Weinstock J, Petry NM. Ethnic difference in HIV risk behaviors among methadone-maintained women receiving contingency management for cocaine use disorders. Drug and Alcohol Dependence. 2008;98:144–153. doi: 10.1016/j.drugalcdep.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RC, Ross MW, Tikkanen R. The effectiveness of MI4MSM: How useful is motivational interviewing as an HIV risk prevention program for men who have sex with men? A systematic review. AIDS Education and Prevention. 2011;23:533–549. doi: 10.1521/aeap.2011.23.6.533. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Compendium of evidence-based interventions and best practices for HIV prevention. 2017a Retrieved January 26, 2017, from https://www.cdc.gov/hiv/research/in-terventionresearch/compendium/rr/index.html.
- Centers for Disease Control and Prevention. Statistics overview: Diagnoses of HIV infection, by transmission category. 2017b Retrieved January 24, 2017, from https://www.cdc.gov/hiv/statistics/overview/
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax G, Shoptaw S. The methamphetamine epidemic: Implications for HIV prevention and treatment. Current HIV/AIDS Reports. 2005;2:194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- Cook RL, Clark DB. Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sexually Transmitted Diseases. 2005;32:156–164. doi: 10.1097/01.olq.0000151418.03899.97. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn D, … Friedman SR. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007;21:231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: The first 25 years and counting. Psychosomatic Medicine. 2008;70(5):606–611. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- Draus PJ, Carlson RG. “The game turns on you”: Crack, sex, gender, and power in small-town Ohio. Journal of Contemporary Ethnography. 2009;38:384–408. [Google Scholar]
- Ebrahim NB, Davis S, Tomaka J. Attitude as a mediator between acculturation and behavioral intentions. Public Health Nursing. 2016;33:558–564. doi: 10.1111/phn.12281. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Gilbert L, Goddard-Eckrich D, Chang M, Wu E, Hunt T, … Witte S. Efficacy of a group-based multimedia HIV prevention intervention for drug-involved women under community supervision: Project WORTH. PLoS One. 2014;9:e111528. doi: 10.1371/journal.pone.0111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bassel N, Schilling RF, Irwin KL, Faruque S, Gilbert L, Von Bargen J, … Edlin BR. Sex trading and psychological distress among women recruited from the streets of Harlem. American Journal of Public Health. 1997;87:66–70. doi: 10.2105/ajph.87.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Center for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2015. 2016 Retrieved from http://www.euro.who.int/__data/assets/pdf_file/0019/324370/HIV-AIDS-surveillance-Europe-2015.pdf?ua=1.
- Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, … Aldrovandi GM. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Research and Human Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpí-Acosta C, Pouget ER, Reilly KH, Hagan H, Neaigus A, Wendel T, Marshall DM., IV Time since migration and HIV risk behaviors among Puerto Ricans who inject drugs in New York City. Substance Use and Misuse. 2016;51:870–881. doi: 10.3109/10826084.2016.1155616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Shangani S, Sabatino K, Rachlis B, Wachira J, Braitstein P, Operario D. Improving engagement in the HIV care cascade: A systematic review of interventions involving people living with HIV/AIDS as peers. AIDS and Behavior. 2016;20:2452–2463. doi: 10.1007/s10461-016-1307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global AIDS Response Progress Reporting. UNAIDS 2015 estimates. 2016 Retrieved from http://www.who.int/hiv/pub/arv/global-AIDS-update-2016_en.pdf?ua=1.
- Hien DA, Campbell ANC, Killeen T, Hu MC, Hansen C, Jiang H, … Nunes EV. The impact of trauma-focused group therapy upon HIV sexual risk behaviors in the NIDA clinical trials network “women and trauma” multi-site study. AIDS and Behavior. 2010;14:421–430. doi: 10.1007/s10461-009-9573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi JA. Crack, crack house sex, and HIV risk. Archives of Sexual Behavior. 1995;24:249–269. doi: 10.1007/BF01541599. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS [UNAIDS] Closing the Gap 2014. 2014. [Google Scholar]
- Knudsen HK, Staton-Tindall M, Oser CB, Havens JR, Leukefeld CG. Reducing risky relationships: A multisite randomized trial of a prison-based intervention for reducing HIV sexual risk behaviors among women with a history of drug use. AIDS Care. 2014;26:1071–1079. doi: 10.1080/09540121.2013.878779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblin BA, Bonner S, Hoover DR, Xu G, Lucy D, Fortin P, … Latka MH. A randomized trial of enhanced HIV risk reduction and vaccine trial education intervention among HIV-negative, high-risk women who use non-injection drugs: The UNITY study. Journal of Acquired Immune Deficiency Syndromes. 2010;53:378–387. doi: 10.1097/QAI.0b013e3181b7222e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Operario D. HIV risk among men who have sex with men who have experienced childhood sexual abuse: Systematic review and meta-analysis. AIDS Education and Prevention. 2012;24:228–241. doi: 10.1521/aeap.2012.24.3.228. [DOI] [PubMed] [Google Scholar]
- Meader N, Li R, Des Jarlais DC, Pilling S. Psychosocial interventions for reducing injection and sexual risk behavior for preventing HIV in drug users. Cochrane Database of Systematic Reviews. 2010;2010(20) doi: 10.1002/14651858.CD007192.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader N, Semaan S, Halton M, Bhatti H, Chan M, Llewellyn A, Des Jarlais DC. An international systematic review and meta-analysis of multisession psychosocial interventions compared with educational or minimal interventions on the HIV sex risk behaviors of people who use drugs. AIDS and Behavior. 2013;17:1963–1978. doi: 10.1007/s10461-012-0403-y. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaigus A, Sufian M, Friedman SR, Goldsmith DS, Stepherson B, Mota P, Pascal J, Des Jarlais DC. Effects of outreach intervention on risk reduction among intravenous drug users. AIDS Education and Prevention. 1990;2:253–271. [PubMed] [Google Scholar]
- Noor S, Ross M, Lai D, Risser J. Use of latent class analysis approach to describe drug and sexual HIV risk patterns among injections drug users in Houston, Texas. AIDS and Behavior. 2014;18:S276–S283. doi: 10.1007/s10461-014-0713-3. [DOI] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, … Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England Journal of Medicine. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Ramsey SE, Bell KM, Engler-Field PA. HIV risk behavior among female substance abusers. Journal of Addictive Diseases. 2010;29:192–199. doi: 10.1080/10550881003684756. [DOI] [PubMed] [Google Scholar]
- Rhoades HM, Creson D, Elk R, Schmitz J, Grabowski J. Retention, HIV risk, and illicit drug use during treatment: methadone dose and visit frequency. American Journal of Public Health. 1998;88:34–39. doi: 10.2105/ajph.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, … Lundgren J. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. Journal of the American Medical Association. 2016;316:171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- Semaan S, Des Jarlais DC, Sogolow E, Johnson WD, Hedges LV, Ramirez G, … Needle R. A meta-analysis of the effect of HIV prevention interventions on the sex behaviors of drug users in the United States. Journal of Acquired Immune Deficiency Syndromes. 2002;30:S73–S93. [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, … Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. British Medical Journal. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Solomon DA, Herman DS, Ramsey SE, Brown RA, Miller IW. Reductions in HIV risk behaviors among depressed drug injectors. American Journal of Drug and Alcohol Abuse. 2005;31:417–432. doi: 10.1081/ada-200056793. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Abramovitz D, Lozada R, Martinez G, Rangel MG, Vera A, … Patterson TL. Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: Results from a randomized controlled trial. PLoS One. 2013;8:e65812. doi: 10.1371/journal.pone.0065812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK. Epidemiology of HIV among injecting and non-injecting drug users: Current trends and implications for interventions. Current HIV/AIDS Reports. 2010;7:99–106. doi: 10.1007/s11904-010-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takah NF, Kennedy IT, Johnman C. Impact of approaches in improving male partner involvement in the prevention of mother-to-child transmission (PMTCT) of HIV on the uptake of PMTCT services in sub-Saharan Africa: A protocol of a systematic review and meta-analysis. British Medical Journal Open. 2016;6:e012224. doi: 10.1136/bmjopen2016-012224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evidence Based Nursing. 2004;1:176–184. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- Tovanabutra S, Robison V, Wongtrakul J, Sennum S, Suriyanon V, Kingkeow DE, … Nelson KE. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. Journal of Acquired Immune Deficiency Syndromes. 2002;29:275–283. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- Tross S, Campbell ANC, Cohen LR. Effectiveness of HIV/STD sexual risk reduction groups for women in substance abuse treatment programs: Results of a NIDA clinical trials network trial. Journal of Acquired Immune Deficiency Syndromes. 2008;48:581–589. doi: 10.1097/QAI.0b013e31817efb6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report 2015. New York, NY: United Nations; 2015. https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. [Google Scholar]
- Villar-Loubet O, Weiss SM, Marks G, O’Daniels C, Jones D, Metsch LR, McLellan-Lemal E. Social and psychological correlates of unprotected anal intercourse among Hispanic-American women: Implications for STI/HIV prevention. Culture, Health & Sexuality. 2016;18:1221–1237. doi: 10.1080/13691058.2016.1182217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KD, Bloom JJ, Hathazi SD, Sanders B, Lankenau SE. Control over drug acquisition, preparation and injection: Implications for HIV and HCV risk among young female injection drug users. ISRN Addiction, 2013. 2013 doi: 10.1155/2013/289012. Article ID 289012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Krupitsky EM, Cheng DM. Implications of cannabis use and heavy alcohol use on HIV drug risk behaviors in Russian heroin users. AIDS and Behavior. 2008;12:662–669. doi: 10.1007/s10461-007-9243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg WM, Jewkes R, Novak SP, Kline T, Myers B, Browne FA, … Parry C. A brief intervention for drug use, sexual risk behaviours and violence prevention with vulnerable women in South Africa: A randomized trial of the Women’s Health CoOp. British Medical Journal Open. 2013;3:e002622. doi: 10.1136/bmjopen-2013-002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK, Lam WKK, Parry CDH, Morojele NK. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS and Behavior. 2006;10:131–137. doi: 10.1007/s10461-005-9036-8. [DOI] [PubMed] [Google Scholar]
- Westreich D, Rosenberg M, Schwartz S, Swamy G. Representation of women and pregnant women in HIV research: A limited systematic review. PLoS One. 2013;8:e73398. doi: 10.1371/journal.pone.0073398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody G, Bruce D, Korthuis PT, Chhatre S, Poole S, Hillhouse M, … Ling W. HIV risk reduction with buprenor-phine-naloxone or methadone: Findings from a randomized trial. Journal of Acquired Immune Deficiency Syndromes. 2014;66:288–293. doi: 10.1097/QAI.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Women’s health. 2013 Retrieved May 5, 2015, from http://www.who.int/mediacentre/factsheets/fs334/en/
- World Health Organization. HIV/AIDS injecting drug use. 2015 Retrieved May 5, 2015, from http://www.who.int/hiv/topics/idu/en.