Abstract
Mental imagery (MI) is the mental rehearsal of movements without overt execution. Brain imaging techniques have made it possible to identify the brain regions that are activated during MI and, for voluntary motor tasks involving hand and finger movements, to make direct comparison with those areas activated during actual movement. However, the fact that brain activation differs for different types of imagery (visual or kinetic) and depends on the skill level of the individual (e.g. novice or elite athlete) raises a number of important methodological issues for the design of brain imaging protocols to study MI. These include instructing the subject concerning the type of imagery to use, objective measurement of skill level, the design of motor tasks sufficiently difficult to produce a range of skill levels, the effect of different environments on skill level (including the imaging device), and so on. It is suggested that MI is more about the neurobiology of the development of motor skills that have already been learned, but not perfected, than it is about learning motor skills de novo.
Keywords: Motor imagery, visual imagery, kinetic imagery, functional brain imaging, motor skill, motor tasks, expertise, motor planning
1. Introduction
Motor imagery (MI) is a term introduced by cognitive neuroscientists to describe the mental rehearsal of voluntary movement without accompanying body movement [1–4]. Motor imagery is typically used by athletes and artists attempting to improve their performance [5–9] and by adults who are trying to regain motor skills lost or impaired by neurological disease [10–12]. Modern methods of brain imaging have made it possible to peer into brain and examine the nature of the neural programming before overt movement occurs [1–4,13–16]. Since the chief advantage of fMRI is its ability to identify the involved brain regions with high spatial resolution, it is not surprising that neuroscientists have used brain imaging to identify the brain regions activated during MI and to compare them those activated during execution of movement [1–4]. A consequence has been that the terminologies and concepts used by functional neuroanatomists and imaging scientists to describe MI are not concordant with the concepts of those who focus on the development of expertise [14,17]. Thus it becomes important to open up discussions between both groups aimed at establishing the use of common descriptors.
Here we review the main features of brain activation during MI as identified by brain imaging techniques. By combining these observations with findings related to expertise we argue that MI relates particularly to the development of expertise in motor skills that have already been learned, but not perfected, rather than to the learning of motor skills de novo.
2. Motor Imagery
There are two different strategies that people ordinarily take when asked to rehearse mentally a voluntary motor task [1,4]. First, they may produce a visual representation of their moving limb(s). This is called visual (motor) imagery (VI). This type of imagery is also referred to as external imagery since for a subject to view their movements they must have an external, or third person, perspective. Alternately, the subject may rehearse the movements using a kinesthetic feeling of the movement. This is called kinetic (motor) imagery (KI). Since the subject is participating as a performer, this type of MI is considered to be an internal, or first person, imagery. Each type of motor imagery has different properties with respect to both psychophysical [1] and physiological [18–22] perspectives, and to the nature of the neural networks that are activated by them [4].
Brain imaging methods demonstrate that multiple distinct regions of cortex become active during MI [4]. Whereas KI activates many of the same motor and sensory regions activated during overt movement, VI activates regions primarily concerned with visual processing, and does not obey Fitt’s law nor is it correlated with excitability of the cortico-spinal path as assessed by transcranial magnetic stimulation (for review see [12]). A point of commonality is that both VI and KI share strong bidirectional connections between premotor cortex (Brodmann 6) and the superior parietal lobule (Brodmann 7). However, considerable, yet partial, overlap exists between the cortical regions activated by KI, VI and motor execution. Thus, an important consideration is the functional organization of these regions of cortical activation into local and global neural networks related to motor control. In other words, how do changes in activation in one region affect activation in other region?; and how do these networks change depending on the nature of the motor task and the skill level of the performer?
Ultimately the poor temporal resolution of fMRI [23] makes it difficult to address questions concerning the nature and functional connectivity of neural networks even when combined with multivariate data analysis techniques such as structural equation modeling(e.g. [4]). Thus great interest has been given to combining fMRI with techniques, such as electro-encephalography (EEG), that have much better temporal resolution, but poor spatial resolution. This approach has, for example, been applied to investigate the dynamics of performance monitoring [24]. Despite the potential promises of fMRI-EEG it is important to keep in mind that fMRI monitors a hemodynamic response and EEG measures a bioelectric response. Although the hemodynamic response is partly related to neuronal activity [25], it also includes processes related to changes in the activity of muscle cells which regulate vasodilation. On the other hand, EEG is primarily a measure of neuronal synchrony [26]. Recently Bayesian approaches have been used to demonstrate that the local hemodynamic and bioelectric responses to a time-locked stimulus do not follow the same time course [25]. Moreover, a positive bioelectric evoked response is locally associated with a negative hemodynamic response.
The above observations emphasize the importance of the use of multimodal techniques to study MI in the context of carefully designed motor paradigms in which the subject can be well trained to use either VI or KI relatively selectively. In contrast, the vast majority of imaging studies on MI simply delineate the number, location, and intensity of the regional activations. Moreover, in practice it can be very difficult for subjects, especially novices, to perform MI at all. For example, studies on MI in patients who have suffered a stroke suggest that these subjects employ a “chaotic motor strategy”, which is neither VI nor KI [12]. The exception to this rule consists of individuals who are highly skilled, such as elite athletes, musicians, and dancers.
In the following discussion we focus on studies related to MI in the context of elite performance. To avoid confusion we restrict the phrase ‘neural networks’ to those studies in which the functional or effective connectivity, and hence the identity of the neural networks, has been established.
3. Imagery versus execution
An important question concerns whether the neural networks activated during MI are the same or different from those activated during actual execution of the movement. Historically, imaging studies have concluded that the only differences between brain activation during MI and during execution of the same motor task were quantitative and not at all qualitative, i.e. that brain activation has the same pattern but is of higher amplitude during execution than imagery (for a review see [4]). However, recent studies have revealed a richer picture when VI and KI are compared, particularly when imaging studies are combined with powerful methods of data analysis such as structural equation modeling [4]. Although these observations indicate that the neural networks activated during movement execution are more similar to KI than to VI, a large gap exists between the pattern of cortical activations for imagery and those for actual movement, as detected by brain imaging. Even when involuntary movements occur during MI, the observed movements typically do not replicate, even in miniature, the exact form of the movements that were supposedly rehearsed [3].
An obvious question is whether the movement itself affects central motor programs. Modern theories of motor control emphasize that control of an actual movement is not managed solely by cortical neural networks, but also heavily depends on inputs from lower centers located in the basal ganglia, spinal cord and peripheral nervous system (e.g. muscle spindles), the mechanical properties of the body, and the environment in which the movement occurs (see for example [14]). Indeed it has recently been demonstrated that brain activation during MI is heavily influenced by the pre-existing configuration of the limbs [27]. Moreover, studies of MI in patients with amyotrophic lateral sclerosis, a disease that solely affects α-motoneurons in the spinal cord, show reduced brain activation; a finding that again emphasizes a role for subcortical neural inputs on cortical activation [28]. Finally, brain activation during MI is not the same in subjects who increase their motor task skill during motor training versus mental practice [29]. Indeed in this two finger tapping task involving the non-dominant hand, subjects who received extra motor training increased activation in supplementary motor cortex and cerebellum whereas those who received extra mental training (VI) increased activation in visual association cortex.
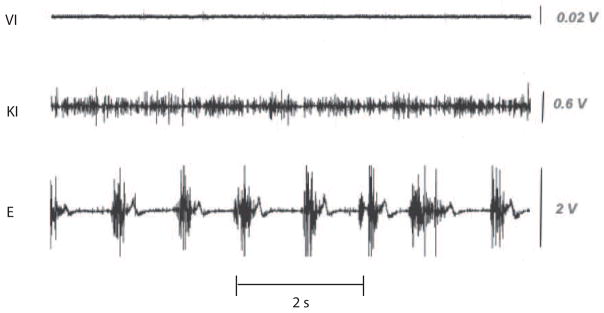
The observation that MI elicits changes in physiological variables that also change during overt movement in the same subjects (e.g., heart rate and electromyogram (EMG)), provides compelling evidence that at least some of the same neural networks are being activated [3–4, 20]. Figure 1 shows surface EMG measured using a surface electrode placed over the first dorsal interosseus (E) as a subject performs a rhythmic thumb-finger opposition task. During KI or VI the EMG activity recorded from this muscle is not the same as with overt movement (E). However, the EMG changes during KI differ from those during VI. In particular, whereas during VI, EMG activity does not differ from that recorded during rest, during KI there is an increase in background amplitude that suggests an increase in muscle tone. These observations are consistent with the observation that every human movement has both a tonic and a phasic component [30]. Observations following hemispherectomy in both primates [31] and humans [32] suggest that the phasic component of voluntary finger movements is most closely associated with activation in primary motor cortex whereas the tonic component is most likely associated more strongly with activation in other cortical and subcortical regions. Thus the observations in Figure 1 suggest that KI is activating regions of cortex involved in the control of the tonic components of movement. This empirical interpretation is strongly supported by structural equation modeling of the changes in neural network activation which show that during KI inputs to primary motor cortex are primarily inhibitory [4].
Figure 1.
Electromyographic (EMG) recordings made during a finger opposition task for three conditions (from top to bottom): visual imagery (VI), kinetic imagery (KI) and overt movement. In all cases the EMG surface electrode was placed over the first dorsal interosseus. See text and [4] for more details.
Despite these differences between VI and KI, it is important to realize that both forms of MI can be used to improve an athlete’s performance in a competitive environment [33]. This observation may, in part, be related to the observation that during both forms of MI there is a strong connection between Brodmann’s area 7 (superior parietal lobule and intraparietal sulcus) and Brodmann’s area 6m (supplementary and pre-supplementary motor areas) [4]. However, the observations in Figure 2 suggest that the explanation for the beneficial effects of MI may be even more complex.
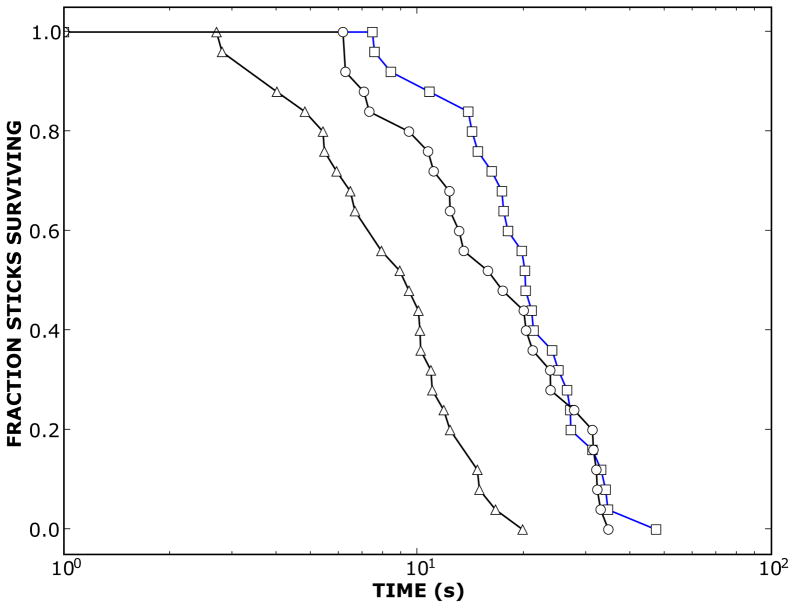
Figure 2.
Comparison of fraction of sticks surviving for time, t, or longer, for stick balancing (△-△) to that obtained when a second task is performed concurrently: rhythmic movement of a leg (○-○); imagined rhythmic movements of leg (□-□). The subject was a moderately skilled stick balancer. The stick length was a one quarter inch wooden dowel of length 55 cm. See text for more details.
Figure 2 shows the effect of a dual task on a subject’s ability to balance a stick at their fingertip with and without MI. Stick balancing at the fingertip is the prototypal example of an “edge of stability” motor task [34–36]. Another recently emphasized example involves the manipulation of a coiled spring; a motor task that is currently though to be beneficial for the rehabilitation of fine finger movements [37–38]. The advantage of studying stick balancing is that skill level can be objectively measured by the time that the stick remains balanced by constructing a survival curve and the degree of analytical insight that can be obtained [34–36]. The difficulties in performing an “edge of stability” motor task stem from the fact that the equilibrium is unstable and hence, due to the effects of random perturbations, the controlled variable (i.e. the upright position of the balanced stick) fluctuates in an unpredictable manner [35]. Increases in skill stability’s edge point to the existence of a very robust neural control strategy [39].
In Figure 2 a subject was asked to repeat the same task (25–50 repetitions) and the time when the stick falls for each trial is measured. The survival curve is a plot of the fraction of sticks still standing at a given time as a function of time [36]. The skill level can be estimated by, for example, the time, t1/2, at which 50% of the sticks are still standing (approximately 10 seconds for the left most condition in Figure 2). As skill increases, the 50% survival time shifts to the right indicating that the sticks on average remain standing longer. The mean survival time, t1/2, for balancing a 55 cm stick (wooden dowel, diameter 6.35 mm) can typically be increases 10–25 times within a 5–10 day practice period (e.g., t1/2 ~ 5–10s to 200–300s for 15 subjects). The practice period requires the subject to obtain a total of 20 minutes of accumulated balance time each day. Over this time, t1/2 increases in a nonlinear “step-wise” manner in which, as predicted from dynamical systems theory [40], increases in skill are preceded by transient worsenings of skill. This progression of skill is characterized by both kinematic and dynamical differences [35]. Briefly, the movements of the limb shift more proximally, i.e. from hand/finger to shoulder/elbow. Using high speed motion capture technologies the distribution of the changes in speed made at the fingertip, described by a Lévy distribution, develop a more pronounced “heavy-tailed” appearance [35].
The dual task paradigm shown in this figure is one designed to enhance stick balancing skill in a moderately skilled individual: t1/2 ~ 20s, heavy-tailed distribution of changes in speed, limb movements confined to shoulder/elbow. The subject was asked to either move one of their legs rhythmically (○-○) or to imagine (likely KI) moving their leg (□-□) while balancing a stick at their fingertip. In each case, stick balancing performance increases and appears to be slightly better with MI (compare with △-△). In contrast, for a novice a decrease in t1/2 occurs (presumably the dual task makes stick balancing too difficult) and for an expert (e.g. t1/2 > 200s) the dual task makes no difference.
To begin to understand how imagery of an unrelated motor task can improve stick balancing it is necessary to understand the distinction between programming a motor skill and actually performing the motor skill. This, in turn, has consequences for the design of paradigms to study MI with functional brain imaging.
3. Motor skill
Individuals clearly differ in their ability to perform particular voluntary motor tasks. The skill level of an individual at a given time is reflected by the accuracy and uniformity of the movements as the task is repeated. Despite the fact that central neural networks for motor control are inherently variable [41], the precision achieved by elite athletes and artists is remarkable [14]. It is quite likely that motor control for a given motor task differs for a low-skilled individual compared to a more highly skilled individual. Thus, it is obvious that careful attention must be given to determining the skill level of the individual who performs MI before interpretations of the possible effects of MI can be advanced. Moreover, it must be emphasized that skill level for the same person dynamically changes [14]. Practice is required to both learn and maintain skill and skill level can dramatically decrease under appropriate environmental conditions. This last point is particularly problematic for brain imaging studies since measurements of skill level while the subject is in the scanner have not generally been systematically obtained.
To date most studies of brain activation during MI have relied on motor tasks that involve hand and finger movements of varying complexity [2,4,42–43]. However, the oft-repeated statement that all of the subjects mastered the task within three weeks or less of practice is discordant with the expectation that each subject would attain different levels of skill. For example it has been estimated that only about 40% of individuals are ever able to master the overhand throwing technique [44] and few golfers attain a single-digit handicap despite years of practice [45].
The relationship between the complexity of the motor task and brain activation during MI has recently been addressed [46–48]. It has been shown that the lateralizing effect of brain activation during MI occurs only if the imagined motor task is sufficiently complex [46]. This observation suggests that motor tasks that are too simple do not optimally engage the motor system. One way to circumvent this issue has been to study MI in the setting of everyday activities [47–48]. The use of everyday activities has two potential advantages for the study of brain activation during MI: 1) these tasks can be easily modulated in their complexity; and 2) they are so familiar to the individual that it is possible, at least in principle, for them to generate a vivid mental representation without prior practice [47].
An alternate approach for examining the effects of skill level on MI is to compare novice and expert athletes [13–17] and performers [49]. Such studies provide a snapshot of the two endpoints of the skill continuum. Comparisons using a variety of techniques have shown that there are differences in neural network activation between novices and elite athletes [14–17]. Moreover, these studies emphasize that is not simply that elite athletes are programming identical tasks in a better way, but is that the elite athlete’s brain is actually programming a different task! To illustrate this point we consider two situations in which the effects of skill level on MI have been examined, one involving simple tasks and the other more complex tasks: Investigations of brain activation elicited by MI that utilize over-learned voluntary motor skills, such as those related to everyday activities, would be anticipated to share similarities with brain activation of MI arising from the more complex skills of elite athletes and performers.
The relevance of brain activation during MI to motor skill is anticipated to be highest in those motor tasks that, under normal circumstances, are programmed in a sequential manner. In other words, the brain first programs the movement and then the body executes the movement. Examples of such motor tasks are those associated with sports such as archery, rifle shooting, and golf. In these sports, the consistency and reproducibility of the preparatory period before the movement, or “pre-shot routine”, is one of the most important differences that distinguish expert from novice [6,14–16,50–52]. Three observations underscore the suitability of such tasks for investigations of the functional neuro-anatomy of motor imagery: 1) the subjects are normally motionless during the pre-shot routine; 2) the length of the pre-shot routine is typically of the order of 7–8s or longer [15,50–52] which places these processes well within the temporal resolution of fMRI, and 3) regardless of skill level, these athletes internalize their experience and feel that they are actively participating in the task. In an fMRI study of imagery in golfers it was observed that every brain region activated during the pre-shot routine of the elite golfer was also activated in the novice golfers [14–15]. However, certain brain regions were activated in the novice golfers that were not activated in the elite golfer, including the basal ganglia and the posterior cingulate region. These observations demonstrate the regions of brain activation during MI depend on the skill level of the individual.
A second situation in which brain activation during MI is not the same for novices and experts occurs in the setting of the so-called “fast ball sports” which include badminton, baseball, cricket, soccer, tennis, hockey and volleyball [53–59]. In these cases, there is now overwhelming behavioral evidence that motor planning occurs concurrently with seeing the movements of others [53–59]. Indeed, skilled cricket batters perform better against human bowlers than mechanical bowling machines [58]. Obviously the functional neuroanatomy for MI of movements that rely on anticipatory clues from others must differ from that of an athlete who performs a self-paced motor task such as a golf swing. Recent studies have suggested the importance of the “mirror system” for anticipatory types of motor programming that facilitate the understanding of another’s movements [54–56]. This mirror system has been suggested to work as an “action-observation” system. Indeed fMRI studies have demonstrated activation of the mirror system in paradigms related to dancing [60] and the tennis serve [61]. Further studies are needed to establish more fully the relationship between MI and the mirror system.
5. Performance
Expertise refers to the ability to maintain a given skill level under a variety of environmental conditions [5,8]. Historically an important question has been to determine how practice sessions should be designed to optimize the performance of athletes in competition or performing artists in recitals and concerts [8]. As expertise increases, the focus of an individual’s MI typically changes from the process of making a movement to that of the goal of the movement. The expected decreases in the attention-demanding sensory system have been observed for subjects who performed a bimanual coordination task as learning-related increases in brain activation occurred in sensorimotor cortical regions [43]. The observation that the level of performance of even the most skilled athlete can dramatically fall in competition (a phenomenon referred to as “choking”) provides convincing evidence that in contrast to skill, expertise is not something that is hardwired in the nervous system, but rather is a dynamic outcome of a large and widely dispersed neural network [66]. One of the major uses for MI is to help athletes and performing artists to improve their performance in challenging settings [33]. However, the need to optimize performance is as important for a person learning to walk again after suffering a stroke as it is for an athlete: Walking in a therapeutic session accompanied by a nurse is not the same as walking in the real world; if the latter cannot be accomplished then the whole rehabilitative exercise becomes pointless.
One of the major determinants of performance is the inverse relationship between attention and expertise [5]. Recent computational studies have suggested that an inverse relationship between selective attention and performance is an inherent feature of any dynamical system, including the nervous system, which operates with time-delayed feedback and is also subjected to the effects of random perturbations [35,67]. The basic problem is that of distinguishing those fluctuations that need to be acted upon by the feedback controller from those do not. This is because, by definition, there is a finite probability that an initial deviation away from a set point will be counterbalanced by one towards the set point just by chance. Too quick a response by a controller to a given deviation can lead to the phenomenon of “over control” leading to destabilization, particularly when time delays are appreciable. On the other hand, waiting too long runs the risk that the control may be applied too late to be effective. Optimal performance requires that an optimal amount of attention be allocated to the task. From this point of view, one interpretation for the results in Figure 2 would be that since the person has only moderate skill for stick balancing, the attention allocated to the task is too high. Thus by diverting away by doing a second task, the amount of attention directed toward stick balancing is lowered and hence skill increases.
A second determinant of performance involves interactions between limbic and cognitive neural systems in motor planning. For example, it has been shown that increased activation of limbic and paralimbic areas is associated with decreased activity in brain areas involved in motor planning and reward [68]. Motor imagery for elite speed skaters is associated with larger autonomic responses than novices, but differences in autonomic activity were uncorrelated with performance on mental arithmetic tasks [20]. On the other, since the amygdalar complex lies at the interface between brain areas involved in cognitive function and those involved in autonomic control, it can become activated as subjects increase their effort to perform tasks in which visual processing is important [15]. Thus the relationship between the cognitive-limbic axes in motor performance is very complex and likely task specific.
There have been no systematic studies of the relationship between MI and performance. However, behavioral observations of novice golfers during their pre-shot routine suggest that they were unnecessarily preoccupied with details that were irrelevant for this task [15]. For example, they raised questions concerning the wind direction even though they had been told to assume that there was no wind. In contrast to elite golfers, the novice golfers with 3–6 months playing experience activated the posterior cingulate region. Although activation of this region has also been associated with learning of novel motor tasks [43], these behavioral observations suggest that a more likely explanation was that the novice golfers had difficulty filtering relevant information from irrelevant information [15]. In other words the poor performance of novice golfers might be because they were putting too much emphasis on irrelevant details and hence trying to program a much more difficult task. This type of “stress”, i.e. an increase in the relative amount of irrelevant information, is very different from definitions of stress that emphasize the degree of activation of the autonomic nervous system [20]. These observations raise the possibility that the altered brain activation during MI observed for patients with diseases of the nervous system such as apraxia [69], Parkinson’s disease [70] and writer’s cramp [71], may be in part relate to difficulties that such individuals have in filtering out irrelevant information.
Concluding remarks
The use of functional brain imaging techniques, such as fMRI, is ultimately limited by the precision by which the imagined motor task can be characterized and its ecological relevance. It cannot be over-emphasized that individuals differ in their ability to perform particular motor tasks and that this ability is itself labile and may not, for example, be maintained by placing the subject into a MRI scanner. The failure to recognize that there is not a single optimal mental representation for motor tasks but a continuum that depends of the level of expertise of the individual represents a major methodological flaw that confounds both the design and interpretation of MI paradigms. Moreover it is quite possible that the relative role of VI versus KI differs as a function of motor task, and that the relationships between MI and performance depend on the particular measured aspects of performance. At the very least these observations demand that an important component of any brain imaging study of MI should be the areful documentation of the skill level of the participant using objective measurements. These effects of skill on performance cannot simply be examined by comparing results obtained for the dominant (skilled) and non-dominant (non-skilled) hand. Indeed the relative slowing and error associated with the non-dominant hand is greater for imagined movements than for real movements [72].
An unsettled question concerns the role of MI in re-learning of motor skills lost because of disease to the nervous system. In our experience MI is most useful for advanced athletes for helping them focus on the goal of the action, rather than novices who are overly concerned with the process of the movement. Indeed focusing attention on two activities of daily living, e.g. balancing and walking, typically degrades performance. This is because the control of these activities is much more heavily dependent on the biomechanical properties of the musculoskeletal system and neural activites arising in the brainstem, spinal cord and peripheral nervous system. Thus, for optimal performance, it is necessary to divert attention away from the process of performing the task. This is exactly opposite to the current philosophy for using MI in the rehabilitation of subjects post-stroke and may, in part, explain the modest and transient benefits obtained using MI in these contexts [12].
The possibility that “edge of stability” motor tasks would identify the more robust mechanisms for motor control makes them ideal candidates for the study of brain activation during MI. In addition to the stick balancing and coiled spring tasks mentioned previously, various paradigms have been developed using time-delayed visual tracking tasks [73–74]. In all cases parameters can be manipulated to make the control task unstable. With the development of computer equipment that can be used by a subject in a MRI scanner and virtual paradigm that involve the interaction of a person with a computer, such as virtual stick balancing [75–77], we anticipate that such studies will become possible in the near future.
There are important unanswered questions concerning the role of MI in normal and expert motor function. Is mental rehearsal necessary to optimize the performance of central motor programs and/or is it necessary to optimize attention by training the person to filter out irrelevant sensory information [15]? Although the latter possibility has received relatively little attention in brain imaging studies of MI, it is supported by the beneficial effects that psychological techniques such as goal setting, positive self-talk, and relaxation, have in improving athletic performance. Answering questions of this nature undoubtedly will require the formation of inter-disciplinary teams of investigators to perform complete studies of even the simplest motor tasks. Such teams of investigators will need to include computer scientists as well as functional neuro-anatomists and individuals with specialties ranging from cognitive sports psychology to kinesiology to successful teachers and coaches. By drawing on the experience of coaches, teachers, and rehabilitation professionals it should be possible to design experimental paradigms to obtain insights into how the brain develops expertise in the performance of motor tasks. The anticipated impact of such collaborations includes the development of novel teaching and rehabilitative strategies.
Footnotes
This research was supported by the William R. Kenan, Jr. Foundation (JM), the National Science Foundation (JM: NSF-0617072) and the National Institutes of Health (AS: NIH-RO1-054942-01A1; SLS: NIH-RO1-DC007488).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeannerod M. Neuropsychologica. 1995;33:1419–1432. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- 2.Jeannerod M. Neuroimage. 2001;14:S103–109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- 3.Annett J. Neuropsychologia. 1995;33:1395–1417. doi: 10.1016/0028-3932(95)00072-b. [DOI] [PubMed] [Google Scholar]
- 4.Solodkin A, Hlustik P, Chen EE, Small SL. Cerebral Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- 5.Fitts PM, Posner MI. Human Performance. Prentice-Hall International; London: 1973. [Google Scholar]
- 6.Feltz DM, Landers DM. Journal of Sports Psychology. 1983;5:25–57. [Google Scholar]
- 7.Landin DK, Hebert EP, Fairweather M. Research Quarterly of Exercise and Sport. 1993;64:232–237. doi: 10.1080/02701367.1993.10608803. [DOI] [PubMed] [Google Scholar]
- 8.Fairweather M. In: The Coaching Process: Principles and Practice for Sport. Cross N, Lyle J, editors. Butterworth-Heinemann; New York: 1999. pp. 113–129. [Google Scholar]
- 9.Yu G, Cole KJ. Journal of Neurophysiology. 1992;67:1114–1117. doi: 10.1152/jn.1992.67.5.1114. [DOI] [PubMed] [Google Scholar]
- 10.Patten BM. Archives of Neurology. 1972;26:25–31. doi: 10.1001/archneur.1972.00490070043006. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein R, Dunsky A, Marcovitz E. Physical Therapy. 2004;84:1167–1177. [PubMed] [Google Scholar]
- 12.Sharma N, Pomeroy VM, Baron JC. Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- 13.Ross JS, Thach J, Ruggieri PM, Lieber M, Lapresto E. American Journal of Neuroradiology. 2003;24:1036–1044. [PMC free article] [PubMed] [Google Scholar]
- 14.Milton J, Small SL, Solodkin A. Journal of Clinical Neurophysiology. 2004;21:134–143. doi: 10.1097/00004691-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Milton J, Solodkin A, Hlustik P, Small SL. NeuroImage. 2007;35:804–813. doi: 10.1016/j.neuroimage.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Milton J, Small SL, Solodkin A, editors. Journal of Clinical Neurophysiology. 2004;21:133–227. [Google Scholar]
- 17.Hatfield BD, Hillman CH. In: Handbook of Sports Psychology. Singer RN, Hausenblas HA, Janelle CM, editors. Wiley & Sons; New York: 2001. pp. 362–386. [Google Scholar]
- 18.Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C. Neuroscience Letters. 1999;263:113–116. doi: 10.1016/s0304-3940(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 19.Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Parvesi G. Neuropsychologica. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- 20.Oishi K, Maeshima T. Journal of Clinical Neurophysiology. 20041;21:70–179. doi: 10.1097/00004691-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Fourkas AD, Avenanti A, Urgesi C, Salvatore SM. Experimental Brain Research. 2005;168:143–151. doi: 10.1007/s00221-005-0076-0. [DOI] [PubMed] [Google Scholar]
- 22.Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Experimental Brain Research. 2006;169:157–164. doi: 10.1007/s00221-005-0078-y. [DOI] [PubMed] [Google Scholar]
- 23.Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Proceedings National Academy Sciences USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debener S, Ullsperger M, Siegel M, Fiehler K, Yves von Cramon D, Engel AK. The Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daunizeau J, Grova C, Marrelec G, Mattout J, Jbabdi S, Pélégrini-Isaac M, Lina JM, Benali H. NeuroImage. 2007;36:69–87. doi: 10.1016/j.neuroimage.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Ebersole JS, Milton J. In: Epilepsy as a Dynamic Disease. Milton J, Jung P, editors. Springer-Verlag; New York: 2002. pp. 51–68. [Google Scholar]
- 27.de Lange FP, Helmich RC, Tori I. NeuroImage. 2006;33:609–617. doi: 10.1016/j.neuroimage.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Stanton BR, Williams VC, Leigh PN, Williams SCR, Blain CRV, Giampierto VP, Simmons A. Brain Research. 2007;1172:145–151. doi: 10.1016/j.brainres.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 29.Nyberg L, Eriksson J, larsson A, Marklund P. Neuropsychologia. 2006;44:711–717. doi: 10.1016/j.neuropsychologia.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Bullock D, Cisek P, Grossberg S. Cerebral Cortex. 1998;8:48–62. doi: 10.1093/cercor/8.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence DG, Kuypers HGJM. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 32.de Bode S, Firsetone A, Mathern GW, Dobkin B. Journal of Child Neurology. 2005;20:64–75. doi: 10.1177/08830738050200011101. [DOI] [PubMed] [Google Scholar]
- 33.Horn TS. Advance in Sports Psychology. Human Kinetics; Urbana-Champaign, IL: 2002. [Google Scholar]
- 34.Cabrera JL, Milton JG. Physical Review Letters. 2002;89:158702. doi: 10.1103/PhysRevLett.89.158702. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera JL, Milton JG. Chaos. 2004;14:691–698. doi: 10.1063/1.1785453. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera JL, Milton JG. Nonlinear Science. 2004;11:305–317. [Google Scholar]
- 37.Venkadesan M, Guckenheimer J, Valero-Cuevas F. Journal of Biomechanics. 2007;40:1653–1661. doi: 10.1016/j.jbiomech.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkadesan M. PhD thesis. Sibley School of Mechanical and Aerospace Engineering, Cornell University; NY: 2007. Dynamic dextrous manipulation: Benefits of the edge of stability in exploring complex dynamical behavior. [Google Scholar]
- 39.Milton JG, Cabrera JL, Ohira T. Europhysics Letters. in press. [Google Scholar]
- 40.Kelso JAS. Dynamic Patterns: The self-organization of brain and behavior. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- 41.Churchland MM, Afshar A, Shenoy KV. Neuron. 2006;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hlustik P, Solodkin A, Noll DC, Small SL. Journal of Clinical Neurophysiology. 2004;21:180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Puttemans V, Wenderoth N, Swinnen SP. The Journal of Neuroscience. 2005;25:4270–4278. doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halverson IE, Roberton MA, Langendorfer S. Research Quarterly for Exercise and Sports. 1982;53:198–205. [Google Scholar]
- 45.Pelz D. Dave Pelz’s Short Game Bible. Broadway Books; New York: 1999. [Google Scholar]
- 46.Szameitat AJ, Shen S, Starr A. European Journal of Neuroscience. 2007;26:3303–3308. doi: 10.1111/j.1460-9568.2007.05920.x. [DOI] [PubMed] [Google Scholar]
- 47.Szameitat AJ, Shen S, Starr A. NeuroImage. 2007;34:702–713. doi: 10.1016/j.neuroimage.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 48.Higuchi S, Imamizu H, Kawato M. Cortex. 2007;43:350–358. doi: 10.1016/s0010-9452(08)70460-x. [DOI] [PubMed] [Google Scholar]
- 49.Kleber B, Birbaumer B, Veit R, Trevorrow T, Lotze M. NeuroImage. 2007;36:889–900. doi: 10.1016/j.neuroimage.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 50.Crews DJ, Landers DM. Medicine and Science in Sports and Exercise. 1993;25:116–126. doi: 10.1249/00005768-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Landers DM, Han M, Salazar W, Petruzzello SJ, Kubitz KA, Gannon TL. International Journal of Sport Psychology. 1994;22:56–71. [Google Scholar]
- 52.Konttinen N, Lyytinen H. Journal of Sport and Exercise Psychology. 1993;15:110–127. [Google Scholar]
- 53.Abernathy B. Australian Journal of Sports Medicine. 1981;13:3–10. [Google Scholar]
- 54.Abernathy B, Russell DG. Australian Journal of Sports Medicine. 1984;16 [Google Scholar]
- 55.Müller S, Abernathy B, Farrow D. Quarterly Journal of Experimental Psychology. 2006;59:2162–2186. doi: 10.1080/02643290600576595. [DOI] [PubMed] [Google Scholar]
- 56.Savelsbergh GJ, Williams AM, Van der Kamp J, Ward P. Journal of Sports Science. 2002;20:279–287. doi: 10.1080/026404102317284826. [DOI] [PubMed] [Google Scholar]
- 57.Penrose JMT, Roach NK. Journal of Human Movement Studies. 1995;29:199–218. [Google Scholar]
- 58.Gibson AP, Adams RD. Australian Journal of Science Medicine and Sports. 1989;21:3–6. [Google Scholar]
- 59.Milton J, Small SL, Solodkin A. In: Your Brain on Cubs. Gordon D, editor. Dana Press; New York: 2008. pp. 43–57. [Google Scholar]
- 60.Calvo-Merino B, Glaser DE, Grèves J, Passingham RE, Haggard P. Cerebral Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- 61.Wright MJ, Jackson RC. International Journal of Psychophysiology. 2007;63:214–220. doi: 10.1016/j.ijpsycho.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Rizzolatti G, Craighero L. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 63.Fadiga L, Craighero L. Journal of Clinical Neurophysiology. 2004;21:157–169. doi: 10.1097/00004691-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Hamzei F, Rijntjes M, Dettmers C, Glauche V, Weiller C, Buchel C. Neuroimage. 2003;19:637–644. doi: 10.1016/s1053-8119(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 65.Buccino G, Vogt S, Ritzi A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 66.Beilock JL, Carr TH. Psychological Science. 2005;16:101–105. doi: 10.1111/j.0956-7976.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 67.Insperger T. IEEE Transactions on Control and Systems Technology. 2006;14:974–977. [Google Scholar]
- 68.Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillo B, Le Bihan D, Dubois B. Proceedings National Academy of Sciences USA. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Neuropsychologia. 2005;43:917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Helmich RC, de lange FP, Bloem BR, Toni I. Neuropsychologia. 2007;45:2201–2215. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant’ Angelo A, Crupi D, Romano M, Messina C, Berardelli A, Girlanda P. Movement Disorders. 2005;20:1488–1495. doi: 10.1002/mds.20626. [DOI] [PubMed] [Google Scholar]
- 72.Maruff P, Wilson PH, De Fazio J, Cerritelli B, Hedt A, Currie J. Neuropsychologia. 1999;37:379–384. doi: 10.1016/s0028-3932(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 73.Milton JG, Longtin A, Beuter A, Mackey MC, Glass L. Journal of Theoretical Biology. 1989;138:129–147. doi: 10.1016/s0022-5193(89)80135-3. [DOI] [PubMed] [Google Scholar]
- 74.Beuter A, Milton J, Labrie C, Glass L. Experimental Neurology. 1990;110:228–335. doi: 10.1016/0014-4886(90)90034-p. [DOI] [PubMed] [Google Scholar]
- 75.Mehta B, Schaal S. Journal of Neurophysiology. 2002;88:942–953. doi: 10.1152/jn.2002.88.2.942. [DOI] [PubMed] [Google Scholar]
- 76.Bormann R, Cabrera JL, Milton JG, Eurich CW. Neuro-computing. 2004;58–60C:517–523. [Google Scholar]
- 77.Cabrera JL, Bormann R, Eurich C, Ohira T, Milton J. Fluctuation and Noise Letters. 2004;4:L107–L117. [Google Scholar]