Abstract
Major surgical procedures often result in significant intra and postoperative bleeding. The ability to identify the cause of the bleeding has the potential to reduce the transfusion of blood products and improve patient care. We present a novel device, the Quantra™ Hemostasis Analyzer, which has been designed for automated, rapid, near patient monitoring of hemostasis. The Quantra is based on SEER Sonorheometry, a proprietary technology that uses ultrasound to measure clot time and clot stiffness from changes in viscoelastic properties of whole blood during coagulation. We present results of internal validation and analytical performance testing of the technology and demonstrate the ability to characterize the key functional components of hemostasis.
Technical Communication
A number of in vitro diagnostic devices, such as the TEG® 5000 (TEG) (Haemonetics Corporation, Braintree, MA) and the ROTEM® Delta (ROTEM) (Tem Innovation GmbH, Munich, Germany), have been shown to be useful in aiding the management of bleeding and guiding transfusion decisions in surgery and trauma, with multiple studies demonstrating significant reductions in blood product utilization and hospital costs1-9. Both the TEG and the ROTEM are viscoelastic devices that measure the time dependent evolution of clot stiffness. A series of parameters can be calculated from the time-stiffness curves to describe some aspects of clot formation, dynamics of clot stiffening, final clot stiffness, and clot dissolution. These devices, however, have a number of limitations including the complexity of operation and interpretation of results10-12. Furthermore, studies have found that the large shear stress applied by these instruments during measurements might disrupt clot formation13,14.
In this work we introduce a novel device, the Quantra™ Hemostasis Analyzer (Quantra) (HemoSonics LLC, Charlottesville, VA), which has been designed for automated, rapid, near patient monitoring of hemostasis. The Quantra is based on SEER (Sonic Estimation of Elasticity via Resonance) Sonorheometry, a proprietary technology that uses ultrasound to measure the changes in viscoelastic properties of whole blood during coagulation ex vivo. Corey and Walker15 describe SEER Sonorheometry in details, whereas other Sonorheometric technologies are described elsewhere16,17. We provide a summary of the Quantra analyzer and SEER Sonorheometry, presents initial results showing the system analytical performance, and demonstrate its ability to characterize the functional role of the treatable components of coagulation.
Methods
The studies presented here were approved by MaGil Institutional Review Board (Rockville, MD), under protocol #0001 Version 1.0. All participating subjects provided written informed consent.
Device Description
The Quantra is an automated instrument that controls all aspects of test sequence, including temperature control, fluidic handling, ultrasound transmission and acquisition, data processing, and data output. The Quantra incorporates an embedded processor, a mechanical assembly that provides connection to a peristaltic pump and solenoid valves, heating elements to heat and maintain the samples at 37°C, and ultrasound transducers operating in the megahertz range. Research Use Only (RUO) development versions of the Quantra, shown in Figure 1, were used for the reported experiments.
Figure 1.
(Left) Research Use Only (RUO) development version of the Quantra Hemostasis Analyzer. The Quantra is a fully automated instrument that requires no sample handling steps from the user. The dimensions of the instrument are comparable to those of a blood gas analyzer. (Right) RUO cartridge used with the Quantra. The cartridge uses four independent channels that are optimized to measure clot time or clot stiffness.
A multi-well plastic cartridge with embedded reagents is used for each test (Figure 1). The RUO cartridge has four test channels that perform four parallel and independent measurements using different reagent combinations in each channel. The cartridge includes a connection mechanism for attachment of a sample container (a 3ml syringe for research purposes). After the cartridge is inserted into the analyzer and a syringe is attached to the cartridge, all the pre-analytical steps of sample heating, metering, and mixing are automated by the analyzer.
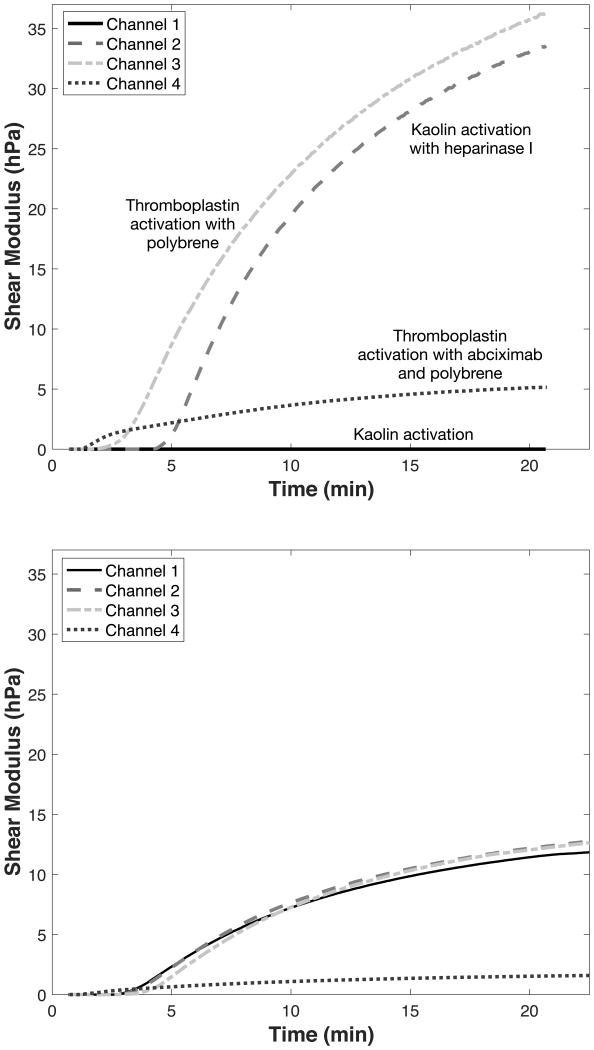
The Quantra Surgical Cartridge uses the reagents listed in Table 1. The first channel is optimized for the measurements of Clot Time, whereas the second channel measures Heparinase Clot Time using heparinase I to neutralize any potential heparin in the sample. Clot Time provides an indication of the functional status of the coagulation factors that lead to fibrin formation. Furthermore, Clot Time and Heparinase Clot Time can be combined to form a Clot Time Ratio, which is used to determine the presence of residual heparin in the sample. Channel 3 is optimized for the measurement of Clot Stiffness, which combines information about platelets and fibrinogen function. Finally, channel 4 is optimized to measure the Fibrinogen Contribution to clot stiffness. Both channels 3 and 4 use hexadimethrine bromide to neutralize residual heparin. The difference between Clot Stiffness and Fibrinogen Contribution represents the Platelet Contribution to clot stiffness18,19. The Quantra test results are summarized in Table 2. Figure 2 shows representative shear modulus vs time curves obtained with the Surgical Cartridge. The upper panel shows curves obtained from a normal subject but in the presence of 6 IU of unfractionat heparin in the sample. The bottom panel shows curves obtained from a sample with reduced fibrinogen levels. The device is designed to generate complete test results within 15 minutes of test initiation.
Table 1.
Reagents utilized in each channel of the Quantra Surgical Cartridge.
| Channel # | Reagents |
|---|---|
| 1 | Kaolin, calcium, buffers and stabilizers |
| 2 | Kaolin, heparinase I, calcium, buffers and stabilizers |
| 3 | Thromboplastin, polybrene, calcium, buffers and stabilizers |
| 4 | Thromboplastin, polybrene, abciximab, calcium, buffers and stabilizers |
Table 2.
Measured and calculated test results reported by the Quantra Surgical Cartridge.
| Quantra Test Result | Units | Description |
|---|---|---|
| Clot Time | Minutes (min) | Clot time measured from channel 1 |
| Heparinase Clot Time | Minutes (min) | Clot time measured from channel 2 in the presence of heparinase |
| Clot Time Ratio | Unit less | Calculated ratio of clot time values from channels 1 and 2 |
| Clot Stiffness | hecto Pascals (hPa) | Clot stiffness measured from channel 3 |
| Fibrinogen Contribution | hecto Pascals (hPa) | Clot stiffness measured from channel 4 in the presence of a platelet inhibitor |
| Platelet Contribution | hecto Pascals (hPa) | Calculated from clot stiffness in channels 3 and 4 |
Figure 2.
(Top panel) Typical SEER Sonorheometry shear modulus curves obtained with the Quantra Surgical Cartridge. Measurements were performed with a whole blood sample from a healthy donor spiked with 6 IU of unfractionated heparin. (Bottom panel) SEER Sonorheometry shear modulus curves obtained in the presence of low fibrinongen levels (Clauss fibrinogen of 95 mg/dl). Estimates of clot time and clot stiffness are generated from all of these curves within 15 minutes of test initiation.
SEER Sonorheometry
SEER Sonorheometry is a patented technology that uses high frequency ultrasound pulses to quantify the shear modulus (i.e., stiffness) of a blood sample during the process of coagulation15. The shear modulus is a parameter that describes the elastic properties of a solid material20. For example, the shear modulus of bone tissue is around 3.3 GPa, whereas natural rubber is typically around 600 Pa. The technology is shown schematically in Figure 3.
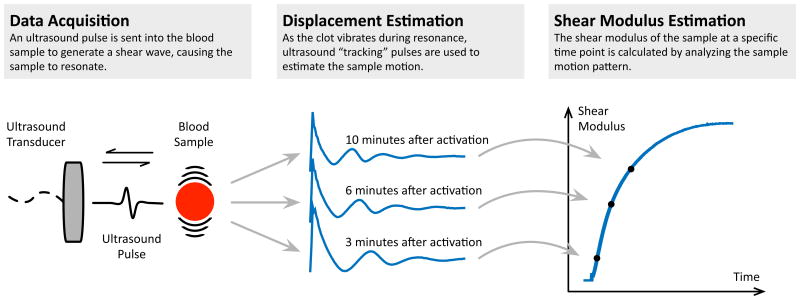
Figure 3.
Schematic representation of SEER Sonorheometry. The technology is composed of three fundamental steps, as represented by the three panels in this figure. First, an ultrasound pulse is transmitted in the blood sample to generate a shear wave, causing the sample to resonate (left panel). A series of ultrasound “tracking” pulses is then sent within the sample and the returning echoes are used to estimate the sample motion (middle panel). The shape of the estimated displacement curve is directly related to the shear modulus of the sample. The time-displacement curve can be compared to theoretical models to determine the actual shear modulus for that specific point in time. This process is repeated every 4 seconds to form a signature curve that shows shear modulus vs time (right panel).
A focused ultrasound pulse is transmitted into the blood sample to generate a shear wave, causing the sample to resonate once the clot begins to form (left panel). As the clot vibrates during resonance, a series of “tracking” ultrasound pulses are transmitted and the returning echoes are analyzed to estimate the sample's motion (middle panel). The shape of the estimated displacement curve is directly related to the shear modulus of the sample. The time-displacement curve can be compared to theoretical models to determine the actual shear modulus for that specific point in time. Repeated acquisitions over time form a signature curve that shows the dynamic changes in shear modulus of the sample during coagulation (right panel). From this curve, the start of clot formation, or clot time, and the stiffness of the clot can be directly estimated. The combination of these two parameters provides information about the functional role of the coagulation factors, fibrinogen, and platelets in the sample.
In these experiments, the shear modulus values were estimated by interrogating the blood sample every 4 seconds. Clot time, expressed in minutes, is estimated by determining the time at which the rate of change in stiffness exceeds a pre-defined threshold value. Clot stiffness is estimated by identifying the shear modulus value at a specific time point after clot time. This parameter is expressed in units of hecto-Pascals or hPa (i.e., 1hPa = 100Pa).
Validation and Comparative Studies
Experiments described here were performed using a plasma-based control material and whole blood samples (obtained in 3.2% sodium citrate) from healthy volunteers. Comparative studies were performed against the TEG 5000 and the Clauss fibrinogen assay implemented in the Stago STart4 Hemostasis analyzer. Detailed descriptions of blood collection, blood processing procedures, and comparative studies are described in the Supplemental Digital Material.
Results
Technology Reproducibility
Assay reproducibility was assessed with both whole blood samples and the plasma-based control materials over a period of 5 days. In the case of whole blood samples, a different volunteer was used for each day. A modified set of reagents was used in the cartridge as heparinase I and abciximab were not included, thus yielding two estimates of Clot Time and two estimates of Clot Stiffness for each run.
Reproducibility results are summarized in Table 3. When pooled over the 5 testing days, the plasma-based control material showed a Clot Time CV of 4.3% (n = 20, 99% CI [2.5% - 6.1%]) and a Clot Stiffness CV of 8.5% (n = 20, 99% CI [4.9% - 12.1%]). Additional experiments with control material on 3 different Quantra analyzers over 5 days yielded an average Clot Time CV of 4.1% (n = 60, 99% CI [3.2% - 5.1%]) and an average Clot Stiffness CV of 8.3% (n = 60, 99% CI [6.4% - 10.4%]).
Table 3.
Average Clot Time and Clot Stiffness values measured in whole blood samples (n = 6 or 8 per day) from normal volunteers or in a plasma-based control material (n = 4 per day).
| Whole Blood Samples | Control Samples | ||||||
|---|---|---|---|---|---|---|---|
| Mean | %CV | n | Mean | %CV | n | ||
| DAY 1 | Clot Time (min) | 2.56 | 4.9% | 6 | 3.52 | 2.9% | 4 |
| Clot Stiffness (hPa) | 11.4 | 2.4% | 6.99 | 3.0% | |||
| DAY 2 | Clot Time (min) | 2.03 | 6.5% | 8 | 3.38 | 4.8% | 4 |
| Clot Stiffness (hPa) | 11.77 | 7.3% | 6.11 | 9.0% | |||
| DAY 3 | Clot Time (min) | 2.22 | 4.2% | 8 | 3.33 | 3.1% | 4 |
| Clot Stiffness (hPa) | 12.20 | 3.8% | 5.71 | 2.9% | |||
| DAY 4 | Clot Time (min) | 2.56 | 7.4% | 8 | 3.43 | 3.1% | 4 |
| Clot Stiffness (hPa) | 10.52 | 8.8% | 5.80 | 4.9% | |||
| DAY 5 | Clot Time (min) | 2.60 | 5.0% | 6 | 3.27 | 5.0% | 4 |
| Clot Stiffness (hPa) | 8.11 | 5.5% | 6.03 | 4.2% | |||
Assessment of Coagulation Function
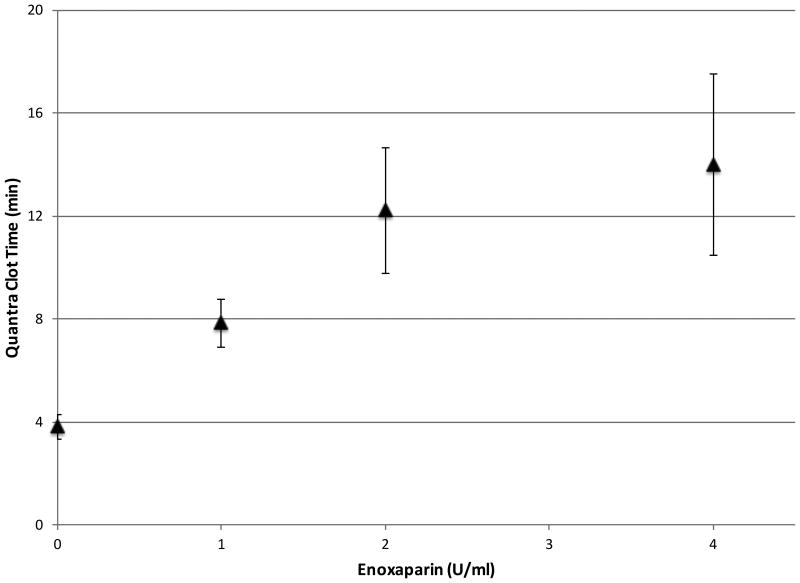
The effects of the coagulation factors on Clot Time were investigated by titrating varying amounts of low molecular weight heparin (LMWH) with samples from healthy volunteers to achieve final concentrations of 0, 1, 2, and 4 IU/ml. All the cartridge channels were loaded with channel 1 reagents (see Table 1), hence activating the sample with kaolin. The results presented in the dose response curve in Figure 4 demonstrate that increasing amounts of LMWH delayed the formation of the fibrin as indicated by a prolonged Clot Time21.
Figure 4.
Quantra Clot Time variation as a function of low molecular weight heparin (LMWH) concentration (n = 5 for each test condition). Error bars show one standard deviation.
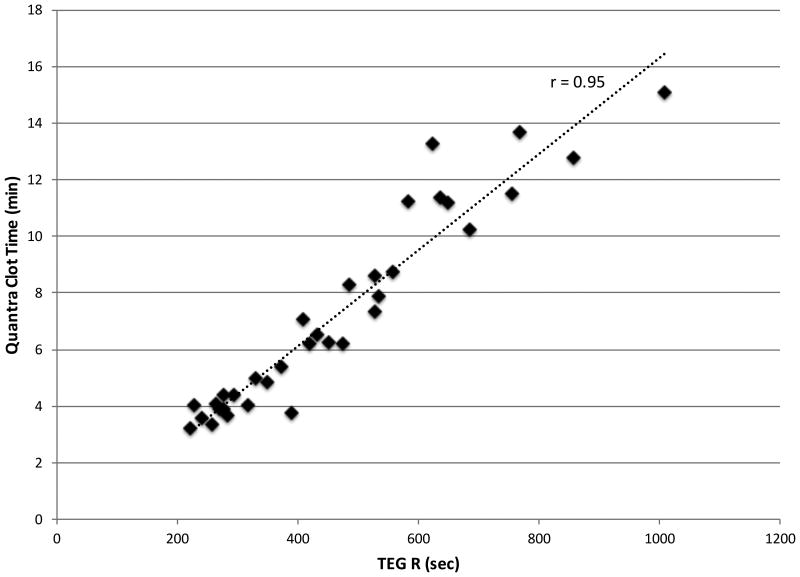
Additional experiments were performed using samples that were untreated, spiked with a low dose of unfractionated heparin, or where the endogenous plasma was replaced with Factor VIII, X, or XII deficient plasmas. The same experimental conditions (same sample and reagents) were assessed in parallel using the TEG, resulting in multiple paired measurements. The results are summarized in Figure 5, which demonstrates that Quantra Clot Time and TEG's R values are well correlated over the range of conditions analyzed (r-value of 0.95, n = 34, 99% CI [0.88 – 0.98]).
Figure 5.
Scatter plot of Quantra Clot Time vs TEG Reaction (R) time using whole blood samples with varying functionality of the coagulation factors. Paired measurements were obtained by running the same sample and reagents on both systems. The r-value of the best line fit is 0.95 (n = 34, 99% CI [0.88 – 0.98]).
Assessment of Platelet Contribution to Clot Stiffness
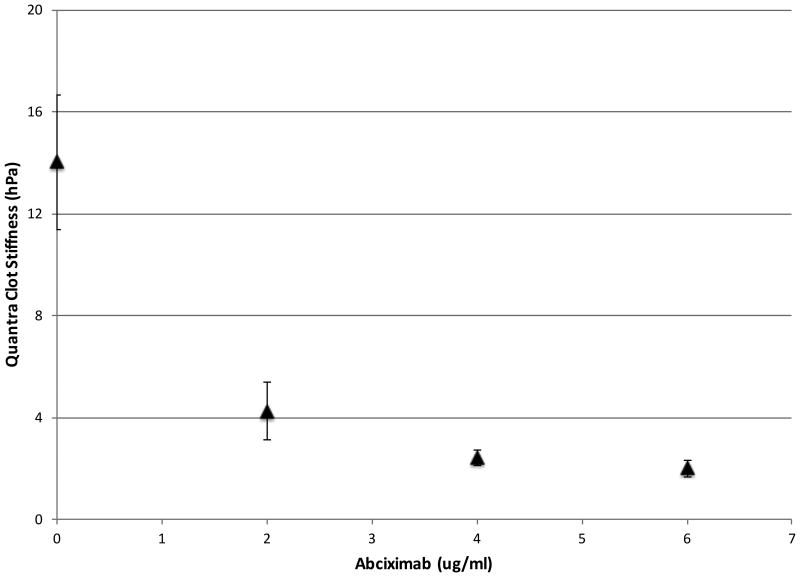
The effects of platelet function on SEER Sonorheometry measurements are shown in Figure 6 (top panel), where samples from healthy volunteers were treated with increasing amounts of abciximab, a potent GPIIb/IIIa receptor antagonist22,23. All the cartridge channels were loaded with channel 3 reagents (see Table 1), hence activating the sample with thromboplastin. In this figure, Clot Stiffness decreases monotonically as a function of abciximab concentration converging towards a plateau at around 6 μg/ml. Above this concentration, the measured stiffness is indicative of the fibrin network alone with little to no contribution from platelets. This figure also shows that Clot Stiffness decreased by roughly 7 fold over the range reported in this figure, emphasizing the role of platelets in determining the final mechanical stiffness of the mature clot.
Figure 6.
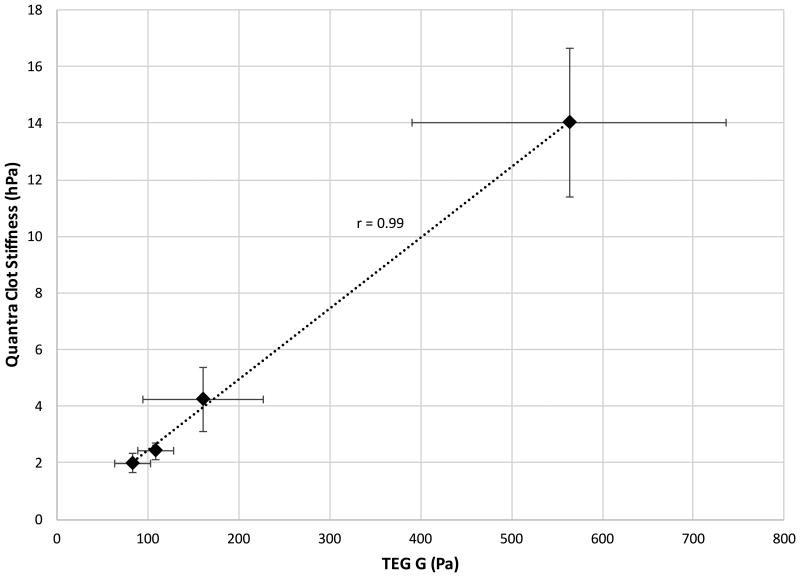
(Top Panel) Dose Response Curve for abciximab (ReoPro) concentration on the Quantra analyzer (n=5 for each test condition) and (Bottom Panel) SEER Sonorheometry Clot Stiffness vs TEG shear elastic modulus strength (G) parameter across a broad range of clot stiffness values. In each plot, error bars indicate one standard deviation.
Experiments were performed in parallel with the TEG using the same blood samples dosed with abciximab. The results are summarized in Figure 6 (bottom panel), which depicts average Clot Stiffness values estimated by SEER Sonorheometry versus the average TEG's G parameter. As shown in this figure, the two measurements exhibit a linear relationship over the broad range of clot stiffness values analyzed.
Assessment of Fibrinogen Contribution to Clot Stiffness
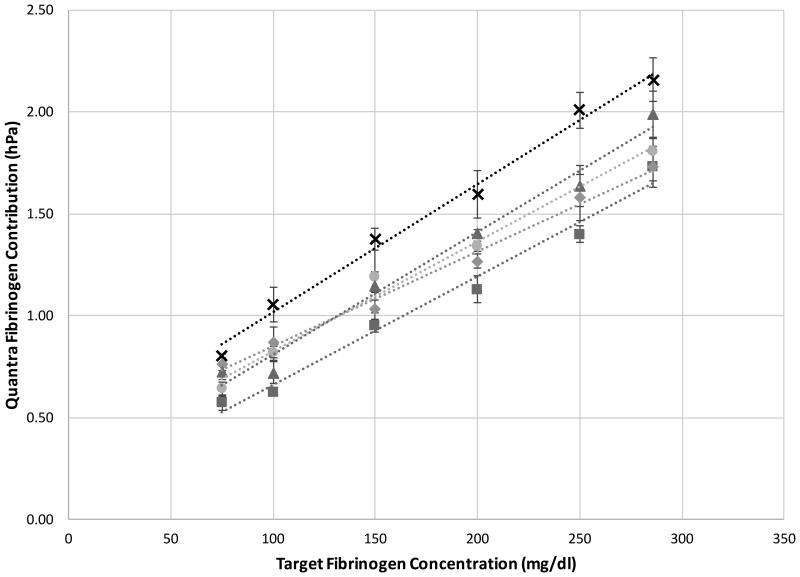
Figure 7 shows the effects of fibrinogen content on SEER Sonorheometry measurements of Fibrinogen Contribution. All the cartridge channels were loaded with channel 4 reagents (see Table 1), hence activating the sample with the combination of thromboplastin and a saturating dose of abciximab to remove the platelet's ability to modulate clot stiffness. For each of the five subjects enrolled, the target fibrinogen concentration was in the range of 75 mg/dl to 286 mg/dl, spanning the pathologically low to normal range.
Figure 7.
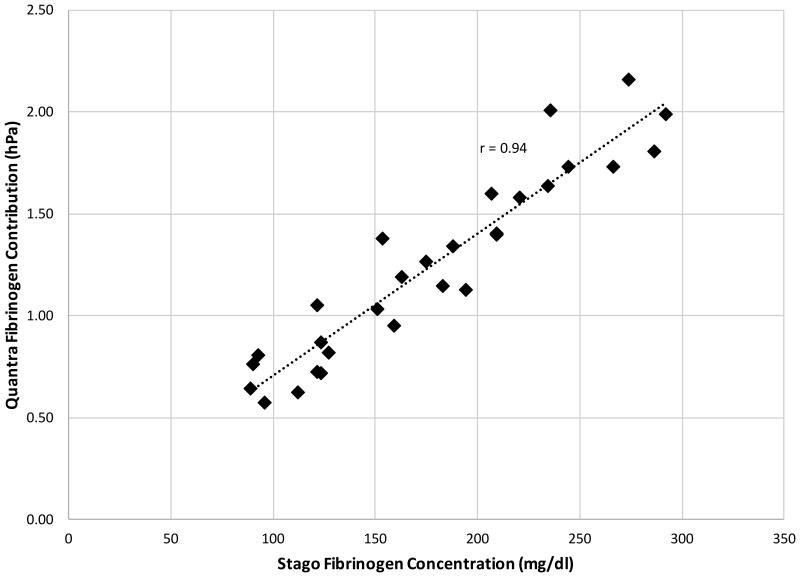
(Top Panel) Quantra Fibrinogen Contribution as a function of target fibrinogen concentration. Whole blood samples from 5 healthy volunteers were mixed with fibrinogen depleted plasma to yield concentrations of 75, 100, 150, 200, 250 and 286 mg/dl (n = 4 for each test condition). For each subject, data show high linearity across the fibrinogen range tested with an average correlation (r-value) with the best line fit of 0.99. (Bottom Panel) Scatter plot of the Quantra Fibrinogen Contribution vs the fibrinogen concentration determined by the Clauss assay as implemented in the Stago STart4 analyzer. The r-value of the best line fit is 0.94 (n = 29, 99% CI [0.87 – 0.98]).
The data shown in the upper panel of Figure 7 indicates that, for each subject, Fibrinogen Contribution varied linearly over the range of 75-286 mg/dl. The lower panel of Figure 7 shows the pooled Quantra data compared with plasma fibrinogen measured with the STart4. The correlation of 0.94 (n = 29, 99% CI [0.87 – 0.98]) observed between the two technologies is high, demonstrating the ability of SEER Sonorheometry to measure low fibrinogen concentrations.
Discussion
In this work, we introduced and characterized the Quantra Hemostasis Analyzer, a novel platform for the viscoelastic assessment of whole blood coagulation, and evaluated its reproducibility and ability to assess the function of key hemostatic components. The Quantra has been designed and optimized for ease of use, complete system automation, and rapid turnaround time in order to enable utilization at the point of care or near patient. The SEER Sonorheometry technology utilized in the Quantra is a patented method that does not require moving mechanical components to be in direct contact with the blood sample being measured. SEER Sonorheometry measures changes in shear modulus of the sample during coagulation to estimate the onset of clot formation and the clot stiffness generated by the combination of fibrin network and activated platelets.
The data presented suggests that SEER Sonorheometry can perform precise measurements for both the determination of clot time and clot stiffness in whole blood with CVs in the range of 2.4% – 8.8%. Figures 4 and 5 show Clot Time measurements over varying anticoagulant concentrations and coagulation factor deficiencies. The data presented in Figures 6 and 7 show the effects of varying levels of functional platelets and fibrinogen content to clot stiffness. These experiments revealed that the shear modulus measured in whole blood is roughly 7 times greater than the contribution provided by fibrinogen alone. We hypothesize that this wide dynamic range stems from the fact that SEER Sonorheometry is non-contact and applies low shear strains, allowing for the measurement of very soft clots that are often associated with clinical bleeding. The data also demonstrate that, under the conditions tested here, the Quantra exhibited good correlation with other well-established devices such as the TEG and the Clauss assay performed on the STart4 (Figures 5-7).
In conclusion, these studies indicate that SEER Sonorheometry combined with the Quantra Surgical Cartridge can provide a rapid and precise measures of Clot Time, Clot Stiffness, Platelet and Fibrinogen Contributions to clot stiffness, and the presence of heparin anticoagulation. Each of these test results could be used, together with other clinical information, to provide comprehensive information and guide treatments intended to restore balanced hemostasis. Additional studies are warranted to demonstrate the clinical utility of the Quantra and the Surgical Cartridge. We hypothesize that the comprehensive information that the device is capable of providing could have numerous potential clinical applications besides the surgical settings, such as in the case of advanced liver disease, cancer, and trauma.
Acknowledgments
We thank Paul J. Braun, Megan K. Shaw, William F. Walker, Deborah A. Winegar, and Anne Zavertnik for their thoughtful review of this manuscript. We thank Eugene Heyman for his support with data analysis. We also thank the technical teams at HemoSonics and Key Tech (Baltimore, MD) for their role in developing the Quantra and the Quantra Surgical Cartridge. This work was supported by grant R44HL103030 from the National Heart Lung and Blood Institute of the NIH and by the investors of HemoSonics LLC.
Footnotes
Contribution: Elisa A. Ferrante, helped design the study, conduct the study, analyze the data, and write the manuscript.
Kiev R. Blasier, helped design the study, conduct the study, and analyze the data.
Thomas B. Givens, helped analyze the data.
Cynthia A. Lloyd, helped design the study and analyze the data.
Timothy J. Fischer, helped design the study and write the manuscript.
Francesco Viola, helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Elisa A. Ferrante has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Kiev R. Blasier has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Thomas B. Givens has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Cynthia A. Lloyd has seen the original study data and approved the final manuscript.
Timothy J. Fischer has seen the original study data and approved the final manuscript.
Francesco Viola has seen the original study data, reviewed the analysis of the data, approved the final manuscript.
Conflicts of Interest: Elisa A. Ferrante is an employee of HemoSonics LLC.
Kiev R. Blasier is an employee of HemoSonics LLC.
Thomas B. Givens is an employee of HemoSonics LLC.
Cynthia A. Lloyd is an employee of HemoSonics LLC.
Timothy J. Fischer is an employee and shareholder of HemoSonics LLC.
Francesco Viola is an employee and shareholder of HemoSonics LLC.
IRB: The studies presented in this manuscript were approved by MaGil Institutional Review Board (Rockville, MD), under IRB #0001.
Contributor Information
Elisa A. Ferrante, HemoSonics LLC, University of Virginia.
Kiev R. Blasier, HemoSonics LLC.
Thomas B. Givens, HemoSonics LLC.
Cynthia A. Lloyd, HemoSonics LLC.
Timothy J. Fischer, HemoSonics LLC.
Francesco Viola, HemoSonics LLC, University of Virginia.
References
- 1.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-Guided Transfusion Algorithm Reduced Transfusions in Complex Cardiac Surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Cammerer U, Dietrich W, Rampf T, Braun SL, Richter JA. The Predictive Value of Modified Computerized Thromboelastography and Platelet Function Analysis for Postoperative Blood Loss in Routine Cardiac Surgery. Anesth Analg. 2003;96:51–57. doi: 10.1097/00000539-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. British journal of anaesthesia. 2009;103:i14–122. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 4.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfusion medicine reviews. 2012;26:1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Bochsen L, Stensballe J, Secher NH. Transfusion packages for massively bleeding patients: The effect on clot formation and stability as evaluated by Thrombelastograph (TEG®) Transfusion and Apheresis Science. 2008;39:3–8. doi: 10.1016/j.transci.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ‘bedside’ thromboelastography (ROTEM) Eur J Cardiothorac Surg. 2007;31:1052–1057. doi: 10.1016/j.ejcts.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Gorlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schochl H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012;39:104–113. doi: 10.1159/000337186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart, Lung and Circulation. 2009;18:277–288. doi: 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Naik BI, Pajewski TN, Bogdonoff DI, Zuo Z, Clark P, Terkawi AS, Durieux ME, Shaffrey CI, Nemergut EC. Rotational thromboelastometry-guided blood product management in major spine surgery. J Neurosurg Spine. 2015;23:239–49. doi: 10.3171/2014.12.SPINE14620. [DOI] [PubMed] [Google Scholar]
- 10.Gorlinger K, Shore-Lesserson L, Dirkmann D, Hanke AA, Rahe-Meyer N, Tanaka KA. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013;27:S20–34. doi: 10.1053/j.jvca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesthesia & Analgesia. 2008;106:1366–1375. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 12.Levy JH, Dutton RP, Hemphill JC, III, Shander A, Cooper D, Paidas MJ, Kessler CM, Holcomb JB, Lawson JH. Multidisciplinary approach to the challenge of hemostasis. Anesthesia & Analgesia. 2010;110:354–364. doi: 10.1213/ANE.0b013e3181c84ba5. [DOI] [PubMed] [Google Scholar]
- 13.Evans PA, Hawkins K, Lawrence M, Williams RL, Barrow MS, Thirumalai N, Williams PR. Rheometry and associated techniques for blood coagulation studies. Med Eng Phys. 2008;30:671–679. doi: 10.1016/j.medengphy.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Burghardt WF, Goldstick TK, Leneschmidt J, Kempka K. Nonlinear viscoelasticity and thromboelastograph. 1 Studies on bovine plasma clots Biorheology. 1995;32:621–630. doi: 10.1016/0006-355X(95)00041-7. [DOI] [PubMed] [Google Scholar]
- 15.Corey FS, Walker WF. Sonic Estimation of Elasticity via Resonance: A New Method of Assessing Hemostasis. Annals of Biomedical Engineering. 2015 doi: 10.1007/s10439-015-1460-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viola F, Mauldin FW, Lin-Schmidt X, Haverstick DM, Lawrence MB, Walker WF. A Novel Ultrasound-Based Method to Evaluate Hemostatic Function: Initial Validation. Clin Chim Acta. 2010;411:106–113. doi: 10.1016/j.cca.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauldin FW, Viola F, Hamer TC, Ahmed EM, Crawford SB, Haverstick DM, Lawrence MB, Walker WF. Adaptive Force Sonorheometry for Assessment of Whole Blood Coagulation. Clin Chim Acta. 2010;411:638–644. doi: 10.1016/j.cca.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang T, Johanning K, Metzler H, Piepenbrock S, Solomon C, Rahe-Meyer N, Tanaka KA. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108:751–8. doi: 10.1213/ane.0b013e3181966675. [DOI] [PubMed] [Google Scholar]
- 19.Solomon C, Ranucci M, Hochleitner G, Schochl H, Schlimp CJ. Assessing the Methodology for Calculating Platelet Contribution to Clot Strength (Platelet Component) in Thromboelastometry and Thrombelastography. Anesth Analg. 2015;121:868–78. doi: 10.1213/ANE.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelb GW, Kamykowski GW, Ferry JD. Rheology of fibrin clots. V. Shear modulus, creep, and creep recovery of fine unligated clots. Biophysical Chemistry. 1981;13:15–23. doi: 10.1016/0301-4622(81)80020-8. [DOI] [PubMed] [Google Scholar]
- 21.Zmuda K, Neofotistos D. Effects of unfractionated heparin, low-molecular-weight heparin, and heparinoid on thromboelastographic assay of blood coagulation. American journal of clinical pathology. 2000;113:725–731. doi: 10.1309/Q4AE-BMCW-CQ7J-NUVT. [DOI] [PubMed] [Google Scholar]
- 22.Packham MA. Role of platelets in thrombosis and hemostasis. Can J Physiol Pharmacol. 1994;72:278–284. doi: 10.1139/y94-043. [DOI] [PubMed] [Google Scholar]
- 23.The EPIC Investigators. Use of monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994;330:956–961. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]