Abstract
In this review we have summarized the role of gut homing molecules with a focus on the heterodimeric integrin α4β7 since the α4β7 has been shown to be important in modulating SIV transmission, disease susceptibility and progression. This review provides an overview of integrins, their structure and function to provide a general background upon which the role of the α4β7 integrin can best be understood. We also describe integrins and their cognate receptors and their potential role in modulating disease that we hope provides some food for thought on how such knowledge can be utilized for vaccine formulation.
Keywords: Integrins, transmission, SIV, α4β7, GIT
I. Introduction
The trafficking of lymphoid cells from the blood into the various tissues and organs has long been known to be influenced and regulated by the interaction between specific chemokines synthesized by cell lineages from the recruiting tissues/organs with their cognate chemokine receptors expressed on the cell surface of the lymphoid cells [1–4]. It is reasoned that chemokines are secreted by cells within the tissues upon sensing the need for cells of a specific lineage and such chemokines send signals like sonar waves with a gradient that is strongest at the source and diminishes with distance. These findings have given rise to the science of “lymphoid cell trafficking” and sometimes termed “lymphocyte homing” that has been a focus of interest for a wide variety of disciplines including immunology, infectious diseases, cancer biology, wound repair/dermatology, tissue/organ specific autoimmune disease, organ/tissue transplantation and inflammation, in general [5–13]. This is true for cell lineages that comprise both the innate and the adaptive immune system and logically implies that such interactions must occur in an ordered fashion so that the first responder innate immune system cells (that include neutrophils, macrophages and NK cells) are directed to the recruiting tissues prior to the cells of the adaptive immune system [14, 15] although, this issue of kinetics and the associated mechanisms involved have not been carefully examined to date. This subject is quite complex because even within one cell lineage, there exists various subsets exemplified by the heterogeneity that exists among NK cells with some possessing cytolytic function and others a non-cytolytic cytokine synthesizing function. These subsets each express an unique repertoire of chemokine and chemokine receptors that guide them to home to distinct tissues and organs with specialized functions [16]. Thus, the gut resident NK cell subsets are unique and quite distinct from those that are present in the blood or the skin and endometrial tissues where each subset performs function that is required within the tissue in which they reside and are guided by the specific micro-environment within the specific tissue. There has also been some effort to distinguish “lymphocyte trafficking” from “lymphocyte homing” in that the former simply implies the movement of cells from one tissue to another and the latter signifying that there are discrete sets of subsets of lymphoid cells that selectively reside (home) to a specific tissue or organ exemplified by cells that selectively home to the secondary lymphoid tissues including regional lymph nodes, gastro-intestinal tissues (GIT) and others to the skin, etc. Lymphocyte trafficking thus can be viewed as a result of chemokine gradients but lymphocyte homing is secondary to the binding of a receptor on a lymphoid cell to its specific ligand expressed by the cells of the homing tissue or organ. It is of interest to note that most of the molecules that serve as “homing” molecules appear to belong to the family of integrins.
The Integrins
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix, cell surface and soluble ligands and are widely distributed in mammalian species and eukaryotes [17]. There is considerable interest in the nature and biology of these integrins that are mostly expressed as heterodimers composed of an α and β chain. Both α and β integrin subunits are type I transmembrane glycoproteins with distinct and large extracellular domains, a single pass transmembrane domain and a short cytoplasmic tail [18, 19]. The extracellular domains of the integrins contain the ligand binding sites that require divalent cations (Ca++/Mg++ and Mn++) that bind to a metal ion-dependent adhesion site termed MIDAS. There are currently 18 different α chains and 8 different β chains that combine in various combinations to give rise to a set of 24 different heterodimeric integrins each of which have distinct substrate specificity and tissue expression profiles [20, 21]. The α and β chains of these heterodimeric integrins can pair such that several of these heterodimers can share one chain or the other (Fig. 1). Thus, as seen, the β1 chain can form heterodimers 11 different alpha chains and a V chain.
Fig. 1.

The various associations of alpha and beta chains of select integrins. Pertinent to this review, note the association of the α4 chain with not only the β7 chain but also the β1 chain and the association of β7 with not only α4 but also αE.
The α4 chain can heterodimerize with the β1 chain or the β7 chain. The β7 chain can heterodimerize with the α4 chain and an αE chain and so on. The mechanisms that lead to specific pairings of one chain with the other are far from clear and subject to further study. There has been no known record of the integrins being expressed as homodimers. The integrin family is composed of 4 sub-families based on the ligands they bind and/or based on phylogenetic relationships of the α chain of the integrin [22]. These include 1) the integrins that recognize a 3 amino acid motif arginine-glycine-aspartic acid (RGD). The RGD containing motifs are present in the plasma proteins fibronectin, vitronectin, fibrinogen and thrombospondins that serve as extracellular ligands for this family of integrins and include α5β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, and αIIbβ3, 2) the laminin receptor sub-family consisting of the heterodimers α3β1, α6β1, α7β1, and α6β4 integrins that facilitate adhesion to basement membranes of various tissues, 3) the RGD-recognition independent integrins α4β1, α4β7, and α9β1 that form a group by themselves and 4) integrins that have a special inserted domain and are denoted by αI or αA (von Willebrand factor type A). The integrins α1, α2, α10 and α11 hetrodimerize with the β1 chain and all bind various collagen types. The integrins αDβ2, αLβ2, αMβ2, αXβ2 and αEβ7 are predominantly expressed by white blood cells including lymphoid cells and their cognate binding partners include counter receptors such as ICAM-1 etc. The heterodimeric forms of the integrins are physically expressed on the cell surface in at least three different forms (Fig. 2) that include a bent form that has a closed head piece and an extended form that has a closed head piece and an extended form that has an open head piece, respectively [23]. Use of antibodies and/or small molecule inhibitors has shown that the bent and extended version with closed head piece have lower affinity to bind ligand whereas the extended version with an open head piece has a much higher affinity to bind its ligand [24]. This is important to know because binding of the integrin to its cognate receptor is clearly influenced by the form by which it is expressed that either promotes/facilitates binding or either binds poorly or fails to bind although the integrin and receptors may both be expressed. One can thus visualize these forms as being expressed in an “activated” or functional form or in an “inactivated” or “partially activated” form (Fig. 2). This concept also brings into question the use of antibodies against the integrins, in general. Thus monoclonal antibodies with specificity for the α chain, the β chain or the heterodimeric form of the integrin are commonly utilized for the detection of the integrins. It is not clear at present whether the specific monoclonal antibodies in question bind selectively to the inactive bent form, the extended forms, or all forms of the integrin. This is important to note because the literature is rampant with the use of an antibody against one chain denoting the detection of the heterodimer but in fact this may not be the case. Thus, for example, use of a monoclonal antibody against the α4 integrin or the β7 integrin have been utilized to denote the detection of the α4β7 heterodimer. However, since the α4 chain can also heterodimerize with the β1 chain to form α4β1 and the β7 chain can heterodimerize with not only the α4 chain but also the αE chain to form αEβ7, the data obtained using monoclonal antibodies against single α or β chains maybe subject to incorrect interpretation? This is especially relevant during an inflammatory response during which different pairings of α and β chains occur and expressed on the cell surface. In addition, it is also important to note that the binding of the integrin to its cognate receptor in a physiological sense can induce the generation of intracellular signals that may or may not be induced by the ligation of the same molecule with monoclonal antibodies that also bind but perhaps to a different non-signal inducing epitope of the integrin. The integrins also serve as part of the cell adhesion complexes that play an integral role in development and as part of a wide range of pathological processes. A study of the composition, organization and dynamics of these adhesion complexes appears to indicate that the complexes consist of both a core and non-canonical components. The adhesion signaling emanates from each of these two distinct components and the mechanisms involved in cell adhesion need to be viewed within this context [25]. A summary of a revised nomenclature for chemokine and their receptors has recently been published [26]. The various integrins, their cognate ligands and predominant function are described in Table 1.
Fig. 2.
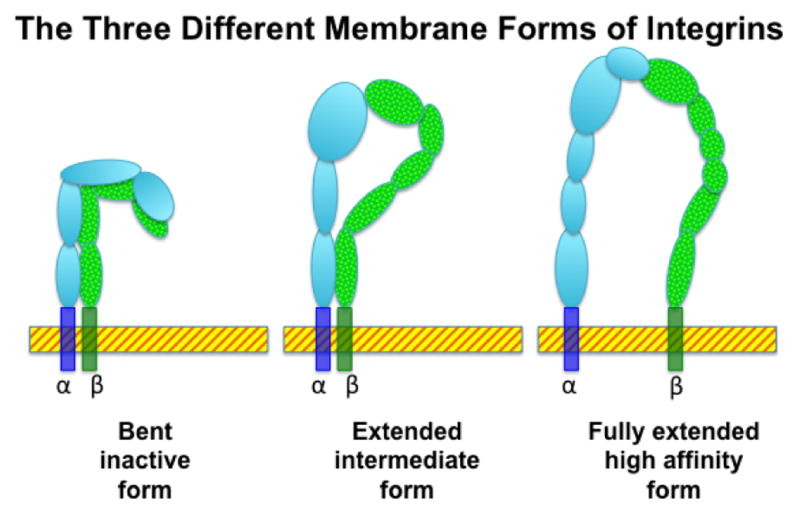
Integrins can be expressed in various forms. As an example, the membrane associated forms of the α4β7 heterodimer are displayed. There are at least 3 distinct forms of the cell membrane associated forms of the α4β7 integrin, the completely bent (inactive) form, the extended but closed head (intermediate) form and the fully extended (active) form. It is generally thought that it is the fully extended form that is functional for ligation to its cognate receptor MAdCAM-1.
Table I.
Integrin/Ligands and their predominant functions
| Integrin | Ligand | Function |
|---|---|---|
| alpha v (αv) | vascular generation | |
| alpha 4 beta1 (α4β1) | vcam-1 | lymphoid cell development |
| beta 1 (β1) | development | |
| alpha v beta3 (αvβ3) | bone sialoprotein | angiogenesis, wound healing, inflammation |
| beta2 integrins (β2) | immune responses | |
| alpha6 beta4 (α6β4) | laminin | skin integrity |
| alpha2b beta3 (α2bβ3) | borrelia burgdorferi | thrombus formation |
| alpha4 beta7 (α4β7) | MAdCAM-1 | gut homing |
| alpha E beta7 (αEβ7) | E-cadherin | mucosal homing |
| alpha l beta2 (αIβ2) | ICAM-1 | |
| CD62L | glycam-1 | lymph node homing |
| CCR7 | CCL19/CCL21 | lymph node homing |
Regulation of integrin expression
Clearly one of the subject areas that has concerned investigators involved in the study of the integrins as homing markers is/are the mechanisms by which the expression of these integrins are regulated. This subject has recently been reviewed [27, 28]. The mechanisms involved have to be understood in the context of the various steps involved in the role integrins play in the interaction between lymphoid cells and the tissues with which they react. Thus, distinct integrins are involved in recruitment and others involved in retention of the cells within the tissue [29]. Thus, when inflammation is induced within a tissue due to microbial insult, chemical or toxic exposure and/or trauma, the micro-environment within the area of insult changes, leading the tissues to generate signals based on the type of insult (step 1). The white blood cells rolling along the vessel walls surrounding the tissue sense the signals (step 2), the integrins expressed on the cell surface of these cells upon interacting with their natural ligands on the endothelial wall initiates adhesion (step 3). This step is followed by increased strengthening of the adhesion (step 4), that is followed by invagination of the vessel wall through which the lymphocyte edges itself through (step 5) and leads to the migration of the lymphoid cell from the vasculature to the inflamed targeted tissue (step 6). This entire process is termed diapedesis [30]. During each step of this process, distinct signaling events are generated leading to the recruitment of the effector cells to the inflamed tissue and the lymphoid cell subsets that express the appropriate integrin ligates with its cognate receptor on the target tissues and thus potentially “homes” [31]. It is important to point out that this entire process has to be regulated to prevent continued migration and uncontrolled tissue destruction and thus becomes a process of checks and balances. While this is certainly true for acute insults secondary to infection, chemical exposure or trauma, it is understood that the cascade of events during chronic infection are quite distinct. Thus, chronic infection and ensuing chronic inflammation leads to the induction of high endothelial venule like vessel neo-formation which now seek to recruit lymphoid cells forming a lymphoid cell tissue site, although this site was not previously a formal lymphoid tissue site. The newly formed vessel of interest promotes lymphocyte recruitment and homing much like the sites previously known to serve as sites of lymphoid tissues. The signals generated by the inflamed tissues once this new site is formed is similar if not identical to sites normally involved in lymphoid cell recruitment [32].
The heterogeneity of the cells combined with the various steps involved in lymphoid cell recruitment and homing make it increasingly clear that there is considerable diversity in the receptor/ligand interactions of chemokines and their receptors [33] but that ligation of a specific chemokine to its cognate receptor induces an unique cascade of signaling molecules resulting in biological function. The signaling pathways are not only due to the translation of the dialog at the cell surface with subsequent transmission of signals via the cytoplasm to the nucleus, such as the role of focal adhesion kinase (FAK), a protein tyrosine kinase and integrin-linked kinase (ILK) which is a serine-threonine kinase [34, 35] but also includes the activation of inside out signaling [36] providing a more refined understanding of the molecular basis of chemokine/receptor interactions.
The regulation of integrin expression, thus, has to be understood within the context of the tissues and organs that are being studied and while they may all share certain common mechanisms, they are likely to be distinct based on the tissue. In general, there are molecules synthesized by cells within a specific tissue due to a local response (such as induced by infection, trauma or injury) that either up-regulate or induce the neo-expression of cell surface molecules on cells that specifically direct the cells to migrate to that specific tissue. Thus changes in the micro-environment within the tissue leads to the generation of such molecules. This is best exemplified by tissues within the gastro-intestinal tract that is the largest immune organ in the vertebrate species. It is now known that dietary nutrients and microbial flora influence the function of intestinal dendritic cells within the GI tract and thereby play a major role in both mucosal and systemic development and function of the immune system [37]. Dysregulation of either the nutritional status, the microbial flora or both play a pivotal role in the decision making process that leads to disease or rapid containment and return to homeostasis. The intestinal dendritic cells when provided the appropriate environmental signal (insult) leads to the synthesis of retinoic acid (although there is some discussion on the cell lineages that synthesize retinoic acid) that in turn leads to the upregulation of the homing molecules such as α4β7 and CCR9 on the lymphoid cells that selectively traffic to the intestinal tissues. This is true for most if not all cell lineages that eventually reside in the intestinal tissues including innate lymphoid cells [14], NK cells [38], T cells [39–41], B cells that are also influenced by lactoferrin [42], and dendritic cells [43] to name a few. It is important to note that differences do exist on the homing molecules that direct cells to home to the large intestine as compared with the small intestine (Fig. 3). This difference is dictated by the expression of the cognate ligands for these homing molecules. Thus, while CCR9 serves to target the homing of cells to the small intestine, the α4β7 integrin serves to target the cells predominantly to the large intestine [44]. It is reasoned that this is because whereas the ligand for α4β7 is expressed by high endothelial cell venules and epithelial cells lining both the large intestine and small intestine, the ligand for CCR9 is CCL25 that is selectively expressed by the small intestine [45, 46]. The importance of CCL25 is highlighted by the finding that HIV-1 infection leads to the down regulation of CCL25 synthesis. This decrease in CCL25 has been shown to be the potential reason for the poor reconstitution of CD4+ T cells to the gut mucosa in HIV-1 infected patients being treated with ART despite undetectable levels of plasma viremia [46]. It is also of interest to note that CCR9 has been shown to inhibit the development of Tregs [47] that are known to contribute to the generation and maintenance of self-tolerance. These data imply that perhaps, Treg facilitated tolerance is a mechanism that operates predominantly in the large as compared with the small intestine. Along these lines, the finding of differences in the phenotype and function of mononuclear cells that reside in the ileum versus the colon (that respond to different bacterial flora/loads and different environmental cues) suggest that there is even evidence of heterogeneity within segments of the large intestine. In contrast to the gut tissues, the skin has developed its own set of signaling molecules and mechanisms to selectively recruit cells to the skin tissues. It is of great interest to note that the inducers for both gut and skin homing molecules turn out to be simple vitamins that for physiological reasons are synthesized differentially by each organ. Thus, sunlight is known to activate vitamin D3 in the skin that serves as a signaling molecule that upregulates CCR10 and down regulates α4β7 and CCR9 leading for the cells to traffic to the skin. The ligand for CCR10 is CCL27 expressed by skin keratinocytes. Thus, it is reasoned that inflammation in skin tissues leads to the activation of vitamin D3 that in turn upregulates CCR10 and down regulates CCR9/α4β7 and combined with the synthesis of CCL27 by skin keratinocytes results in the rapid recruitment of lymphoid cells to the skin where such lymphoid cells attempt to contain inflammation. It is assumed that the action of vitamin A in the gut and vitamin D3 in the skin has reciprocal homing molecule inducing potential (Fig. 4) [48]. Besides the recent discovery of gut and skin homing molecules, there have ben a plethora of previous advances made on molecules that facilitate homing of cells to lymphoid tissues such as lymph nodes. These include the expression of CD62L (L-selectin) and CCR7 [49, 50]. The natural ligands for L-selectin are GlyCAM-1 expressed by HEV of lymph nodes, CD34 expressed by endothelial cells, MAdCAM-1 expressed by endothelial cells lining the vessels within the gut associated lymphoid tissues and PSGL-1 expressed by white blood cells and endothelial cells (although its preferred ligand is P-selectin). The natural ligands for CCR7 are the chemokines CCL19 and CCL21[51]. As noted, the list of homing molecules and their cognate receptors keeps growing as results of additional studies provide a more refined view on this subject and more precise cell lineages/subsets get identified specially those that play critical roles in either promoting/facilitating and/or regulating immune responses. This view is best captured by the recent discovery of a discrete population and subset of CD4+ T cells termed T follicular helper T cells (Tfh) that have been shown to play an important role in facilitating antibody production by B cells and germinal center formation [52]. This subset synthesizes IL-21 and its receptor IL-21R is widely distributed on immune cells. The Tfh are phenotypically characterized by the expression of CXCR5 whose ligand is CXCL13 and it is now becoming clear that the synthesis of CXCL13 leads to the selective trafficking of Tfh (see Fig. 5) to local lymph nodes [53]. The Tfh also are characterized by the expression of CXCR3, CCR5, PD-1++ [54]. Thus, it may turn out that discrete subsets express a discrete set of cell surface molecules that help in the identification of the subset and that some of these molecules are integrins that selectively bind to their cognate chemokine ligands that are synthesized by specific cells within a specific tissue and that such chorus of events leads to the trafficking of this discrete subset to a specific tissues or organ where they mediate their specific immune function [55]. In other cases, there are chemokines that bind to their corresponding chemokine ligands and induce the activation of intracellular pathways initiating the synthesis of cytokines and thus mediating biological function (a list of some of the chemokines listed by their common and by their proper chemokine nomenclature and their respective ligands are listed in Table 2. Increasing evidence indicates this to be the case for a number of hematopoietic and progenitor stem cells and mesenchymal stem cells that traffic to specific anatomic sites when they are needed [56]. Thus, the concept of cell trafficking and homing is now accepted as a general phenomenon for a variety of cell lineages including the neural progenitor cells (NPCs) that express CXCR4 and respond to the chemokine CXCL12 and functions during neuronal injury to guide cells for repair mechanisms to become initiated [57].
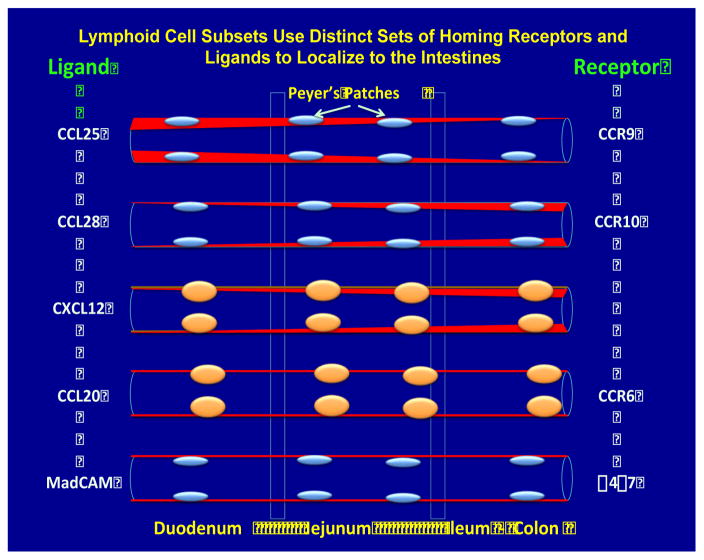
Fig. 3.
Lymphoid cell subsets can be distinguished by their expression of specific chemokine receptors that have specific ligands. The ligands are expressed at either different densities along the intestinal tissues or at the same level (red) denoting sites for high versus low levels of specific lymphoid cell subset homing. Some of these pairs of receptor:ligands are illustrated primarily to exemplify how the expression of the receptors promote the differential trafficking of lymphoid cells to distinct anatomical sites of the intestine. The receptor for CXCL12 is currently not known.
Fig. 4.
Recent data highlights the potential role of different vitamins in influencing the trafficking of immune cells to distinct tissue sites. Thus Vitamin A is metabolized to all trans retinoic acid by dendritic cells in the GI tissues that markedly upregulates the expression of α4β7 and CCR9 (the gut homing molecules) while retinoic acid down regulates the expression of CCR10 (the skin homing molecule). On the contrary, on the exposure to sunlight the activated form of Vitamin D3 upregulates CCR10 (skin homing molecule) but down regulates α4β7 and CCR9 (gut homing molecules). This distinct functions of the vitamins leads to the differential homing of retinoic acid activated lymphoid cells to the GI tissues where it ligates with its cognate receptor MAdCAM-1 and vitamin D3 activated lymphoid cells to the skin where it ligates with CCL27 expressed by keratinocytes.
Fig. 5.

A prominent role for T follicular helper T cells (antigen experienced CD4+ T cells found in the periphery within B cell follicles of secondary lymphoid organs) has recently been documented. The figure illustrates the preferential homing of Tfh via their expression of CXCR5 that responds to the chemokine CXCL13. Perturbations in the synthesis of CXCl13 and/or the expression of CXCR5 results in immune dysfunction highlighting the important role of the cheomine and chemokine receptor interactions.
Table 2.
Chemokine and their cognate ligands
| Common name | Chemokine nomenclature | Cognate ligand |
|---|---|---|
| IL-8 | CXCL8 | CXCR1 |
| IP-10 | CXCL10 | CXCR3 |
| SDF-1 | CXCL12 | CXCR4 |
| MCP-1 | CCL2 | CCR2 |
| MIP-1β | CCL4 | CCR5 |
| RANTES | CCL5 | CCR5 |
| MIP-3α | CCL20 | CCR6 |
| TECK | CCL25 | CCR9 |
| FRACTALKINE | CX3CL1 | CX3CR1 |
The gut homing α4β7 integrin
Our laboratory for the past decade has chosen to focus our studies on the α4β7 integrin, one of the major gut homing molecules that has been studied by a number of laboratories worldwide so far (Fig 6). It is known to interact with the HIV-1 gp120 trimer and is shown herein to illustrate both its proximity to and size relative to the CD4 molecule. The rationale for focusing on α4β7 was based on the following facts:
Fig. 6.

The conformational structure of the membrane expressed form of the activated α4β7 heterodimer and its size relative to the CD4 molecule is illustrated. It has been documented that the HIV-1 gp120 trimer associates with the α4β7 molecule via various env sequences.
The CD4+ T cells residing within the gastro-intestinal tract (GIT, the largest immune organ in the human body) are the major target of both HIV-1 infection in humans and pathogenic SIV infection of the macaque species of nonhuman primates (NHP). The experimentally SIV infected macaque species of NHP is the accepted prototype model to study human HIV-1 infection.
The CD4+ T cells are rapidly eliminated from the GIT during acute infection leaving the tissues that comprise the GIT dysfunctional and what is most important is the finding that even if potent anti-retroviral drug therapy is initiated during early acute infection, the GIT never returns back to “normal” despite the reduction of viremia to undetectable levels. This irreversible state has been thought to be secondary to fibrosis of the GIT that does not allow for proper re-seeding of the lymphoid cells.
The compromised GIT leads to a lifetime of abnormal GI function associated with sub-optimal immunological function and also thought to lead to GIT leakage promoting bacterial translocation and chronic immune activation.
It was thus reasoned that limiting and/or preventing gut tissue damage and dysfunction particularly during acute infection might provide a window of opportunity for the host immune system to respond physiologically in a manner that would be beneficial to the host in the long run.
Towards this goal we sought to capitalize on the knowledge that the α4β7 integrin serves as one of the major gut homing molecules on CD4+ T cells and targeting this molecule may achieve the objective of limiting the migration/trafficking of CD4+ T cells to the gut tissues.
A murine monoclonal antibody termed clone Act-1 was available in our laboratory that has specificity for the heterodimeric form of the α4β7 integrin.
The murine anti-α4β7 monoclonal antibody was primatized by transplanting/grafting the CDR’s of this antibody onto a recombinant rhesus IgG1.
The recombinant rhesus anti-α4β7 mAb is stable and was utilized for all the studies described henceforth below.
In vivo pharmacokinetics (PK) studies showed that a single bolus dose of 50 mg/kg of the anti-α4β7 mAb was sufficient to completely block α4β7 expressing cells in the blood, gastro-intestinal tissues and vaginal tissues for a period of approximately 21 days.
The administered antibody at this concentration was very well tolerated with no detectable adverse hematologic, biochemical or liver/kidney/cardiac changes.
Tissue distribution of α4β7 integrin
A varying frequency of most hematopoietic cell lineages is known to express the α4β7 integrin in the majority of cases in an inactivated form. Thus, naïve CD4+ and CD8+ T cells and B cells constitutively express a low mean density level of expression of the α4β7 integrin. The α4β7 integrin is also expressed by NK cells, monocytes, subsets of dendritic cells, eosinophils, IgA secreting plasma cells and mast cells, to name a few [58]. Detailed flow cytometric based analysis of the distribution of α4β7integrin on lymphoid cells has been published [59]. In the gastro-intestinal tissues, the CD4+ T cells that express α4β7 are predominantly resting cells (CD25−/CD69−/HLA-DR−/Ki67−) with a central memory phenotype (CD28+/CCR7+/CD45RA−) that also express CCR5. In addition, the majority of the Th17 subset of CD4+ T cells also express α4β7. It is important to keep in mind that the α4β7 integrin is expressed in various forms on the cell membrane (Fig. 2). This includes a form that is flat and parallel with the cell membrane, an intermediate activated for whereby the extracellular domains of the α and β chain are half extended (bent form) and the fully extended activated form. The Act-1 and the primatized anti-α4β7 monoclonal antibodies both appear to react to all 3 forms of the α4β7 integrin and thus staining with this reagent does not help distinguish the form being expressed. What is important to note is that the binding of α4β7 to its natural ligand MAdCAM requires divalent cations and the evidence so far indicates that its ligation requires an activated for of the α4β7 molecule. It is generally known that activated cells express higher mean densities of the α4β7 integrin and of importance is the finding that it is precisely this subset of CD4+ T cells with high densities of α4β7 expression that are the principal target of both HIV-1 and SIV infection. As indicated above, the α4β7high expressing CD4+ T cells were identified as one of the major targets of HIV-1 infection in humans and SIV infection in rhesus macaques [60–62]. These data indicate that the targeting of CD4+ T cells that express α4β7 and co-express CCR5 and Th17 from trafficking to the gut may lead to decreased numbers of target cells and thus potentially decrease GIT pathology. In other words, prevent the addition of fuel to the fire in the GIT.
The molecular basis of antibody mediated inhibition of integrin binding
While it is generally assumed that antibodies against the integrins bind to the integrins and in so doing inhibit their binding to their cognate receptors such as anti-α4β7 binding to MAdCAM-1 and anti-α4 binding to VCAM-1, the details of the specific binding sites/motifs of such antibodies on the integrin molecule and how such antibodies mediate their effect is far from clear. Use has been made of electron microscopy and crystallographic techniques to gain at least some partial understanding of the domains of the integrins that are bound by the antibodies and the domains of the integrins that bind to their natural ligands [63] in efforts to define how such antibodies may function in vivo. Thus, the anti-α4 monoclonal antibody (also called Natalizumab) binds to the α4β-propeller domain of the α4β1 integrin that is outside the ligand binding domain 1 of VCAM-1. But by binding, it induces sufficient change in the α4β1 molecule so that such binding non-competitively antagonizes the binding of α4β1 to VCAM [63]. The anti-α4β7 monoclonal antibody (also termed Vedoluzimab) appears to recognize an extended, disulphide-bonded loop known as the specificity determining loop (SDL) that projects from the β7 βl domain near its metal ion dependent adhesion site (MIDAS) at the interface with the α4 β-propeller domain. The antibody interestingly appears to contact just the β7 subunit but only when it is in its heterodimeric form with the α4 chain. In this regard, it is important to note that the α4β7 integrin binds to its cognate receptor MAdCAM-1, but one of the intriguing question with regards to the binding of α4β7 to MAdCAM-1 is that such binding is known to facilitate both rolling adhesion (relatively fluid) along the endothelial wall and also firm binding of the cells to MAdCAM-1 expressed by vascular cells specially within the GIT. Thus unlike other integrin:receptor binding which are known to be quite firm such as the vascular cell adhesion molecules, ICAM-1, 2, 3 and 5, the MAdCAM-1 structure is either relatively flexible or is expressed in different forms. Conformational changes within MAdCAM-1, which involve displacement of the α1 and α7 helices in the β1 domain, suggest that the location of these helices play an important role in conferring the state of affinity of this molecule for the α4β7 molecule [63, 64]. While some controversy still exists as to the nature of the specificity of the sequences of the α or β chain that are bound by the respective anti-α4 (Natalizumab) or anti-α4β7 (Vedoluzimab) monoclonal antibodies, full crystal structures of these integrins may help resolve such issues. However, these are pretty big molecules and therefore hard to define their crystal structure.
Role of vitamins as regulators of immune responses
The fact that our body is constantly exposed to micro-organisms via the skin (surface exposure), lungs and pulmonary tissues (air), and the gastro-intestinal tissues (ingested food) logically dictates that a site specific immune system has developed phylogenetically to allow us to continuously interact with such diverse environmental insults. This complex network of immune cells are therefore distinct for each site of exposure and have specialized functions unique to the tissue/organ site based on the type of micro-organisms that they are likely to encounter. Thus, compartmentalization of the immune system developed in which specific tissue sites acquired the ability to recruit the cell types required dealing with the group of micro-organisms that are uniquely present at that specific site. Exposure to distinct micro-organisms at distinct sites also helped develop a memory response at the specific site to rapidly interact with specific types of micro-organisms increasing the efficiency of the immune system. In order to recruit the specialized cells to specific tissue sites, a signaling system developed in the form of chemokines and their corresponding receptors. Thus, sensing of the micro-organism at a specific tissue site led to the generation of chemokine signals that would selectively attract the cell lineage that expressed the specific chemokine receptor required for the specialized function at the specific tissue site. One of the first sets of experiments that documented the homing of cells to specific tissue sites, were studies in which gut immune cells when isolated and then transfused were shown to home preferentially to the gut tissues upon passive transfer [65]. Isolation of distinct subsets of lymph cells were also labeled and re-infused in sheep and their in vivo tracking post infusion led to the identification of 3 distinct subsets that migrated either to the skin, lymph nodes or the gut tissues [66]. These initial findings led to the identification of cell surface markers on these cells that were associated with specific tissue tropism and to the identification of chemokines that selectively recruited cells to a specific tissue. These pioneering studies followed by the successful preparation of monoclonal antibodies against cell surface molecules led to the description of a series of tissue homing molecules that serve as zip codes for cells to home to specific tissues and organs. These include the α4β1, α4β7, CCR9, CCR4, CLA (cutaneous lymphocyte antigen), CD103 (αEβ7), to name a few. More recently, a series of elegant studies of tissue specific homing led to the knowledge that simple vitamins such as vitamin A and vitamin D3 play antagonist roles in the expression of homing molecules on lymphoid cells specifically to the gut and the skin tissues, respectively (Fig. 4). The most physiologically relevant form of vitamin D is vitamin D3 that is synthesized in the skin from 7-dehydrocholesterol, a process that is dependent on sunlight [67]. Locally produced vitamin D3 can act on immune cells both in an autocrine and paracrine manner. Vitamin D3 has a general inhibitory effect on adaptive immune responses. It has been shown to inhibit T-cell proliferation, and inhibit chronically activated T cells [68], inhibit the expression of IL-2, IFN-γ, CTL function and enhance non-specific T-cell suppressor activity. It does enhance IL-4 synthesis and thus reasoned to shift TH1 type immune responses to TH2 type immune responses. Its role in maintaining gut tissue homeostasis has recently been summarized [69]. In addition, it has been shown to play a role in regulating host versus graft specific immune responses at the maternal-fetal interface [70]. Vitamin A on the other hand is obtained from the diet either as all-trans-retinol, retinyl esters of β-carotene [71, 72]. All-trans retinol or β-carotene are oxidized to all-trans retinal by alcohol dehydrogenase (ubiquitously expressed enzymes). All-trans-retinal is then oxidized to all-trans retinoic acid by retinal dehydrogenase (RALDH), the expression of which is tightly controlled. A simplified version of the metabolism of vitamin is illustrated in Fig. 7. All-trans retinoic acid has been known to be synthesized by dendritic cells in the gut tissues that are CD123−, CD11c+, CD103+. The ligands for α4β7 and CCR9 are MAdCAM-1 and CCL25, respectively expressed by the HEV and gut epithelial cells (Fig. 8). Retinoic acid synthesis leads to the upregulation of the α4β7 and CCR9 expressed by resting multiple cell lineages including NK cells and CD4+ T cells that then home to the GI tissues and ligate with their cognate receptors MAdCAM-1 and CCL25. However, it is important to note that there is some data that also support that retinoic acid is synthesized by gut epithelial cells. It is also important to keep in mind that there are a number of cytokines and their corresponding ligands that function in homing. This is best illustrated by the study of innate immune cells such as NK cells. Thus, the synthesis of IL-23 and its ligation with its receptor IL-23R on NK cells, in particular CD56+, NKp44+ and the CD56−, NKp44− subsets leads to the synthesis of CCL20 that binds to CCR6 that in turn leads to the synthesis of the cytokines IL-22 and IL-26. These 2 cytokines (IL-22 and IL-26) then can bind to their cognate receptors IL26R and IL-22R expressed by epithelial cells lining the GI tract. Fig. 9 illustrates in summary form the synthesis and interactions between these cytokines/cytokine receptors and the cells of the innate immune system such as the NK cell subsets.
Fig. 7.
The extracellular and intracellular pathways involved in the metabolism of Vitamin A are illustrated. Vitamin A is metabolized to yield all trans retinol that forms a complex with pre-albumin and SRBP and the complex binds to STRA6 which leads to its internalization and association with CRBP. The retinol is then converted to retinal by the enzyme ROLDH which is converted to retinoic acid (RA) by the action of RALDH. RA is then transported to the nucleus where it binds to a sequence termed the RAR and initiates transcription of a number of genes that have such a RAR element. The dictionary for the acronyms is shown on the right. Thus, while the pathways by which RA activates transcription have been defined, how these activation pathways are involved in the upregulation of α4β7 and CCR9 is a subject of study.
Fig. 8.
A summary of the sequence of events that leads to the trafficking of gut homing cells is illustrated. As seen, dendritic cells (CD123−, CD11c+, CD103+), and/or gut epithelial cells synthesize retinoic acid from vitamin A. The local followed by the gradual systemic release of retinoic acid leads to the up regulation (activation) of the gut homing molecules α4β7 and CCR9 on a variety of cell lineages including CD4+ T cells and NK cells. Such α4β7 hi and CCR9hi expressing cell lineages traffic to the gut where they ligate and anchor with their corresponding ligands MAdCAM-1 and CCL25.
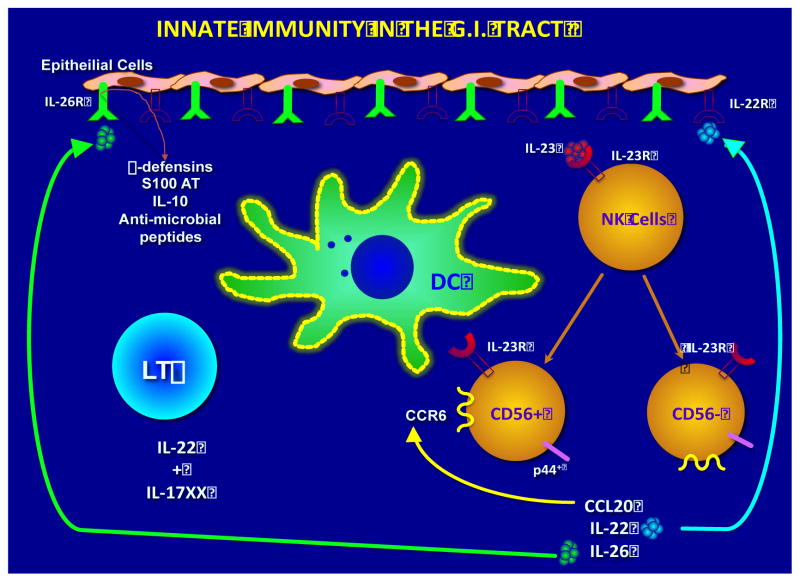
Fig. 9.
A variety of cytokines and their cognate cytokine receptors also play a role in the trafficking of lymphoid cells within the GI tissues in concert with chemokines. Thus, gut tissue resident dendritic cells upon sensing infection synthesize IL-23 (part of the IL-12 family of pro-inflammatory cytokines). The cytokine IL-23 ligates with its cognate receptor IL-23R expressed NK cells that upon ligation differentiate into CD56+, p44+ and CD56−, p44− subsets. These subsets in turn synthesize the chemokine CCL20 and the cytokines IL-22 and IL-26. The IL-22 then appears to bind to the IL-22R and the IL-26 to the IL-26R expressed by gut epithelial cells whose ligation leads to the synthesis of a variety of anti-microbial molecules including β-defensins, S100AT, IL-10 etc. CCL20 binds in an autocrine fashion to its ligand CCR6 expressed by the 2 NK cell subsets and the cycle is perpetuated until the regulatory mechanisms are activated.
As opposed to vitamin D3, vitamin A enhances adaptive immune responses including increased IL-2, increased CTL function, inhibits IL-4 synthesis and thus promotes TH1 over Th2 responses, increases the synthesis of metalo-proteinases, enhance antigen presentation in the presence of TNF-α, and of great interest drives the development of Tregs and TH17 cells.
The vitamins A and D3 also influence homing of cells. Effector and memory lymphocytes migrating to the small bowel express α4β7 and CCR9 in the presence of retinoic acid whereas those that migrate to the skin rely on the expression of CCR4 and CCR10 in the presence of vitamin D3, although this requirement is not seen in murine cells. Vitamin D3 does decrease the production of the chemokines CCL2, CCL5, CXCl10 and thus influence the migration of monocytes in EAE models. The studies of the effect vitamins have on the immune system has been elegantly summarized elsewhere [73].
Effect of in vivo anti-α4β7mAb administration on SIV infection of rhesus macaques
As outlined above, our rationale for focusing on the α4β7 integrin was primarily driven by the hypothesis that such antibodies may inhibit and/or limit the trafficking of CD4+ T cells that express α4β7 and are the primary target of both human HIV and SIV infection of humans and nonhuman primates, respectively. In short, prevent adding fuel to the fire and thus limit gut pathology. In efforts to establish proof of principle, we first initiated studies in which groups of rhesus macaques were administered either the primatized anti-α4β7 mAb or for purposes of control normal rhesus IgG intravenously 2–3 days prior to infection. We infected groups of monkeys with a single high dose of SIVmac239 either intravenously or intra-rectally on day 0 and then administered the anti-α4β7 or normal rhesus IgG every 3 weeks based on previously defined pharmaco-kinetic data. The results of these studies showed that while the administration of the anti-α4β7 mAb had only some marginal effect on plasma viral loads (0.5 to 1 log10 decrease), the administration of the anti-α4β7 mAb markedly decreased gut tissue viral loads as compared with the monkeys that received normal rhesus IgG (p< 0.0001) for prolonged periods of time (>1 year). The administration of the anti-α4β7 mAb also appeared to preserve the CD4+ T cells both in the blood and the gut tissue and markedly delayed the onset of any AIDS like disease in these animals up to 3–5 years. In comparison, 12/12 monkeys that received normal rhesus IgG developed high plasma and gut tissue viral loads and developed AIDS within 18 months of the infection [74, 75].
These studies prompted us to determine whether the anti-α4β7 mAb administration would have any effect in monkeys challenged intra-vaginally, the most predominant route of HIV infection worldwide. Groups of 12 female monkeys were administered the anti-α4β7 mAb and for purposes of control another group of 12 female monkeys were administered normal rhesus IgG (each at 50 mg/kg intravenously) on day −2 and then every 3 weeks, thereafter. The entire group of 24 monkeys were then infected repeatedly at weekly intervals with 1 ml of a low dose of a single stock of SIVmac251. Under the pre-determined protocol, animals were re-challenged every week until 10/12 control IgG treated animals became infected. Based on plasma viral loads, the administration of the anti-α4β7 mAb delayed infections, as 10/12 control-treated animals were infected after a median of 2–3 exposures, compared to 5–6 exposures that were required to infect the anti-α4β7 treated macaques. At this point only 6/12 anti-α4β7 treated macaques were infected. Thus, the # of challenges required to infect animals was increased in the presence of anti-α4β7 (p=0.002 log-rank test for differences in survival curves; HR reduction = 4.3 (95% CL 1.5, 12.2), p-value=0.007; proportional hazards regression model). A comparison of infection rates per SIV challenge showed that whereas the anti-α4β7 administered monkeys required 71 challenges to infect 6/12 monkeys, 10 of the control 12 monkeys that received IgG only required 44 challenges. There was thus a 2.7 fold decreased risk in infections per SIV challenge (Fisher’s exact test, p < 0.05) in the monkeys that received the anti-α4β7 mAb. The results of the gut viral loads were even more striking. Thus, whereas all 10/12 control IgG treated macaques showed between 10–20 copies of pro-viral DNA/ng DNA of the gut tissues, the 6 monkeys that did get infected in the anti-α4β7 treated group showed consistently <3–5 copies/ng DNA throughout the study. Of further importance was the finding that the anti-α4β7 treated animals that became infected maintained significantly higher peripheral CD4+ T cells numbers and gut tissue levels of CD4+ T cells suggesting that the anti-α4β7 treatment had protected the otherwise normal depletion of CD4+ T cells that occurs in the control IgG treated animals. Thus, blocking the α4β7 integrin significantly impedes both trans-vaginal transmission and subsequent tissue dissemination of the virus in this rhesus macaque model of HIV/AIDS [76].
In summary, the in vivo administration of this primatized monoclonal antibody against the α4β7 integrin prior to and during acute infection markedly reduces gut tissue viral loads in monkeys infected intravenously, intra-rectally or with low repeated doses of SIV intra-vaginally. Such administration also reduces vaginal transmission, protects the depletion of peripheral CD4+ T cells and markedly delays progression to disease in animals that do become infected.
Potential mechanisms by which anti-α4β7 administration mediates its beneficial effect
The potential biological effects of anti-α4β7 mAb administration presumably depends on its ability to bind to the α4β7 heterodimers on the surfaces of activated CD4+ T cells, but it is not yet clear which consequences of this binding are most relevant for protection. In our study, the in vivo administration of this α4β7-mAb did not greatly appreciably alter the numbers of CD4+ T cells within the gastro-intestinal tissues and the cervicovaginal compartment; however, the α4β7-mAb did bind to > 99.9% of the α4β7 heterodimers on the surfaces of those cells. The resulting masking of α4β7 might suppress infection by reducing the physical interaction of potential target CD4+ T cells with SIV strains whose Env proteins bind specifically to α4β7. Alternatively, or in addition, α4β7-mAb may, by interfering with α4β7+/CD4+ cells binding to MAdCAM-1, act to suppress the subsequent trafficking of infected α4β7+/CD4+ cells from the genital tract to the GALT, thereby preventing spread of a nascent infection into the largest depot of vulnerable target cells in the body. We hypothesize that these two potential mechanisms by which α4β7-mAb functions are intrinsically linked. A gp120 derived from an SIVmac251 founder isolate binds to α4β7 in such a way that it competes with MAdCAM. Moreover, the α4β7-mAb inhibits binding of both SIVmac 251 gp120 and MAdCAM to α4β7. The capacity of the α4β7-mAb to interfere with both of these interactions is important to note. It is consistent with a degree of mimicry between gp120 and MAdCAM [40] that underscores the importance of α4β7+/CD4+ cells in vaginal transmission. The protection afforded by the α4β7-mAb in the series of studies conducted by our lab strongly suggests that SIVmac251 utilizes this subset of CD4+ T cells at some key early point during transmission. The importance of accessing α4β7+/CD4+ T cells early after transmission is further supported by the delayed infection observed in a number of the α4β7-mAb treated animals. Of note, one of our treated animals did not become viremic until three weeks after viral challenges were discontinued and that we detected persistent proviral DNA in cervical tissues of the infected treated monkeys we tested – but neither of the two uninfected controls – in necropsies performed at 16–18 weeks. Those observations raise the possibility that blocking α4β7 delays the spread of an initial, localized cervicovaginal infection by blocking migration of the infected T cells. Finally, it is also possible that binding of α4β7-mAb to its cognate integrin modulates signaling pathways within CD4+ T cells in a way that impedes SIV replication, or that it disrupts cell-cell interactions necessary for efficient viral transmission. There are clearly other explanations for our findings and include a potential role for FcR binding of the anti-α4β7 mAb, the potential ability of the anti-α4β7 mAb to induce a significant number of functional Tregs that do not allow CD4+ T cells to proliferate and become activated, the ability of the anti-α4β7 mAb to prevent fibrosis of the GIT allowing for the more efficient seeding of a normal complement of gut homing cells in the gut and thus return to homeostasis, etc. Clearly, much has still to be done to ferret out the potential mechanisms involved that is precisely the future goals of our laboratory.
II. Conclusions
Clearly much still needs to be learnt with regards to how the network of immune cells traffic, reside and mediate their respective function in specific tissues and organs. Studies using murine models while quite informative represent a limitation due to the obvious species-specific differences. Thus, the distribution and regulation of α4β7, for example, and other integrins are quite distinct between mice and humans. For practical reasons, we study peripheral blood as a source of cells from humans and nonhuman primates. The studies of how cells within the GIT, lung and other organs function and interact with each other are difficult to study except from biopsy tissues and/or post mortem. Thus, the studies conducted so far need to be viewed with a healthy dose of skepticism. In addition, we tend to study responses against micro-organisms using proteins and peptides that are constituents of the micro-organism. Yet, we know, that these proteins are to a large extent glycosylated or associated with lipids. It is now finally being recognized that such glycosylation plays a major role in how an antigen is recognized by the immune system and that lipid specific and vitamin specific immune responses do occur and are recognized in the context of non-polymorphic MHC like molecules. Thus, vitamins and their metabolites are now known to bind to monomorphic MR1 (non-classical MHC molecules) molecules particularly in the GIT and are recognized by a subset of T cells termed mucosal associated invariant T cells (MAIT) that constitute a large frequency of T cells in the liver, for example. A major role for MHC-E, F and G molecules is also beginning to emerge. Thus, the study of integrins expressed by these cells and their cognate receptors would be very important if we are to make advances against diseases that target the mucosal tissues and require unique vaccine formulations and strategies to be successful. We have made some in roads with the study of the α4β7 integrin and its role in SIV and by implication human HIV infection. However, much has yet to be learnt.
Acknowledgments
This study was supported by NIH-RO1 AI 98628 to AAA, and NIH-RO1 AI113883 to SNB.
References
- 1.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29(11):514–22. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10(3–4):313–23. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 3.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1(2):96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 4.Zabel BA, Rott A, Butcher EC. Leukocyte chemoattractant receptors in human disease pathogenesis. Annu Rev Pathol. 2015;10:51–81. doi: 10.1146/annurev-pathol-012513-104640. [DOI] [PubMed] [Google Scholar]
- 5.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol. 2015;15(3):172–84. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 6.Nolz JC. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol Life Sci. 2015;72(13):2461–73. doi: 10.1007/s00018-015-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koivisto L, Heino J, Hakkinen L, Larjava H. Integrins in Wound Healing. Adv Wound Care (New Rochelle) 2014;3(12):762–83. doi: 10.1089/wound.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–74. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 9.Barroca P, Calado M, Azevedo-Pereira JM. HIV/dendritic cell interaction: consequences in the pathogenesis of HIV infection. AIDS Rev. 2014;16(4):223–35. [PubMed] [Google Scholar]
- 10.Allocca M, Fiorino G, Vermeire S, Reinisch W, Cataldi F, Danese S. Blockade of lymphocyte trafficking in inflammatory bowel diseases therapy: importance of specificity of endothelial target. Expert Rev Clin Immunol. 2014;10(7):885–95. doi: 10.1586/1744666X.2014.917962. [DOI] [PubMed] [Google Scholar]
- 11.Turner DL, Gordon CL, Farber DL. Tissue-resident T cells, in situ immunity and transplantation. Immunol Rev. 2014;258(1):150–66. doi: 10.1111/imr.12149. [DOI] [PubMed] [Google Scholar]
- 12.Comerford I, Kara EE, McKenzie DR, McColl SR. Advances in understanding the pathogenesis of autoimmune disorders: focus on chemokines and lymphocyte trafficking. Br J Haematol. 2014;164(3):329–41. doi: 10.1111/bjh.12616. [DOI] [PubMed] [Google Scholar]
- 13.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309–20. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 14.Kim MH, Taparowsky EJ, Kim CH. Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity. 2015;43(1):107–19. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng H, Tian Z. NK cell trafficking in health and autoimmunity:a comprehensive review. Clin Rev Allergy Immunol. 2014;47(2):119–27. doi: 10.1007/s12016-013-8400-0. [DOI] [PubMed] [Google Scholar]
- 16.Morris MA, Ley K. Trafficking of natural killer cells. Curr Mol Med. 2004;4(4):431–8. doi: 10.2174/1566524043360609. [DOI] [PubMed] [Google Scholar]
- 17.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 20.Byrareddy SN, Sidell N, Arthos J, Cicala C, Zhao C, Little DM, et al. Species-specific differences in the expression and regulation of alpha4beta7 integrin in various nonhuman primates. J Immunol. 2015;194(12):5968–79. doi: 10.4049/jimmunol.1402866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128(5):839–52. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim Biophys Acta. 2009;1788(4):779–89. doi: 10.1016/j.bbamem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24(1):107–15. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Zhu J, Mi LZ, Walz T, Sun H, Chen J, et al. Structural specializations of alpha(4)beta(7), an integrin that mediates rolling adhesion. J Cell Biol. 2012;196(1):131–46. doi: 10.1083/jcb.201110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries JD, Paul NR, Humphries MJ, Morgan MR. Emerging properties of adhesion complexes: what are they and what do they do? Trends Cell Biol. 2015;25(7):388–97. doi: 10.1016/j.tcb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol. 2015;36:41–7. doi: 10.1016/j.ceb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29(2):87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller WA. The regulation of transendothelial migration: new knowledge and new questions. Cardiovasc Res. 2015;107(3):310–20. doi: 10.1093/cvr/cvv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herter J, Zarbock A. Integrin Regulation during Leukocyte Recruitment. J Immunol. 2013;190(9):4451–7. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M, Yamamoto K, Ogiwara N, Matsumoto T, Shigeto S, Ota H. Helicobacter heilmannii-like organism in parietal cells: A diagnostic pitfall. Pathol Int. 2015 doi: 10.1111/pin.12349. [DOI] [PubMed] [Google Scholar]
- 33.Pawig L, Klasen C, Weber C, Bernhagen J, Noels H. Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front Immunol. 2015;6:429. doi: 10.3389/fimmu.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim ST. Nuclear FAK: a new mode of gene regulation from cellular adhesions. Mol Cells. 2013;36(1):1–6. doi: 10.1007/s10059-013-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oloumi A, McPhee T, Dedhar S. Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta. 2004;1691(1):1–15. doi: 10.1016/j.bbamcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg MH. Integrin activation. BMB Rep. 2014;47(12):655–9. doi: 10.5483/BMBRep.2014.47.12.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev. 2014;260(1):86–101. doi: 10.1111/imr.12194. [DOI] [PubMed] [Google Scholar]
- 38.Reeves RK, Evans TI, Gillis J, Johnson RP. Simian immunodeficiency virus infection induces expansion of alpha4beta7+ and cytotoxic CD56+ NK cells. J Virol. 2010;84(17):8959–63. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic A, Alpdogan O, Willis LM, Eng JM, Greenberg AS, Kappel BJ, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103(4):1542–7. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 40.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–9. doi: 10.1038/ni1566. Epub 2008/02/12. [DOI] [PubMed] [Google Scholar]
- 41.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2(5):439–49. doi: 10.1038/mi.2009.90. Epub 2009/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang SH, Jin BR, Kim HJ, Seo GY, Jang YS, Kim SJ, et al. Lactoferrin Combined with Retinoic Acid Stimulates B1 Cells to Express IgA Isotype and Gut-homing Molecules. Immune Netw. 2015;15(1):37–43. doi: 10.4110/in.2015.15.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan X, Dee MJ, Altman NH, Malek TR. IL-2Rbeta-dependent signaling and CD103 functionally cooperate to maintain tolerance in the gut mucosa. J Immunol. 2015;194(3):1334–46. doi: 10.4049/jimmunol.1400955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann ER, Bernardo D, English NR, Landy J, Al-Hassi HO, Peake ST, et al. Compartment-specific immunity in the human gut: properties and functions of dendritic cells in the colon versus the ileum. Gut. 2015 doi: 10.1136/gutjnl-2014-307916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7(2):291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 46.Zaballos A, Gutierrez J, Varona R, Ardavin C, Marquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J Immunol. 1999;162(10):5671–5. [PubMed] [Google Scholar]
- 47.Evans-Marin HL, Cao AT, Yao S, Chen F, He C, Liu H, et al. Unexpected Regulatory Role of CCR9 in Regulatory T Cell Development. PLoS One. 2015;10(7):e0134100. doi: 10.1371/journal.pone.0134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiter B, Patil SU, Shreffler WG. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin Exp Allergy. 2015;45(7):1214–25. doi: 10.1111/cea.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 50.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1(4):247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 51.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 52.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Ren M, Zeng H, Guo Y, Zhuang Z, Feng Z, et al. Elevated follicular helper T cells and expression of IL-21 in thyroid tissues are involved in the pathogenesis of Graves’ disease. Immunol Res. 2015;62(2):163–74. doi: 10.1007/s12026-015-8647-z. [DOI] [PubMed] [Google Scholar]
- 54.Allam A, Majji S, Peachman K, Jagodzinski L, Kim J, Ratto-Kim S, et al. TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Sci Rep. 2015;5:10443. doi: 10.1038/srep10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryant VL, Slade CA. Chemokines, their receptors and human disease: the good, the bad and the itchy. Immunol Cell Biol. 2015;93(4):364–71. doi: 10.1038/icb.2015.23. [DOI] [PubMed] [Google Scholar]
- 56.Bari S, Seah KK, Poon Z, Cheung AM, Fan X, Ong SY, et al. Expansion and homing of umbilical cord blood hematopoietic stem and progenitor cells for clinical transplantation. Biol Blood Marrow Transplant. 2015;21(6):1008–19. doi: 10.1016/j.bbmt.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 57.Merino JJ, Bellver-Landete V, Oset-Gasque MJ, Cubelos B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: focus in CNS repair. J Cell Physiol. 2015;230(1):27–42. doi: 10.1002/jcp.24695. [DOI] [PubMed] [Google Scholar]
- 58.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153(2):517–28. [PubMed] [Google Scholar]
- 59.Farstad IN, Halstensen TS, Kvale D, Fausa O, Brandtzaeg P. Topographic distribution of homing receptors on B and T cells in human gut-associated lymphoid tissue: relation of L-selectin and integrin alpha 4 beta 7 to naive and memory phenotypes. Am J Pathol. 1997;150(1):187–99. [PMC free article] [PubMed] [Google Scholar]
- 60.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3(3):356–61. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Schurpf T, Springer TA. How natalizumab binds and antagonizes alpha4 integrins. J Biol Chem. 2013;288(45):32314–25. doi: 10.1074/jbc.M113.501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, Fu T, Peng B, Sun H, Chu H, Li G, et al. The hydrophobic contacts between the center of the betaI domain and the alpha1/alpha7 helices are crucial for the low-affinity state of integrin alpha4 beta7. FEBS J. 2014;281(13):2915–26. doi: 10.1111/febs.12829. [DOI] [PubMed] [Google Scholar]
- 65.Cahill RN, Poskitt DC, Frost DC, Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977;145(2):420–8. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackay CR, Andrew DP, Briskin M, Ringler DJ, Butcher EC. Phenotype, and migration properties of three major subsets of tissue homing T cells in sheep. Eur J Immunol. 1996;26(10):2433–9. doi: 10.1002/eji.1830261025. [DOI] [PubMed] [Google Scholar]
- 67.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 68.Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci. 2014;1317:70–5. doi: 10.1111/nyas.12408. [DOI] [PubMed] [Google Scholar]
- 69.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239(11):1524–30. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD. Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol. 2015;224(3):R107–21. doi: 10.1530/JOE-14-0642. [DOI] [PubMed] [Google Scholar]
- 71.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66(7):606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 72.Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46(15):4449–58. doi: 10.1021/bi7003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186(2):1044–59. doi: 10.4049/jimmunol.1003052. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwa S, Kannanganat S, Nigam P, Siddiqui M, Shetty RD, Armstrong W, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118(10):2763–73. doi: 10.1182/blood-2011-02-339515. Epub 2011/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, et al. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20(12):1397–400. doi: 10.1038/nm.3715. Epub 2014/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]