Abstract
Background
Patients with type 2 diabetes treated with pharmacotherapy should be adherent to and persistent with their medications to experience glycemic control and prevent associated complications.
Objective
To compare medication adherence and persistence among patients with type 2 diabetes who are newly initiating a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a sulfonylurea.
Methods
This was a retrospective, observational cohort study using the MarketScan claims databases. The patients who were selected for the study had newly initiated treatment with an SGLT-2 inhibitor or a sulfonylurea between January 1, 2015, and December 31, 2015 (index date; class of earliest medication is defined as the index class); were aged ≥18 years on the index date; were continuously enrolled with health insurance for 12 months before and 6 months after (ie, follow-up) the index date; and had ≥1 baseline diagnoses of type 2 diabetes. Study exclusions were type 1 diabetes, pregnancy, and gestational diabetes. Medication adherence was measured by the proportion of days covered (PDC) with the index class during the follow-up period and dichotomized as adherent (PDC ≥80%) or nonadherent. Persistence was defined as the number of days from the index date until a >60-day continuous gap in days without the index drug class (ie, discontinuation) or the end of follow-up. A propensity score model was used to match patients receiving an SGLT-2 inhibitor to patients receiving a sulfonylurea in a 1:1 ratio based on patient characteristics. Logistic (ie, adherence) and Cox (ie, persistence) regression models were fit to the matched samples.
Results
Initially, the study included 17,724 patients who received an SGLT-2 inhibitor and 25,490 patients who received a sulfonylurea. After propensity score matching, 13,657 patients remained in each cohort. Compared with patients receiving a sulfonylurea, a statistically significantly greater percentage of patients receiving an SGLT-2 inhibitor were adherent to therapy (61.4% vs 53.9%, respectively; odds ratio of adherence, 1.364; 95% confidence interval [CI], 1.30–1.43; P <.001) and persistent (76.1% vs 68.9%, respectively; hazard ratio of discontinuation, 0.746; 95% CI, 0.71–0.78; P <.001).
Conclusion
Maintaining adherence to and persistence with antidiabetes medication is vital to glycemic control among patients with type 2 diabetes. In this real-world study, patients who newly initiated treatment with SGLT-2 inhibitors were more likely to adhere to treatment and persist with the initiated therapy than similar patients who newly initiated treatment with sulfonylureas.
Keywords: adherence, antidiabetes medication, glycemic control, persistence, SGLT-2 inhibitor, sulfonylureas, type 2 diabetes
Type 2 diabetes mellitus is a chronic disease that affects more than 29 million individuals in the United States.1 Patients with type 2 diabetes have insulin resistance, a condition in which the body does not produce enough insulin to maintain normal levels of blood glucose.2 Prolonged elevated blood glucose, typically measured as glycated hemoglobin (HbA1c), is associated with damage to the eyes, kidneys, and nerves.2 Type 2 diabetes is frequently managed through multimodal strategies, including diet and exercise, with a key focus on glycemic control.3 The American Diabetes Association recommends lowering HbA1c levels to <7% in most nonpregnant adults with diabetes, with a primary goal of reducing the risk for microvascular disease.3
KEY POINTS
-
▸
It is well-known that maintaining adherence to antidiabetes medications is vital to glycemic control.
-
▸
This is the first real-world study using claims data to compare adherence and persistence between patients receiving SGLT-2 inhibitors or sulfonylureas.
-
▸
Overall, the proportion of patients who were adherent to their initiated medication was significantly greater (P <.001) among patients who initiated SGLT-2 inhibitors than among patients who initiated sulfonylureas.
-
▸
The proportion of patients who were persistent with their index therapy was also significantly greater (P <.001) among patients who initiated SGLT-2 inhibitors than among patients who initiated sulfonylureas.
-
▸
Treatment initiation with an SGLT-2 inhibitor increased the likelihood of adherence by 36% and reduced the risk for treatment discontinuation by 25% versus initiation of a sulfonylurea.
-
▸
Further research is needed to determine if these findings correlate with better glycemic control and fewer complications in patients with type 2 diabetes.
According to current diabetes treatment guidelines, the recommended first-line pharmacologic treatment for type 2 diabetes is metformin.3 For patients who do not achieve or maintain HbA1c targets using metformin, or for patients who do not tolerate metformin, a therapeutic option is the addition of another antidiabetes medication class, such as dipeptidyl peptidase-4 (DPP-4) inhibitors, sulfonylureas, or thiazolidinediones.3 Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are a relatively new class of medications indicated for the treatment of patients with type 2 diabetes. Canagliflozin, the first SGLT-2 inhibitor available in the United States, was approved by the US Food and Drug Administration (FDA) in March 2013; dapagliflozin was approved by the FDA in January 2014; and empagliflozin was approved shortly thereafter, in August 2014.
Historically, for patients who had been receiving monotherapy with metformin, sulfonylureas have been the most common add-on or replacement medication.4 In a randomized, double-blind, active-controlled, 52-week, noninferiority trial, dapagliflozin had similar efficacy to the sulfonylurea glipizide as add-on therapy to metformin, and resulted in weight loss and reduced hypoglycemic events.5 In a separate 52-week, double-blind, active-controlled noninferiority trial, patients who were inadequately controlled with metformin and received canagliflozin or the sulfonylurea glimepiride experienced different HbA1c reductions, with canagliflozin 300 mg showing superiority to glimepiride.6
It is well-known that maintaining adherence to antidiabetes medication is vital to glycemic control and other favorable clinical outcomes, such as those examined in the clinical trials.7 However, patient adherence to medications in observational studies or in real-world populations may differ from that observed in an experimental clinical trial setting where study participants are provided medications and have regular follow-up with study personnel.
To our knowledge, no analyses have compared real-world adherence and persistence between SGLT-2 inhibitors and sulfonylureas. Therefore, this study used a very large, contemporary, real-world population to compare medication adherence and persistence between patients with type 2 diabetes who were newly initiating an SGLT-2 inhibitor or a sulfonylurea.
Methods
This study used US administrative health insurance claims data extracted from the Truven Health MarketScan Commercial Claims and Encounters (Commercial), Medicare Supplemental and Coordination of Benefits (Medicare Supplemental), and Early View databases. These databases comprise enrollment information, demographic information, and inpatient medical, outpatient medical, and outpatient pharmacy claims data collected from more than 300 large, self-insured US employers and more than 25 health plans.
The Commercial database includes information for individuals who are insured through private health insurance plans. The Medicare Supplemental database includes information for individuals who are Medicare-eligible (primarily aged ≥65 years) and have supplemental health insurance paid for by their current or former employer. The Medicare Supplemental database includes the Medicare-paid and supplemental-paid components of reimbursed administrative claims. The Early View database includes all the components in the standard Commercial and Medicare Supplemental databases, but it has a short lag time and includes adjudicated claims for healthcare services incurred up to 30 days before data extraction completion. Of the outpatient prescription claims, 97% are fully adjudicated within 30 days from the prescription fills. The databases used in this study contained data for more than 70 million unique individuals during the study period.
Study Design and Patient Selection Criteria
This was a retrospective, observational cohort study. Table 1 outlines the patient selection criteria. Adults aged ≥18 years with ≥1 outpatient pharmacy claims for an SGLT-2 inhibitor or a sulfonylurea (including fixed-dose combinations) between January 1, 2015, and December 31, 2015, were selected from the databases. The date of the first outpatient pharmacy claim was designated the index date, and the medication class (ie, SGLT-2 inhibitors or sulfonylureas) initiated on the index date was designated the index medication class. Patients were required to have ≥12 months of continuous enrollment in medical and pharmacy benefits before the index date (ie, baseline period) and ≥6 months of continuous enrollment in medical and pharmacy benefits after the index date (ie, follow-up period).
Table 1.
Patient Selection Criteria
| Selection criteria | Patients using sulfonylurea, N (%) | Patients using SGLT-2 inhibitor, N (%) |
|---|---|---|
| Patients with ≥1 pharmacy claims for an SGLT-2 inhibitor or a sulfonylurea (including fixed-dose combinations) between January 1, 2015, and December 31, 2015 (earliest claim = index date; class of earliest medication = index class)a | 470,284 (100.0) | 151,514 (100.0) |
| Age ≥18 years at index date | 470,157 (100.0) | 151,469 (100.0) |
| Continuous enrollment in medical and pharmacy benefits for ≥12 months before the index date (baseline period) | 371,836 (79.1) | 124,367 (82.1) |
| Continuous enrollment in medical and pharmacy benefits for ≥6 months after the index date (follow-up period) | 302,246 (64.3) | 87,040 (57.4) |
| ≥1 nondiagnostic medical claimsb with a diagnosis code for type 2 diabetes in any position during the baseline period | 280,717 (59.7) | 82,542 (54.5) |
| No medical claims with a diagnosis code for type 1 diabetes during the baseline or follow-up periods | 255,498 (54.3) | 72,846 (48.1) |
| No medical claims with a diagnosis code for gestational diabetes during the baseline or follow-up periods | 255,216 (54.3) | 72,808 (48.1) |
| No medical claims with a diagnosis code for any pregnancy condition during the baseline or follow-up periods | 253,453 (53.9) | 72,092 (47.6) |
| No use of the index medication class during the baseline period | 27,314 (5.8) | 29,228 (19.3) |
| No use of the comparator medication class during the baseline period or on the index date | 25,490 (5.4) | 17,724 (11.7) |
| Final unmatched population | 25,490 (5.4) | 17,724 (11.7) |
Patients with more than 1 type of SGLT-2 inhibitor or sulfonylurea on the index date were excluded from the study.
Claims that are not associated with a diagnostic workup used to rule out the presence of a condition, such as claims for laboratory tests.
SGLT-2 indicates sodium-glucose cotransporter 2.
In addition, patients were required to have a diagnosis of type 2 diabetes during the baseline period and to have no claims with diagnosis or procedure codes for type 1 diabetes, gestational diabetes, or pregnancy during the baseline or follow-up periods. To ensure that patients were naïve to their index medication class, patients were not allowed to have outpatient pharmacy claims for SGLT-2 inhibitors or sulfonylureas during the baseline period. Finally, patients with claims for an SGLT-2 inhibitor or a sulfonylurea on the index date and claims for more than 1 type of SGLT-2 inhibitor or more than 1 type of sulfonylurea on the index date were excluded.
Measurement of Adherence and Persistence
Medication adherence was measured using the proportion of days covered (PDC) by the index medication class during the 6-month follow-up period.8 The PDC was calculated by dividing the number of days the patient was “covered” by the medication (ie, had the medication “on hand” according to the days of supply recorded on each prescription) during the follow-up period (numerator) by 180 days (denominator). If a patient had prescriptions for the medication class with overlapping days of supply (ie, if a patient refilled a prescription early), it was assumed that the patient completed the first prescription and started taking the second prescription on the day after completing the first; thus, the calculation extended the end of the days of supply of the second prescription by the number of days that it overlapped with the first prescription.
The PDC was computed across all medications within the index medication class (ie, individual medications within the SGLT-2 inhibitor class or the sulfonylurea class were considered interchangeable). The PDC had a value between 0% and 100% but was dichotomized at the clinically meaningful threshold of <80% versus ≥80%, which has been shown to be predictive of hospitalization and mortality among patients with diabetes taking oral antidiabetes medications.9,10 Furthermore, the threshold of ≥80% PDC for antidiabetes medications is used by the Centers for Medicare & Medicaid Services as a quality measure for Part D Star Ratings.11
Persistence with the index medication class was measured as the number of days from the index date until the earliest of a discontinuation of the index medication class or the end of follow-up. Medication discontinuation was defined as a gap in therapy with the index medication class of >60 days. Like adherence, persistence was calculated across all medications within the index medication class.
Measurement of Covariates
The covariates included the patients' demographic and clinical characteristics, which were measured to describe the study sample and for use in the propensity score models. The patients' demographics were measured on the index date using health insurance enrollment records, and are shown in Table 2. The clinical characteristics were measured throughout the 12-month baseline period, and are shown in Table 3.
Table 2.
Patient Demographics Measured as of Index Date
| Demographics | Propensity score matched | Unmatched | ||||
|---|---|---|---|---|---|---|
| Patients using sulfonylurea (N = 13,657) | Patients using SGLT-2 inhibitor (N = 13,657) | Standardized difference for matched cohorts, % | Patients using sulfonylurea (N = 25,490) | Patients using SGLT-2 inhibitor (N = 17,724) | P value for unmatched cohorts | |
| Age, yrs, mean (SD) | 54.3 (9.7) | 54.3 (9.5) | 0.522 | 57.7 (11.8) | 54.0 (9.2) | <.001 |
| Age-group | <.001 | |||||
| 18-34 yrs | 2.6% | 2.5% | 0.880 | 2.1% | 2.4% | |
| 35-44 yrs | 12.6% | 12.6% | 0.229 | 10.2% | 12.8% | |
| 45-54 yrs | 32.9% | 32.2% | 1.528 | 26.0% | 33.6% | |
| 55-64 yrs | 42.6% | 42.8% | 0.572 | 39.4% | 42.8% | |
| 65-79 yrs | 8.7% | 9.3% | 2.109 | 17.2% | 7.9% | |
| ≥80 yrs | 0.6% | 0.6% | 0.692 | 5.0% | 0.5% | |
| Men | 53.9% | 53.4% | 1.005 | 56.1% | 52.9% | <.001 |
| Insurance plan type | <.001 | |||||
| Comprehensive | 5.3% | 5.4% | 0.227 | 13.9% | 4.5% | |
| EPO | 0.5% | 0.3% | 2.691 | 0.4% | 0.3% | |
| HMO | 8.9% | 8.9% | 0.281 | 10.6% | 8.4% | |
| POS | 5.2% | 5.1% | 0.068 | 5.4% | 5.1% | |
| PPO | 60.2% | 60.8% | 1.078 | 53.1% | 62.0% | |
| POS with capitation | 0.4% | 0.7% | 3.647 | 0.3% | 0.7% | |
| CDHP | 11.0% | 10.7% | 0.813 | 8.7% | 11.1% | |
| HDHP | 3.7% | 3.4% | 1.556 | 3.3% | 3.3% | |
| Unknown | 4.9% | 4.7% | 0.909 | 4.3% | 4.7% | |
| Payer | <.001 | |||||
| Commercial | 90.0% | 89.7% | 1.043 | 77.0% | 91.2% | |
| Medicare | 10.0% | 10.3% | 1.043 | 23.0% | 8.8% | |
| Geographic region | <.001 | |||||
| Northeast | 17.0% | 17.0% | 0.125 | 16.3% | 16.8% | |
| North Central | 16.6% | 16.9% | 0.739 | 23.4% | 15.8% | |
| South | 58.3% | 57.8% | 1.143 | 49.7% | 59.9% | |
| West | 7.9% | 8.2% | 1.320 | 10.6% | 7.5% | |
| Unknown | 0.2% | 0.1% | 0.637 | 0.1% | 0.1% | |
| Population density | .022 | |||||
| Urban | 83.7% | 83.8% | 0.344 | 83.3% | 84.3% | |
| Rural | 16.2% | 16.1% | 0.236 | 16.6% | 15.7% | |
| Unknown | 0.1% | 0.1% | 1.334 | 0.1% | 0.1% | |
| Index copay, mean (SD)a | $4 ($6) | $48 ($70) | 88.163 | $4 ($6) | $48 ($83) | <.001 |
Not used in the propensity score model.
CDHP indicates consumer-directed health plan; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; SD, standard deviation; SGLT-2, sodium-glucose cotransporter 2.
Table 3.
Patient Clinical Characteristics Measured During 12-Month Baseline Period
| Clinical characteristics | Propensity score matched | Unmatched | ||||
|---|---|---|---|---|---|---|
| Patients using sulfonylurea (N = 13,657) | Patients using SGLT-2 inhibitor (N = 13,657) | Standardized difference for matched cohorts, % | Patients using sulfonylurea (N = 25,490) | Patients using SGLT-2 inhibitor (N = 17,724) | P value for unmatched cohorts | |
| Deyo-Charlson Comorbidity Index, mean (SD) | 1.7 (1.3) | 1.7 (1.3) | 2.560 | 2.0 (1.6) | 1.7 (1.3) | <.001 |
| Adapted Diabetes Complications Severity Index, mean (SD) | 0.7 (1.2) | 0.7 (1.2) | 2.115 | 0.9 (1.5) | 0.7 (1.2) | <.001 |
| Number of unique 3-digit ICD-9-CM diagnosis codes,a mean (SD) | 10.7 (7.0) | 10.8 (6.8) | 1.549 | 11.3 (8.0) | 10.9 (6.8) | <.001 |
| Number of unique NDC codes, mean (SD) | 12.5 (8.4) | 12.7 (7.9) | 2.994 | 11.5 (8.3) | 13.6 (8.2) | <.001 |
| Total healthcare expenditures, mean (SD) | $12,295 ($23,921) | $12,824 ($23,580) | 2.228 | $15,096 ($38,103) | $13,670 ($22,526) | <.001 |
| Endocrinologist visit | 10.2% | 12.2% | 6.439 | 6.6% | 17.3% | <.001 |
| Microvascular complications of diabetes | 16.3% | 17.4% | 2.939 | 18.0% | 19.1% | <.001 |
| Diabetic nephropathy | 3.2% | 3.5% | 1.604 | 4.5% | 3.7% | <.001 |
| Diabetic retinopathy | 6.5% | 7.0% | 2.076 | 6.8% | 7.9% | <.001 |
| Diabetic peripheral neuropathy | 9.0% | 9.6% | 1.860 | 9.5% | 10.6% | <.001 |
| Macrovascular complications of diabetes | 14.3% | 14.6% | 0.881 | 20.7% | 14.6% | <.001 |
| Atherosclerosis | 11.3% | 11.5% | 0.535 | 16.0% | 11.7% | <.001 |
| Stroke | 1.1% | 1.1% | 0.191 | 2.3% | 1.0% | <.001 |
| Myocardial infarction | 0.8% | 0.8% | 0.110 | 1.6% | 0.8% | <.001 |
| Unstable angina pectoris | 0.9% | 0.8% | 0.433 | 1.4% | 0.7% | <.001 |
| Heart failure | 2.2% | 2.3% | 0.674 | 4.9% | 2.1% | <.001 |
| Percutaneous coronary intervention | 0.9% | 0.8% | 0.887 | 1.2% | 0.7% | <.001 |
| Coronary artery bypass graft | 0.3% | 0.2% | 0.835 | 0.4% | 0.2% | <.001 |
| Peripheral vascular disease | 2.4% | 2.6% | 1.213 | 4.0% | 2.5% | <.001 |
| Other comorbidities | ||||||
| Renal impairment | 6.4% | 6.8% | 1.974 | 11.4% | 6.5% | <.001 |
| Hypertension | 76.3% | 76.5% | 0.518 | 76.8% | 77.3% | .223 |
| Dyslipidemia | 79.2% | 79.5% | 0.716 | 75.1% | 81.6% | <.001 |
| Depression | 8.5% | 8.4% | 0.288 | 8.1% | 8.7% | .028 |
| Hypoglycemia | 2.9% | 3.1% | 1.182 | 3.1% | 3.2% | .347 |
| Proteinuria | 1.9% | 1.9% | 0.439 | 2.0% | 2.0% | .706 |
| Antihypertensive medications | ||||||
| Renin-angiotensin system antagonists | 65.6% | 65.8% | 0.421 | 60.6% | 68.4% | <.001 |
| ACE inhibitors | 42.5% | 42.0% | 0.850 | 40.7% | 43.0% | <.001 |
| ARBs | 25.7% | 26.4% | 1.504 | 22.1% | 28.3% | <.001 |
| Direct renin inhibitors | 0.2% | 0.2% | 0.000 | 0.1% | 0.2% | .227 |
| Diuretics | 35.4% | 35.5% | 0.272 | 35.0% | 36.3% | .006 |
| Other antihypertensives | 34.8% | 34.9% | 0.294 | 37.9% | 35.0% | <.001 |
| Number of antidiabetes medication classes, mean (SD) | 1.2 (0.8) | 1.3 (0.8) | 7.250 | 1.0 (0.7) | 1.5 (0.9) | <.001 |
| Antidiabetes medication use in baseline period | ||||||
| Alpha-glucosidase inhibitors | 0.3% | 0.2% | 0.409 | 0.2% | 0.3% | .104 |
| Metformin | 75.4% | 74.7% | 1.595 | 65.1% | 76.5% | <.001 |
| DPP-4 inhibitors | 26.5% | 28.0% | 3.392 | 18.3% | 30.9% | <.001 |
| Meglitinides | 1.0% | 1.1% | 0.391 | 0.8% | 1.4% | <.001 |
| TZDs | 5.7% | 6.0% | 1.579 | 4.1% | 7.6% | <.001 |
| Insulins | 14.8% | 17.8% | 8.026 | 9.4% | 26.9% | <.001 |
| GLP-1 receptor agonists | 7.8% | 10.4% | 8.840 | 4.4% | 17.7% | <.001 |
| Amylin analogs | 0.0% | 0.1% | 4.474 | 0.0% | 0.1% | <.001 |
| Concurrent antidiabetes medication use | ||||||
| Alpha-glucosidase inhibitors | 0.0% | 0.0% | 0.856 | 0.0% | 0.0% | .706 |
| Metformin | 78.4% | 77.2% | 3.083 | 77.1% | 75.5% | <.001 |
| DPP-4 inhibitors | 5.8% | 6.3% | 2.033 | 4.0% | 7.0% | <.001 |
| Meglitinides | 0.3% | 0.3% | 0.549 | 0.2% | 0.3% | .066 |
| TZDs | 1.4% | 1.6% | 1.438 | 1.0% | 2.2% | <.001 |
| Insulins | 2.9% | 4.0% | 5.985 | 1.8% | 7.3% | <.001 |
| GLP-1 receptor agonists | 1.2% | 1.9% | 5.466 | 0.7% | 3.2% | <.001 |
| Amylin analogs | 0.0% | 0.0% | — | 0.0% | 0.0% | .168 |
| Any mail-order prescriptionb | 13.0% | 13.1% | 0.326 | 17.3% | 12.5% | <.001 |
The baseline period for all patients occurred before the implementation of the International Classification of Diseases, Tenth Revision coding.
Mail-order prescription was measured during the follow-up period.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NDC, National Drug Code; SD, standard deviation; SGLT-2, sodium-glucose cotransporter 2; TZD, thiazolidinedione.
The Deyo-Charlson Comorbidity Index12; the number of unique 3-digit International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes13; the number of unique National Drug Codes13; and the total healthcare expenditures were captured as measures of overall health. The diabetes-related characteristics included the adapted Diabetes Complications Severity Index,14 the presence of a visit to an endocrinologist, and diagnoses and procedures that are indicative of the macrovascular and microvascular complications of diabetes.
Medication use was also captured, including baseline use of antihypertensive and antidiabetes medications, as well as concurrent use of antidiabetes medications, because previous antidiabetes medication use and polypharmacy may be indicative of type 2 diabetes that is difficult to manage and shows greater medication burden. The concurrent use of antidiabetes medications was determined based on a previously published algorithm evaluating the overlapping use of the index medication and other antidiabetes medications immediately before and after the index date.15
Statistical Analyses
Bivariate descriptive analyses were conducted on the unmatched and propensity score–matched samples. Categorical variables were compared between the cohorts using chi-square tests. Continuous variables were compared between the cohorts using t-tests.
Propensity score matching was conducted to reduce the potential for confounding that was introduced by differences in the measured demographic and clinical characteristics between the SGLT-2 inhibitor and sulfonylurea cohorts. Propensity scores were estimated using a logistic regression model, with the dependent variable being a binary indicator for membership in the SGLT-2 inhibitor cohort, and a group of independent variables comprising the patient demographic and clinical characteristics listed in Tables 1 and 2 (except the index copay, which was considered a characteristic of the index medication).
Once the propensity score was estimated, patients receiving an SGLT-2 inhibitor were matched to patients receiving a sulfonylurea at a 1:1 ratio.16 The balance in patient characteristics achieved by the propensity score matching was assessed with the standardized difference.17 A standardized difference of <10% was considered an adequate match.18,19
Ultimately, a bivariate logistic regression model was used to compare the odds of achieving the PDC ≥80% threshold between the matched SGLT-2 inhibitor and sulfonylurea cohorts, and a bivariate Cox proportional hazards regression model was used to compare the hazards of medication discontinuation between the matched SGLT-2 inhibitor and sulfonylurea cohorts.
Results
In 2015, there were 151,514 patients with ≥1 claims for an SGLT-2 inhibitor and 470,284 patients with ≥1 claims for a sulfonylurea in the 3 databases combined. After applying the study inclusion and exclusion criteria, the final unmatched sample comprised 17,724 initiators of an SGLT-2 inhibitor and 25,490 initiators of a sulfonylurea (Table 1). Before matching, the sulfonylurea cohort was significantly older, on average, and had a significantly larger proportion of men, in addition to having other demographic differences compared with the SGLT-2 inhibitor cohort (Table 2).
Compared with SGLT-2 inhibitor initiators, sulfonylurea initiators had significantly higher average Deyo-Charlson Comorbidity Index scores, significantly higher total baseline healthcare expenditures, and a significantly greater proportion of patients with macrovascular diabetes complications (Table 3). A significantly lower proportion of patients in the sulfonylurea cohort had a baseline visit to an endocrinologist compared with the SGLT-2 inhibitor cohort.
For both cohorts, the most common antidiabetes medications used during the baseline period were metformin, DPP-4 inhibitors, and insulin. The use of all 3 medications was significantly less common among sulfonylurea initiators than among SGLT-2 inhibitor initiators. A large proportion of both cohorts was receiving metformin concurrently with their index drug on their index date.
After propensity score matching, 13,657 patients were in each cohort. Adequate balance was achieved on the covariates included in the propensity score model, as evidenced by standardized differences of <10% (Tables 2 and 3). On average, the matched cohorts were aged 54 years, and nearly 54% were men. Approximately 75% of the matched patients received metformin during the baseline period, and just more than 25% received a DPP-4 inhibitor.
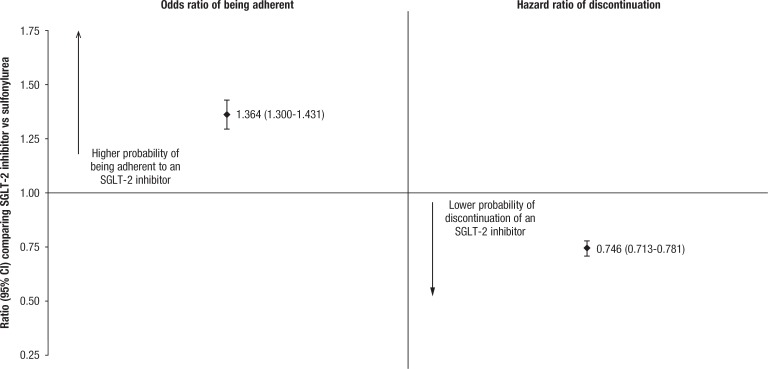
Among matched patients, the average PDC during the 6-month follow-up period was significantly lower for sulfonylurea initiators than for SGLT-2 inhibitor initiators (71.8% vs 75.6%, respectively; P <.0001; Table 4). Similarly, the proportion of patients classified as adherent was significantly lower in the sulfonylurea cohort than in the SGLT-2 inhibitor cohort (53.9% vs 61.4%, respectively), with a corresponding odds ratio of 1.364 (P <.0001; Figure).
Table 4.
Adherence and Persistence Outcomes Measured During 6-Month Follow-Up Period
| Demographics | Propensity score matched | Unmatched | ||||
|---|---|---|---|---|---|---|
| Patients using sulfonylurea (N = 13,657) | Patients using SGLT-2 inhibitor (N = 13,657) | P value for matched cohorts | Patients using sulfonylurea (N = 25,490) | Patients using SGLT-2 inhibitor (N = 17,724) | P value for unmatched cohorts | |
| PDC by index medication class (adherence),a mean (SD) | 71.8% (29.3%) | 75.6% (27.5%) | <.0001 | 72.1% (29.6%) | 75.8% (27.3%) | <.001 |
| PDC ≥80% | 53.9% | 61.4% | <.0001 | 54.7% | 61.8% | <.001 |
| Nonpersistent with index medication classb | 31.1% | 23.9% | <.0001 | 31.4% | 23.6% | <.001 |
| Days to nonpersistence among patients who discontinued, mean (SD) | 80.7 (50.8) | 77.0 (48.7) | <.0001 | 80.6 (50.3) | 77.2 (48.6) | <.001 |
Proportion of days covered calculated as the number of days with index medication class “on hand” divided by 180 days and capped at 100%.
Nonpersistent is defined as the presence of a >60-day gap past the end of the previous fill's days' supply (fill date plus days' supply).
PDC indicates proportion of days covered; SD, standard deviation; SGLT-2, sodium-glucose cotransporter 2.
Figure. Odds Ratio of Being Adherent, Hazard Ratio of Medication Discontinuation During 6-Month Follow-Up Period After Matching.
CI indicates confidence interval; SGLT-2, sodium-glucose cotransporter 2.
A significantly larger proportion of patients receiving a sulfonylurea than patients receiving an SGLT-2 inhibitor discontinued their index medication class during follow-up (31.1% vs 23.9%, respectively; P <.0001). In a Cox proportional hazards model fit on the matched patient sample, patients who received an SGLT-2 inhibitor had 25% lower hazards of discontinuing their treatment than patients receiving a sulfonylurea (0.746; P <.0001).
Discussion
This claims-based study compared propensity score–matched populations of adults with type 2 diabetes initiating SGLT-2 inhibitors or sulfonylureas. In this analysis, patients who were newly initiating an SGLT-2 inhibitor were 36% more likely to be adherent to their medication and 25% less likely to discontinue their medication than patients who initiated a sulfonylurea. Given the large number of antidiabetes medications available in the United States and the importance of adherence and persistence, real-world research comparing and differentiating between medication classes in terms of compliance is important. This analysis adds to the body of literature from clinical trials comparing SGLT-2 inhibitors and sulfonylureas.
Several published analyses have compared adherence and persistence between sulfonylureas and other classes of antidiabetes medication. Patients using sulfonylureas have been shown to have poorer persistence compared with those receiving DPP-4 inhibitors in a German study of primary care practices20 and in a US claims-based study.15 Another claims-based study comparing the DPP-4 inhibitor sitagliptin with sulfonylureas as an add-on therapy to metformin showed that patients adding a sulfonylurea had lower adherence and persistence than patients adding sitagliptin.21
A Canadian analysis showed that patients newly initiating a sulfonylurea had a greater likelihood of discontinuing their antidiabetes medication and a lower likelihood of starting another antidiabetes medication after their first treatment compared with patients initiating metformin.22 Poorer persistence for patients receiving a sulfonylurea compared with patients receiving metformin was also reported in an Irish claims analysis.23 By contrast, SGLT-2 inhibitors have been associated with better adherence and persistence than DPP-4 inhibitors and glucagon-like peptide-1 receptor agonists in US claims analyses.24,25 The results of these other published studies are consistent with the analysis presented here.
Adherence and persistence are often compared between antidiabetes medications and/or medication classes because of the association between adherence to antidiabetes medications and improved outcomes. A 2011 review article identified 37 published articles that examined the association between adherence to antidiabetes medication and several health outcomes.7 Of those studies, 22 used pharmacy claims or refill records to measure adherence.7 Asche and colleagues found that better adherence was associated with better glycemic control.7 Glycemic control is important in diabetes management to prevent microvascular disease.3 The previously cited review article also showed that better adherence to antidiabetes medications was associated with decreased healthcare utilization.7
Asche and colleagues reported that the association between better antidiabetes medication adherence and decreased healthcare costs is less clear,7 although some analyses have reported a significant relationship between the two, with better adherence associated with lower costs.26–28
Although the benefits of adherence to antidiabetes medications are well-documented, adherence remains a challenge for many patients. In this analysis, a little more than 50% of the patients were considered adherent, which was defined as having PDC ≥80%. Additional research to determine the modifiable drivers of nonadherence and nonpersistence and to test potential adherence interventions among adults with type 2 diabetes is needed.
Limitations
This research has limitations. Administrative claims are generated for billing purposes, not for research. It was assumed that patients took their medications for the duration of the days of supply on the medication claim.
Certain patient characteristics that may affect provider prescribing decisions or influence adherence and persistence were not available in the databases. These characteristics include race, socioeconomic status, glycemic control, weight, physical activity, and family history. If these differed by cohort after matching and are associated with adherence and persistence, the study findings may be biased.
In addition, reasons for medication discontinuation are not available in the data.
Finally, this analysis did not attempt to associate adherence and persistence with clinical outcomes.
Conclusions
In this retrospective analysis using real-world data, we found that adults with type 2 diabetes who initiated an SGLT-2 inhibitor medication had better adherence and persistence than patients who initiated a sulfonylurea medication. Further research is needed to determine if the better adherence and persistence associated with taking an SGLT-2 inhibitor translates into better glycemic control and fewer complications in patients with type 2 diabetes.
Author Disclosure Statement
Dr Bell is currently an employee of GlaxoSmithKline. Dr Cappell, Mr Liang, and Ms Kong are employees of Truven Health Analytics, which received funding from AstraZeneca to conduct this study.
Source of Funding
This study was funded by AstraZeneca.
Contributor Information
Kelly F. Bell, The time of study, a Director, Health Economics and Outcomes Research, AstraZeneca, Wilmington, DE.
Katherine Cappell, Director, Custom Data Analytics, Truven Health Analytics, an IBM Company, Ann Arbor, MI.
Michael Liang, Analyst, Truven Health Analytics, an IBM Company.
Amanda M. Kong, Research Leader, Truven Health Analytics, an IBM Company..
References
- 1. Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 5, 2017.
- 2. American Diabetes Association. Facts about type 2. Updated October 27, 2015. www.diabetes.org/diabetes-basics/type-2/facts-about-type-2.html. Accessed March 21, 2017.
- 3. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013; 36(suppl 1): S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hazel-Fernandez L, Xu Y, Moretz C, et al. Historical cohort analysis of treatment patterns for patients with type 2 diabetes initiating metformin monotherapy. Curr Med Res Opin. 2015; 31: 1703–1716. [DOI] [PubMed] [Google Scholar]
- 5. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011; 34: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 7. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011; 33: 74–109. [DOI] [PubMed] [Google Scholar]
- 8. Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007; 10: 3–12. [DOI] [PubMed] [Google Scholar]
- 9. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006; 166: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 10. Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004; 27: 2149–2153. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Medicare & Medicaid Services. Trends in Part C & D Star Rating measure cut points. Updated November 8, 2016. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/2017-Trends-in-Part-C-and-D-Star-Ratings-Cut-Points.pdf. Accessed January 5, 2017.
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 13. Fowler R, Johnston SS. Comparative performance of risk adjustment measures in a sample of commercially-insured patients under age 65—two simple measures outperform current standards. Value Health. 2010; 13: A4. [Google Scholar]
- 14. Chang HY, Weiner JP, Richards TM, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012; 18: 721–726. [PubMed] [Google Scholar]
- 15. Farr AM, Sheehan JJ, Curkendall SM, et al. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014; 31: 1287–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baser O. Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health. 2006; 9: 377–385. [DOI] [PubMed] [Google Scholar]
- 17. D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001; 54: 387–398. [DOI] [PubMed] [Google Scholar]
- 20. Rathmann W, Kostev K, Gruenberger JB, et al. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013; 15: 55–61. [DOI] [PubMed] [Google Scholar]
- 21. Bloomgarden ZT, Tunceli K, Liu J, et al. Adherence, persistence, and treatment discontinuation with sitagliptin compared with sulfonylureas as add-ons to metformin: a retrospective cohort database study. J Diabetes. 2017; 9: 677–688. [DOI] [PubMed] [Google Scholar]
- 22. Grégoire JP, Sirois C, Blanc G, et al. Persistence patterns with oral antidiabetes drug treatment in newly treated patients—a population-based study. Value Health. 2010; 13: 820–828. [DOI] [PubMed] [Google Scholar]
- 23. Grimes RT, Bennett K, Tilson L, et al. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br J Clin Pharmacol. 2015; 79: 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diels J, Neslusan C. Comparative persistency with newer agents used to treat type 2 diabetes (T2DM) in the United States: canagliflozin versus dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Value Health. 2015; 18: A68–A69. [Google Scholar]
- 25. Cai J, Wang Y, Baser O, et al. Comparative persistence and adherence with newer anti-hyperglycemic agents to treat patients with type 2 diabetes in the United States. J Med Econ. 2016; 19: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 26. Breitscheidel L, Stamenitis S, Dippel FW, Schöffski O. Economic impact of compliance to treatment with antidiabetes medication in type 2 diabetes mellitus: a review paper. J Med Econ. 2010; 13: 8–15. [DOI] [PubMed] [Google Scholar]
- 27. Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011; 30: 91–99. [DOI] [PubMed] [Google Scholar]
- 28. White TJ, Vanderplas A, Chang E, et al. The costs of non-adherence to oral antihyperglycemic medication in individuals with diabetes mellitus and concomitant diabetes mellitus and cardiovascular disease in a managed care environment. Dis Manage Health Outcomes. 2004; 12: 181–188. [Google Scholar]