Summary
Scaffold proteins are ubiquitous chaperones that bind proteins and facilitate physical interaction of multi-enzyme complexes. Here we used a biochemical approach to dissect the scaffold activity of the flotillin-homolog protein FloA of the multi-drug-resistant human pathogen Staphylococcus aureus. We show that FloA promotes oligomerization of membrane protein complexes, such as the membrane-associated RNase Rny, which forms part of the RNA-degradation machinery called the degradosome. Cells lacking FloA had reduced Rny function and a consequent increase in the targeted sRNA transcripts that negatively regulate S. aureus toxin expression. Small molecules that altered FloA oligomerization also reduced Rny function and decreased the virulence potential of S. aureus in vitro, as well as in vivo using invertebrate and murine infection models. Our results suggest that flotillin assists in the assembly of protein complexes involved in S. aureus virulence, and could thus be an attractive target for the development of new antimicrobial therapies.
Keywords: flotillin, Staphylococcus, Rny, membrane organization
Introduction
Spatial organization of proteins in cells can select for or against specific protein interactions and optimize the efficiency of biological reactions (DeLoache and Dueber, 2013; Diekmann and Pereira-Leal, 2013). Scaffold proteins are non-catalytic chaperones that function as spatial organizers by binding proteins and facilitating multi-enzymatic biological reactions (Bauer and Pelkmans, 2006; Chapman and Asthagiri, 2009; Good et al., 2011). These proteins have been studied intensively in eukaryotic cells due to their role in assembling the components of signaling transduction cascades (Kolch, 2005; Langeberg and Scott, 2015). Yet, scaffold proteins are also found in prokaryotes; for instance, UspC, a scaffold of the KdpDE signal cascade, activates potassium uptake in Escherichia coli (Heermann et al., 2009), and GraX of Staphylococcus aureus facilitates assembly of the GraSR signal transduction cascade, which responds to the presence of antimicrobials (Li et al., 2007).
The prokaryotic cell membrane was recently found to contain a type of scaffold protein termed flotillin (Bach and Bramkamp, 2013; Dempwolff et al., 2012; Donovan and Bramkamp, 2009; Lopez and Kolter, 2010; Mielich-Süss et al., 2013; Yepes et al., 2012). Bacterial flotillins localize in functional membrane microdomains (FMM) that spatially confine diverse signal transduction cascades and multi-protein reactions (Donovan and Bramkamp, 2009; Lopez and Kolter, 2010), and in certain organizational and functional features, resemble the lipid rafts of eukaryotic cells (Simons and Ikonen, 1997). Eukaryotic lipid rafts also have flotillins; they assist in recruiting signal transduction proteins that must be located in rafts for activation, and facilitate their interaction and oligomerization (Babuke and Tikkanen, 2007; Morrow and Parton, 2005; Otto and Nichols, 2011; Stuermer, 2011; Zhao et al., 2011). However, the role of bacterial flotillins is not completely understood (Dempwolff et al., 2016; Schneider et al., 2015a). The most direct hypothesis suggests that, similar to the eukaryotic flotillins, bacterial flotillins act as scaffolds to promote protein interaction and oligomerization (Good et al., 2011; Langhorst et al., 2005). Bacillus subtilis is the best-established cell model to study the importance of FMM in bacterial physiology (Bach and Bramkamp, 2013; Dempwolff et al., 2012; Donovan and Bramkamp, 2009; Lopez and Kolter, 2010; Mielich-Süss et al., 2013; Yepes et al., 2012). The B. subtilis FMM have two flotillin-like proteins, FloT and FloA; cells lacking these flotillins show altered FMM-associated signal transduction pathway function (Lopez and Kolter, 2010), including a defect in the protease FtsH (Yepes et al., 2012) and in the Sec protein secretion machinery (Bach and Bramkamp, 2013).
Flotillins are found in most bacterial and archaeal species (Bramkamp and Lopez, 2015; Rivera-Milla et al., 2006; Tavernarakis et al., 1999), and the S. aureus flotillin protein is 84% identical to B. subtilis FloA. Foundational work in B. subtilis suggests that inhibition of flotillin activity interferes with oligomerization of FMM-associated proteins; as many FMM-associated proteins have a role in S. aureus virulence, such inhibition could be an important strategy against infections by staphylococcal strains with intrinsic resistance to conventional antibiotics. Due to the evolution of strains resistant to a wide range of β-lactam antibiotics (methicillin-resistant S. aureus; MRSA), S. aureus is currently a major problem in both clinical and community settings (Kreiswirth et al., 1993). MRSA infections are difficult to treat, and have a ~20% mortality rate in clinical settings (Klevens et al., 2007).
Here we analyzed how FloA scaffold activity influences the spatial confinement of FMM-associated protein complexes in S. aureus cells. As a case study, we evaluate the influence of FloA on oligomerization of the RNase Rny from the S. aureus degradosome. We found that FloA interacts physically with Rny and helps to stabilize the degradosome. Cells that lacked FloA showed reduced Rny function; this led to an increase in its targeted sRNA transcripts, which downregulate cytolytic toxin expression and reduce the virulence of this mutant in in vivo infections. We identified several small-molecule inhibitors of flotillin activity that phenocopy a flotillin-deficient mutant, and used these molecules to reduce MRSA progression in in vivo infection models. Our results suggest that flotillin assists in the assembly of virulence-related protein complexes and influences the infectivity potential of S. aureus.
Results
Rny localizes in the FMM of S. aureus
The connection between Rny and FloA was identified while analyzing a FMM-enriched membrane fraction from S. aureus. We used a biochemical approach designed for eukaryotic lipid raft purification (Brown, 2002; Shah and Sehgal, 2007), which is based on the ability of eukaryotic lipid rafts to resist disaggregation by treatment with non-ionic detergent (Triton X-100, Brij). Differences in lipid composition make lipid rafts more compact than the remainder of the cell membrane and more resistant to detergent disaggregation (Brown, 2002; Shah and Sehgal, 2007). After detergent treatment, membrane fragments can be separated by size using zonal centrifugation; this generates a membrane fraction sensitive to detergent disaggregation (detergent-sensitive membrane fraction; DSM) and another that is resistant to disruption and contains larger membrane fragments that are FMM-enriched (detergent-resistant membrane fraction; DRM) (Bramkamp and Lopez, 2015).
The most abundant proteins found in the S. aureus DRM and not in the DSM fraction were identified by mass spectrometry. A number of proteins previously defined as FMM components in other bacterial systems were detected in the S. aureus DRM fraction, including the Sec protein secretion machinery (Bach and Bramkamp, 2013), the Kdp potassium transporter machinery, and several ABC (ATP-binding cassette) protein complexes (Lopez and Kolter, 2010) (Fig. S1A). The Rny endoribonuclease was highly represented in the DRM fraction (Fig. S1B). Rny is a component of the degradosome multimeric complex (Kaito et al., 2005; Kang et al., 2010; Marincola et al., 2012; Nagata et al., 2008), constituted by the RNases J1 and J2, two additional ribonucleases PNPase and Rny, the RNA helicase CshA, and the glycolytic enzymes phosphofructokinase and enolase (Marincola et al., 2012; Oun et al., 2013; Redder and Linder, 2012; Roux et al., 2011). The degradosome is responsible for RNA turnover in bacteria, and thus has a central role in gene regulation (Bandyra et al., 2013; Lehnik-Habrink et al., 2012; Mackie, 2013). In S. aureus cells, the degradosome has an important function in degrading non-coding RNAs, such as rsaA and sau63, which inhibit expression of toxin-coding genes responsible for disrupting host tissue during infection (Thoendel et al., 2011).
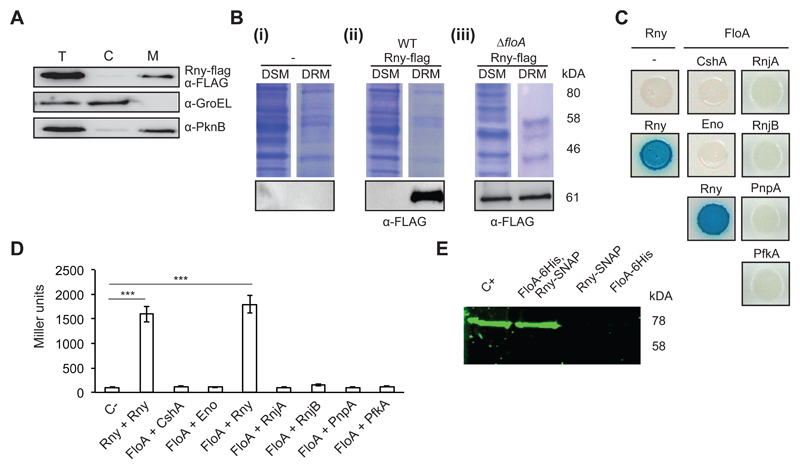
We hypothesized that Rny is spatially confined to the S. aureus FMM. To explore Rny subcellular localization, we generated a S. aureus strain in which a chromosomally integrated rny-flag construct was expressed under the control of the rny native promoter. Cell fractionation followed by immunodetection experiments revealed that Rny concentrated preferentially in the membrane fraction (Fig. 1A). To confirm that Rny associates with the DRM fraction, S. aureus cell membranes were disaggregated using non-ionic detergent treatment, to allow Rny detection in DSM and DRM by immunoblot. Rny was observed preferentially in the DRM fraction (Fig. 1B i and ii). We generated a FloA deletion mutant (ΔfloA) and tested for Rny in the DRM fraction and found Rny at comparable concentrations in the ΔfloA DRM and DSM fractions (Fig. 1B i and iii); this suggests FloA-dependent, heterogeneous Rny distribution in S. aureus membranes, consistent with spatial confinement of Rny to the FMM.
Figure 1. Rny is a membrane-bound protein associated with the DRM fraction.
(A) Immunoblot detection of Rny in the total cell (T), cytoplasmic (C) and membrane fractions (M) of Staphylococcus aureus cells using antibodies to the Flag epitope. The control for cytoplasm was an antibody to the soluble chaperone GroEL and for membrane, antibody to the membrane-bound sensor kinase PknB. (B) Immunodetection of Rny in DSM and DRM fractions of S. aureus cells. (i) Control lanes; (-) indicates an unlabeled strain. (ii) Detection of Rny in an Rny-Flag-labeled strain using anti-Flag antibodies. (iii) Detection of Rny in an Rny-Flag-labeled strain of the ΔfloA mutant. Top, Coomassie-stained gel; bottom, immunoblot. (C) Bacterial two-hybrid (BTH) analysis to study Rny and FloA interactions. (i) Interaction activates lacZ expression, leading in turn to degradation of X-Gal (blue signal). Positive control, Rny self-interaction; negative control, Escherichia coli strains bearing empty plasmids (pKNT25 + pUT18). (D) Quantification of interaction efficiency in a β-galactosidase assay; a 700 Miller Units threshold limit defines positive and negative interaction signals. Significance was measured by one-way ANOVA. p <0.005, ***. Data shown as mean ± SD of three independent biological replicates (n = 3). Each biological replicate included three technical replicates. (E) In-gel detection of Rny-SNAP using the SNAP-binding fluorescence probe TMR-Star to detect Rny in protein samples pulled down with FloA-His6. The arrow indicates band of the size predicted for Rny. Positive control (C+) is the Rny-SNAP-labeled wild-type (WT) membrane fraction. Negative controls (FloA-His6 and Rny-SNAP) were eluted from nickel-charged columns. Protein samples pulled down with FloA-His6/Rny-SNAP are shown in lanes FloA-His6 and Rny-SNAP.
Rny interacts physically with FloA
To test for Rny and FloA interactions, we generated a bacterial two-hybrid (BTH) heterologous system, in which FloA and Rny were tagged with T25 or T18 catalytic domains of an adenylate cyclase that reconstitute the enzyme after FloA:Rny interaction (Karimova et al., 1998). A fully active adenylate cyclase produces cAMP and triggers expression of a cAMP-inducible lacZ reporter (Karimova et al., 1998). Using this assay, we detected a strong interaction signal for Rny self-interaction (Fig. 1C and D); as Rny is able to self-interact and oligomerize (Roux et al., 2011), this confirmed the suitability of the BTH assay. We also observed a strong interaction between Rny and FloA, but did not detect interaction between FloA and the remaining cytoplasmic proteins that constitute the degradosome. Rny is the only degradosome protein with a membrane-anchoring region and is thought to be responsible for the membrane localization of the entire structure (Marincola et al., 2012; Oun et al., 2013; Redder and Linder, 2012; Roux et al., 2011). Rny thus appears to be the only degradosome protein that interacts with FloA.
We attempted to copurify FloA and Rny directly from S. aureus cell extracts using a double-labeled strain that expressed a His6-tagged FloA and a SNAP-tagged Rny variant. The membrane fraction was solubilized and loaded onto a column of nickel-charged resin (Qiagen) that selectivity binds His6-tagged proteins and thus proteins that bind directly or indirectly to FloA. The resin-bound proteins were eluted with an imidazole-containing buffer. We added the fluorescence substrate TMR-Star, which binds covalently to SNAP-tag fusions and permits in-gel detection of protein-bound fluorophores (Cole, 2013; Kobayashi et al., 2012). In this approach, SDS-PAGE presented a fluorescence signal attributable to Rny-SNAP in the positive control with purified membranes of Rny-SNAP-labeled cells; a similar band was detected in the eluted fraction of the FloA-His6/Rny-SNAP double-labeled strain (Fig. 1E), suggesting that Rny-SNAP co-eluted with FloA-His6. In contrast, no signal was detected in the elution fraction of strains carrying only FloA-His6 or Rny-SNAP reporters (Fig. 1E), which implied that Rny retention on the column was FloA-His6-dependent. In addition, this in-gel protein detection is a particularly interesting approach for S. aureus research, as this is an antibody-free protein identification technique and it avoids interference from several S. aureus non-specific immunoglobulin-binding proteins that often generate strong false positives in immunoblotting (Atkins et al., 2008).
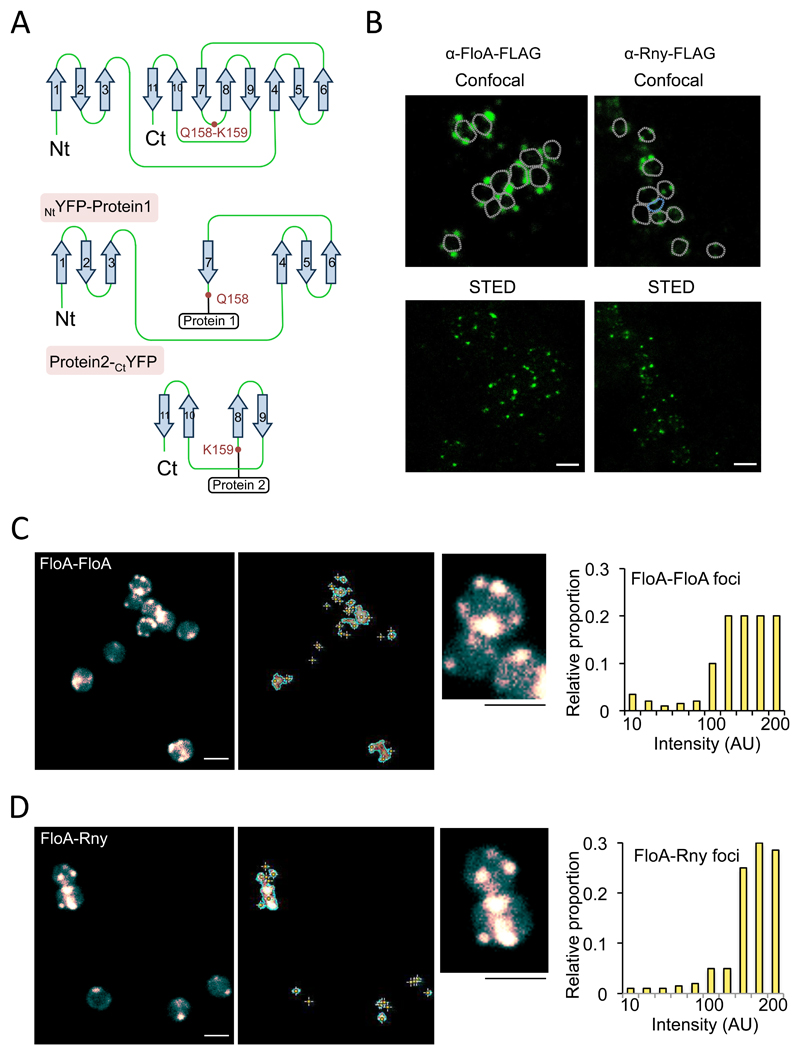
We used stimulated emission depletion microscopy (STED), a super resolution microscopy technique to study subcellular localization of FloA or Rny. FLAG-tagged variants of FloA and Rny were expressed in different strains and α-FLAG antibodies were used to detect the proteins. Alexa488 conjugated secondary antibody was used to visualize signal at the microscope. We detected FloA and Rny signal distributed in discrete membrane foci, showing a comparable distribution pattern, thus likely that both signals colocalize and interact (Fig. 2A). To image FloA-Rny interactions, we created a split-protein reassembly approach in which a yellow fluorescent protein (YFP), codon-optimized for use in gram-positive bacteria, was dissected at a surface loop between glutamine 158 and lysine 159 residues (Fig. 2B), based on published procedures (Blakeley et al., 2012; Magliery et al., 2005; Sakamoto and Kudo, 2008; Wilson et al., 2004). Protein dissection generated N- and C-terminal YFP fragments (NtYFP; CtYFP) (Fig. 2B) that require antiparallel interaction for reassembly (Blakeley et al., 2012; Cabantous et al., 2005; Magliery et al., 2005), whereas random oligomerization does not allow YFP reassembly (Fig. S3A). If the fused proteins have affinity for one another and antiparallel interaction brings NtYFP and CtYFP into close proximity, the fluorophore is folded and can be detected by fluorescence microscopy (Fig. 2C). As the YFP reassembly reaction is essentially irreversible in physiological conditions, it also can be of use to detect transient interactions (Blakeley et al., 2012; Cabantous et al., 2005; Magliery et al., 2005). NtYFP and CtYFP fragments were fused to FloA and Rny at the N- and C-terminal regions to yield NtYFP-FloA and Rny-CtYFP constructs. NtYFP-FloA and FloA-CtYFP constructs were also generated as a control to test split-protein reassembly (Fig 2C). FloA oligomerization caused YFP folding in discrete membrane foci. The number of foci increased with cell culture maturation, exhibiting on average >4 foci/cell at stationary phase. The strain harboring NtYFP-FloA Rny-CtYFP constructs showed a fluorescence signal distributed in membrane foci similar to the FloA pattern (Fig. 2D), although focus number represented ~40% of the total foci detected in the control strain, and interacting foci were usually detected in the proximity of the septal membrane during cell division.
Figure 2. Construction of a split-YFP reassembly assay in S. aureus to test flotillin interactions.
(A) STED microscopy analyses of FloA and Rny subcellular localization. Fluorescence signal is represented in green. Upper row shows signal using confocal microscopy and bottom row show the same cells imaged by STED microscopy. Scale bar is 2 μm. (B) Tertiary structure of a codon-improved YFP suitable for gram-positive bacteria. Dissection of YFP at a surface loop between residues Q158 and K159 generated NtYFP and CtYFP fragments. Below are the NtYFP and CtYFP fragments generated by dissection. (C) Fluorescence microscopy analyses of split-YFP reassembly in control FloA-FloA interaction after incubation (37°C, 72 h, 200 rpm agitation). Center panel shows signal detection. Right panel shows semi-quantitative determination of the fluorescence signal. Scale bar is 2 μm. (D) Fluorescence microscopy analyses of split-YFP reassembly in FloA-Rny interaction (37°C, 72 h, 200 rpm agitation). Center panels shows signal detection and detail of subcellular localization of fluorescence foci. Right panel shows semi-quantitative determination of signal. To quantify fluorescence signal, we counted 700 random cells from each of three microscopic fields for each strain (n = 2100 cells in total for each strain). Scale bar is 2 μm.
Absence of FloA affects degradosome activity
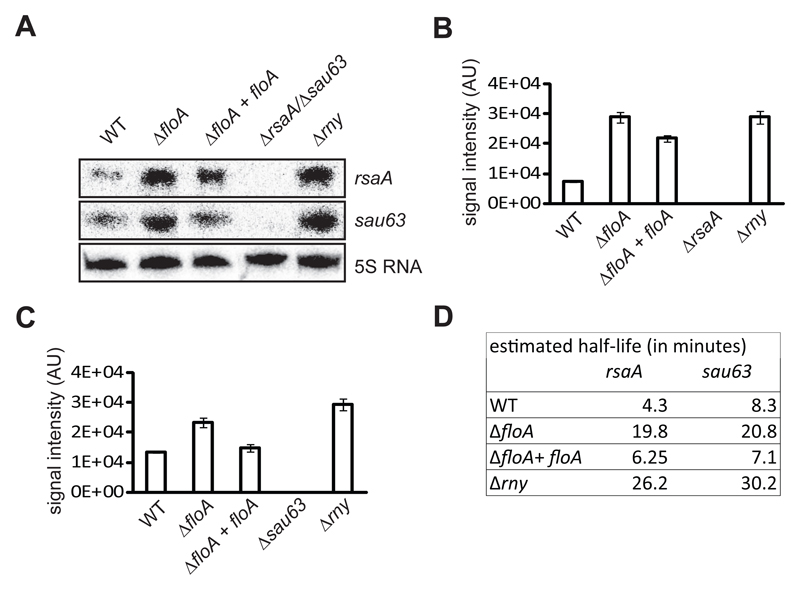
Having determined that Rny is confined to the FMM and interacts directly with FloA, we examined the influence of FloA on Rny and degradosome activity. The S. aureus degradosome is involved in the decay of specific RNA targets (Marincola et al., 2012; Oun et al., 2013; Redder and Linder, 2012; Roux et al., 2011), including the sRNA transcripts rsaA and sau63 (Marincola et al., 2012). In rny-deficient mutants, rsaA and sau63 half-life is prolonged and their concentration increased compared to WT controls (Durand et al., 2012; Marincola et al., 2012). We used Northern blot analysis to semi-quantitatively determine rsaA and sau63 levels in FloA-deficient cells and compare them to those in WT cells, both cultured synchronously to stationary phase (Fig. 3A-C). The Δrny mutant exhibited a more intense signal for both transcripts, as reported (Durand et al., 2012; Marincola et al., 2012). We detected an increase in the sau63 and rsaA signals in the ΔfloA mutant, whereas the complemented ΔfloA mutant (ΔfloA+floA), with a WT copy of floA integrated in the neutral chromosomal locus amy, showed a slight decrease in rsaA and sau63 signals. Half-lives of the target sRNAs were extended in the Δrny and in the ΔfloA mutants, and were considerably shorter in the ΔfloA+floA complemented strain (Fig. 3D). No transcriptional differences between WT and ΔfloA mutant were detected in mRNA that are not targeted by the degradosome (Fig. S3B).
Figure 3. rsaA and sau63 transcripts are more abundant in the ΔfloA mutant.
(A) Northern blot analysis of rsaA and sau63 expression on distinct genetic backgrounds. RNA was hybridized with probes specific for rsaA (top) and sau63 (center). 5S rRNA detection is indicated as loading control (bottom). (B) Relative amounts of rsaA transcripts determined by quantification of signal intensity (arbitrary units, AU). (C) Relative amounts of sau63 transcripts determined by quantification of signal intensity. (D) Northern blot analysis of half-life of rsaA and sau63 transcripts on distinct genetic backgrounds. Transcript stability is measured over time (minutes) after rifampicin treatment.
Rny oligomerization is less efficient in the absence of FloA
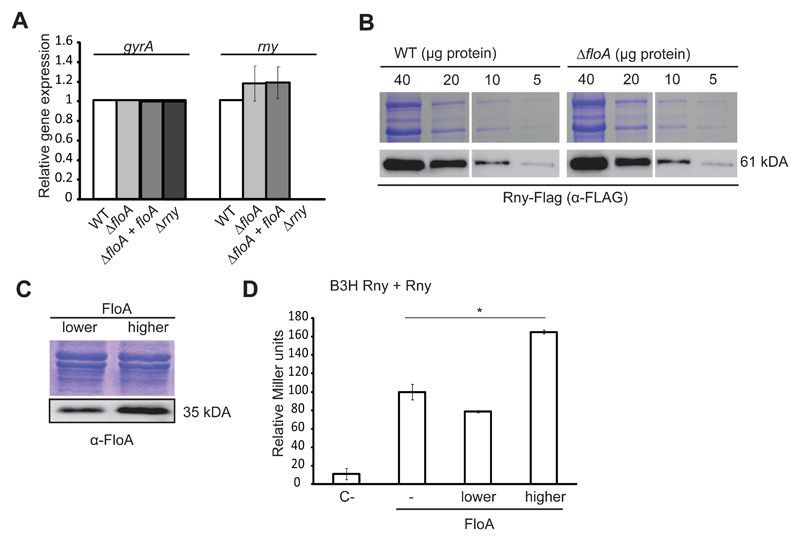
How does FloA scaffold activity influence Rny activity? The most direct hypothesis is that scaffold proteins promote stability of protein complexes by tethering interacting partners, which reinforces proximity and increases likelihood of interaction (Good et al., 2011). In the absence of FloA, Rny might thus oligomerize less efficiently and negatively affect degradosome activity. We tested whether Rny stability is compromised in the absence of FloA. As control, qRT-PCR analyses confirmed that lack of FloA did not affect rny gene expression (Fig. 4A) and immunodetection assay revealed that Rny remained associated with the membrane fraction in the ΔfloA mutant (Fig. S4A); these data confirms the presence of Rny in WT and ΔfloA mutant strains, with no notable differences between the two genetic backgrounds (Fig. 4B).
Figure 4. Absence of FloA does not affect Rny transcription and translation levels.
(A) qRT-PCR analysis of expression of rny on distinct genetic backgrounds (Student’s t-test, p ≤0.05). gyrA expression is used as a reference. (B) Immunodetection of Rny-Flag in gradually diluted samples of WT strain and ΔfloA mutant. Top, Coomassie-stained gel; bottom, immunoblot. (C) BTH assay to quantify the Rny interaction at distinct FloA concentrations. Low- (pSEVA631) and high-copy (pSEVA641) plasmids expressing FloA rendered lower and higher concentrations of FloA in immunoblot analysis (bottom). SDS-PAGE shown for the loading control. (D) Graph showing β-galactosidase activity of the B3H strains (in Miller Units). C- is the negative control strain bearing empty plasmids. Rny-Rny interaction in the absence of FloA (–), or at lower or higher FloA concentrations. Significance was measured by one-way ANOVA. * p <0.05. Data shown as mean ± SD of three independent biological replicates (n = 3). Each biological replicate included three technical replicates.
We designed a bacterial three-hybrid assay (B3H) to monitor Rny homodimerization efficiency quantitatively at different FloA concentrations (Fig. 4C and D). We used a classical BTH assay in which Rny was tagged with T25 or T18 catalytic domains. This was complemented with a modulable vector system (Silva-Rocha et al., 2013) in which floA was cloned in two vectors that replicated at different copy number. This generated two B3H strains that produced lower and higher FloA levels as a direct function of floA copy number, which was confirmed by immunoblotting (Fig. 4C). The assay indicated no increase in Rny interaction at lower FloA concentrations, whereas a higher FloA concentration led to a significant increase in the Rny interaction (Fig. 4D). This result showed there is an optimal FloA concentration that promotes protein-protein interaction and that it acts as scaffold protein to promote Rny oligomerization.
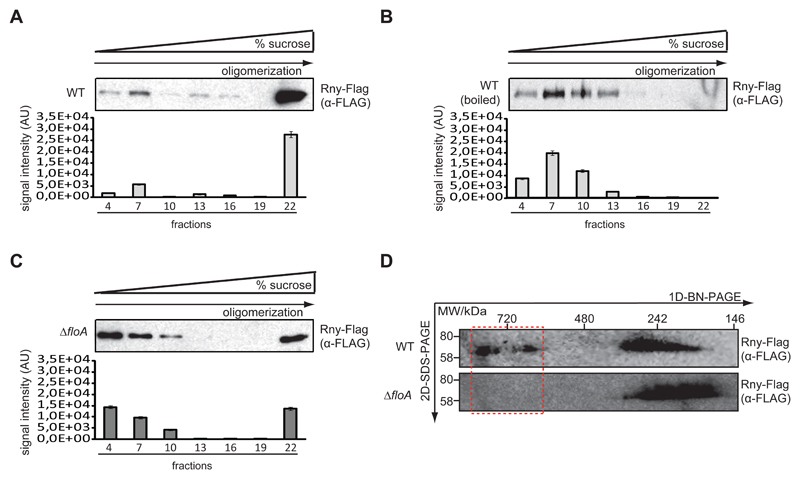
We next tested whether FloA scaffold activity affects Rny oligomerization directly, given that Rny must tetramerize to assemble an active degradosome (Bandyra et al., 2013; Callaghan et al., 2005; Koslover et al., 2008). To semi-quantitatively determine Rny oligomerization efficiency in WT and ΔfloA strains, the membrane fraction of both strains was solubilized in Tris buffer with 1% DDM [n-dodecyl β-d-maltoside] and resolved in using velocity sucrose gradient centrifugation (sucrose 5-40%) and Western blot analysis. In WT samples, the Rny signal was concentrated in high-density fractions 20-22 at the bottom of the tube (Fig. 5A) where high molecular weight protein complexes accumulated, which indicated large amounts of higher MW Rny-containing oligomers. As control, the WT cell membrane fraction was boiled (1 h) to denature proteins (Fig. 5B), resolved and blotted as above. Signal was detected in low-density fractions 1-10 at the top of the tube, in which low MW protein complexes accumulated, indicating degradation of the Rny-containing protein complexes. In the ΔfloA mutant, the signal was equally distributed between the two gradient regions (Fig. 5C). The signal in fractions 20-22 was attributable to a correctly assembled degradosome, whereas the remaining of the signal, in fractions 1-10, was similar to that in control samples of degraded protein.
Figure 5. Absence of FloA affects degradosome oligomerization.
(A) Western blot analysis to detect Rny-Flag in a fractioned sucrose gradient (fractions 1- 22) from the membrane fraction of WT cells (top). Quantitative estimate of signal intensity (AU; bottom). (B) Western blot analysis to detect Rny-Flag in a fractioned sucrose gradient using a denatured membrane fraction of WT cells. Protein was denatured by boiling the sample. (C) Western blot analysis to detect Rny-Flag in a fractioned sucrose gradient from the membrane fraction of ΔfloA mutant cells. (D) Immunodetection of Rny-Flag in DDM-solubilized membrane oligomers of WT strain and ΔfloA mutant using 2D-BN-PAGE. Protein complexes were separated by BN-PAGE, lanes excised and resolved by SDS-PAGE, then immunoblotted.
The WT and ΔfloA strain membrane fractions were resolved in Blue Native PAGE (BN-PAGE; Life Sciences). BN-PAGE allows the separation of membrane protein complexes in their natural oligomeric states (Wittig et al., 2006) (Fig. 5D). We used BN-PAGE with a 3-12% polyacrylamide gradient to resolve membrane-bound oligomers between 150-1000 kDa, coupled to SDS-PAGE 2D-eletrophoresis and detected Rny by immunoblotting. WT samples showed two signals of ~300 and 900 kDa, whereas the ΔfloA mutant exhibited only the ~300 kDa signal, which suggests that the degradosome is partially disorganized in FloA-defective S. aureus cells.
Small-molecule inhibitors of FloA oligomerization reduce Rny activity and attenuate virulence in staphylococcal infections
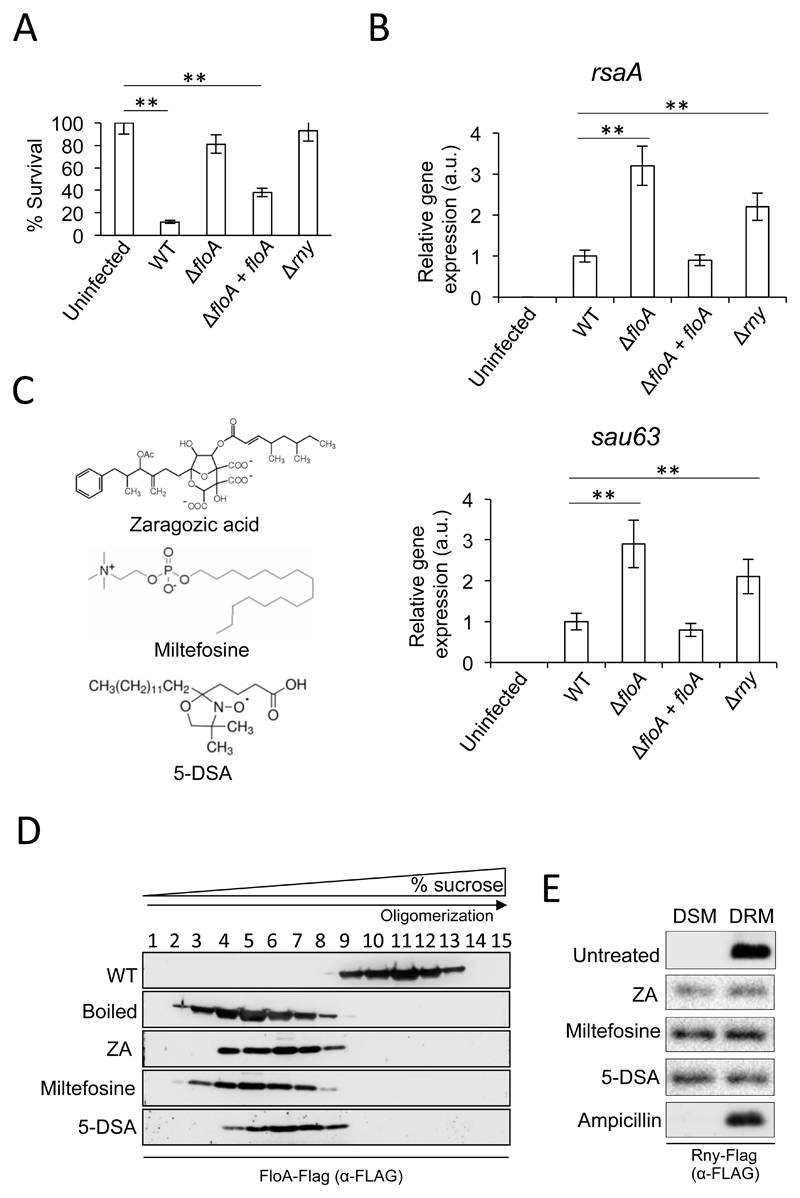
FloA contributes to efficient Rny oligomerization and to the establishment of S. aureus infections. The rny gene was initially identified as a virulence regulator in S. aureus because an rny mutant attenuated virulence in invertebrate worm (Kaito et al., 2005) and murine infection models (Marincola et al., 2012). Using the invertebrate wax moth larva Galleria mellonella, we evaluated whether the ΔfloA mutant shows a virulence-attenuating phenotype. Three cohorts of 15 larvae received injections of 5 x 106 WT S. aureus cells, were incubated (37°C, 48 h), and the number of surviving larvae counted (Fig. 6A). Infection of larvae with the WT staphylococcal strain resulted in survival of ~15% of infected larvae (p <0.01). Larvae injected with Δrny or ΔfloA mutant cells (5 x 106) presented an 80% survival rate; both mutants thus attenuated virulence in this invertebrate infection model. In contrast, larvae that received the complemented ΔfloA+floA strain had a 35% survival rate. In another experiment, infected larvae were incubated (37°C, 12 h) before hemolymph collection and quantification of rsaA and sau63 expression by qRT-PCR analysis (Fig. 6B). Expression was significantly higher in Δrny and ΔfloA mutants than in the WT strain, which contrasted with reduced expression in the complemented ΔfloA+floA strain.
Figure 6. Small molecules that perturb flotillin oligomerization affect Rny activity in S. aureus.
(A) Galleria mellonella worms were infected by injection with various S. aureus strains (5 x 106 cells); percent survival is shown. Control larvae were infected with saline solution. Larvae were incubated (37°C, 48 h) and surviving larvae counted. Data shown as mean ± SEM (n = 15 larvae/group; three independent experiments). Significance was measured by one-way ANOVA with Tukey’s comparison. ** p <0.01. Data shown as mean ± SD of three independent biological replicates (n = 3). (B) qRT-PCR analysis of rsaA and sau63 expression in WT, ΔfloA, ΔfloA+floA and Δrny mutants of S. aureus. Relative gene expression is normalized to WT levels. Significance was measured by one-way ANOVA with Tukey’s comparison. ** p <0.01. Data shown as mean ± SD of three independent biological replicates (n = 3). Each biological replicate included three technical replicates. (C) Molecular structure of flotillin-perturbing small molecules. (D) Immunodetection of flotillin in a fractioned sucrose gradient from the membrane fraction of cells treated with ZA, miltefosine and 5-DSA. FloA-containing protein complexes were detected using anti-FloA antibodies. (E) Immunodetection of Rny in DSM and DRM fractions of S. aureus cells treated with ZA, miltefosine and 5-DSA. Ampicilin-treated samples are negative control since ampicillin does not affect FMM.
Targeting flotillin scaffold activity could thus be a strategy appropriate for fighting bacterial infection by simultaneously perturbing oligomerization of virulence-related protein complexes, of particular interest for MRSA infections that cannot be eradicated by conventional antibiotics. The small molecule zaragozic acid (ZA) (Fig. 6C) is a potential inhibitor of flotillin activity (Lopez and Kolter, 2010), as it inhibits S. aureus squalene synthase (Bergstrom et al., 1993), necessary for the production of the polyisoprenoid lipids that stabilize flotillin in the FMM. When bacteria are exposed to nanomolar concentrations of ZA, flotillin organizes in a smaller number of membrane foci, concomitant with a reduction in its chaperone activity (Lopez and Kolter, 2010). Using a Flag-tagged S. aureus strain, we tested whether ZA perturbs flotillin oligomerization. The membrane fraction of ZA-treated and untreated cells was resolved by gradient centrifugation and analyzed by immunoblotting, as before. In untreated controls, the FloA signal was concentrated in the high-density, high MW protein complex fractions, whereas in ZA-treated samples, the signal was detected low-density, low MW fractions (Fig. 6D). The split-protein reassembly assay was also used to evaluate the ZA effect on flotillin oligomerization. The strain harboring NtYFP- and CtYFP-tagged FloA showed a reduction of the fluorescence signal upon addition of ZA (Fig. S4 B and C), which suggests that ZA treatment interferes with FloA oligomerization in S. aureus.
Using this split-YFP reassembly strain as a reporter to screen for inhibition of FloA oligomerization, we tested a group of small molecules selected for their capacity to interact with cell membranes thus they might interfere with flotillin oligomerization, since many of these compounds are known to interact with eukaryotic lipid rafts or to affect lateral segregation of membrane lipids (Table S1). Among the molecules tested, we detected significant inhibition of the FloA oligomerization signal using the broad-spectrum phospholipid antimicrobial miltefosine (hexadecylphosphocholine) (20 μM) and the lipid probe 5-DSA (5-doxyl-stearic acid) (10 μM) (Fig. 6C, S4B and C) at concentrations that did not affect S. aureus growth (Fig. S5). Miltefosine is a synthetic alkylphospholipid (ALP) that accumulates in lipid rafts and decreases lipid raft ordering in membrane models (van der Luit et al., 2002). ALP thus alter the plasma membrane, specifically lipid raft composition and biophysical properties (Castro et al., 2013; de Sa et al., 2015). The probe 5-DSA accumulates in biological membranes and is conventionally used to monitor membrane fluidity in studies of lipid raft organization (Grammenos et al., 2010; Kardash and Dzuba, 2014). 5-DSA can also displace certain membrane lipids and alter the function of membrane-associated proteins (Jezek et al., 1995; Wu and Gaffney, 2006).
To test FloA oligomerization in the presence of miltefosine or 5-DSA, we resolved the membrane fraction of treated and untreated cultures by sucrose gradient centrifugation and immunoblot analysis, as above. Miltefosine or 5-DSA in S. aureus cultures caused a negative effect on FloA oligomerization, with the signal concentrated in the low MW protein fractions (Fig. 6D). S. aureus Rny-Flag strain cultures were then treated with ZA, miltefosine or 5-DSA, and DSM and DRM fractions were tested for Rny by immunoblotting (Fig. 6E). We detected Rny in both fractions, which suggested that the small molecules altered the heterogeneous Rny distribution in S. aureus membranes. qRT-PCR analyses of rsaA and sau63 expression in ZA-, miltefosine- and 5-DSA-treated cultures presented significantly higher expression concomitant to lower hemolysis activity compared to untreated controls (Fig. 7A and S5A-C), which indicated that drug-mediated alterations in flotillin oligomerization lead to an Rny activity defect. The presence of these drugs also affected flotillin activity in other Gram-positive species. Flotillin promotes efficient homodimerization of the FMM-associated sensor kinase KinC, which regulates biofilm formation in Bacillus subtilis (Schneider et al., 2015b). Thus, B. subtilis cultures treated with ZA, miltefosine or 5-DSA showed reduced biofilm formation compared to untreated sampled (Fig. S6D).
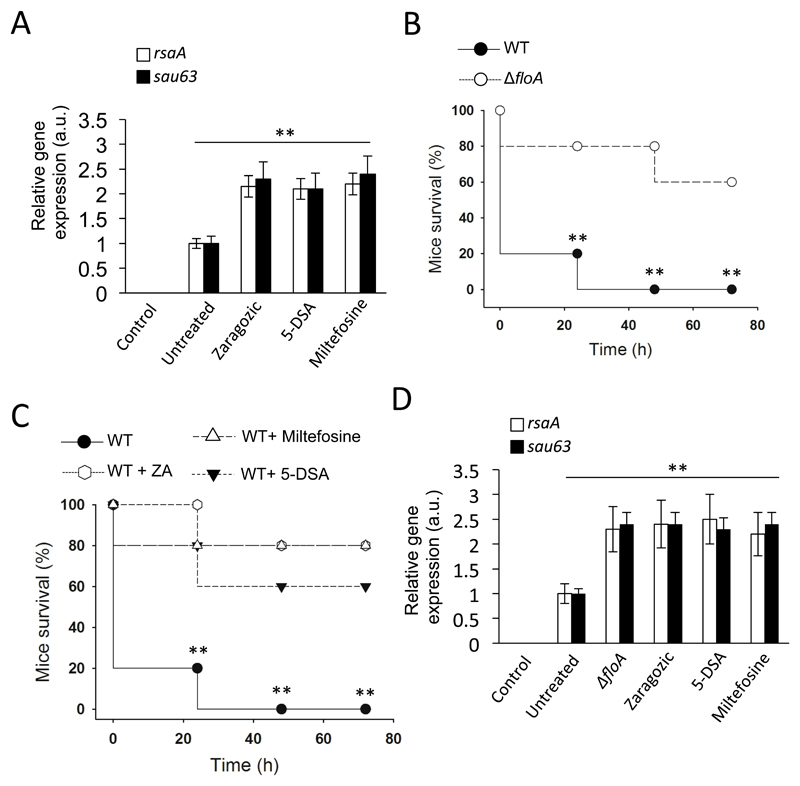
Figure 7. Inhibition of flotillin oligomerization in S. aureus attenuates virulence in a murine infection model.
(A) qRT-PCR analysis of rsaA and sau63 expression in ZA-, miltefosine- and 5-DSA-treated cells. Significance was measured by one-way ANOVA with Tukey’s comparison. For untreated vs. treated samples, ** p <0.01. Data shown as mean ± SD of three independent biological replicates (n = 3). Each biological replicate included three technical replicates. (B) Survival (%) of S. aureus-infected mice (n = 5) with WT and ΔfloA. Pneumonia caused by intranasal instillation of 3 x 108 colony-forming units in 20 μl saline solution. Infections were allowed to progress for four days. Differences in survival were analyzed by the log-rank test. Statistical significance was measured by unpaired Student's t-test, ** p <0.01. Data shown as mean ± SD of endpoint vital measurements. (C) Survival (%) of S. aureus-infected mice (n = 5), untreated or treated with ZA, milfefosine or 5-DSA (50, 20, 10 mg/kg/day respectively). Pneumonia caused by intranasal instillation of 3 x 108 colony-forming units in 20 μl saline solution. Small molecules were administered to infected mice 30 min post-infection via intraperitoneal injection. Infections were allowed to progress for four days. Differences in survival were analyzed by the log-rank test. Statistical significance was measured by unpaired Student's t-test between; Untreated vs. 5-DSA-treated; Untreated vs. ZA-treated and Untreated vs. Miltefosie-treated samples. ** p <0.01. Data shown as mean ± SD of endpoint vital measurements. (D) qRT-PCR analysis of rsaA and sau63 expression in mice. Lungs were collected 48 h post-infection and rsaA/sau63 expression levels determined. Significance was measured by one-way ANOVA with Tukey’s comparison. For untreated vs. treated samples, ** p <0.01. Data shown as mean ± SD of three independent biological replicates (n = 3). Each biological replicate included three technical replicates.
We tested for a connection between FloA inactivation and reduced virulence potential in vivo using a murine infection model in which cohorts of BALB/c mice (n = 5) were infected intranasally (3 x 108 colony-forming units, CFU) with WT and ΔfloA strains (Fig. 7B); additional infection experiments were performed in which WT infections were treated with ZA, miltefosine or 5-DSA at 30 min post-bacterial challenge (Fig. 7C). We monitored the survival rate of infected mice four days after infection. Mice infected with the WT strain showed high mortality, while ΔfloA strain-infected mice exhibited delayed, significantly decreased mortality, with a 50% survival rate (p<0.01). ZA-, miltefosine- and 5-DSA-treated mice also presented decreased mortality, with a 70%, 80% and 60% survival rate respectively and thus comparable to that of the ΔfloA strain. In a different infection experiment, mice were infected intranasally (3 x 108 CFU) and infections were allowed to progress during two days before animals were sacrificed, lungs were collected and qRT-PCR analyses of rsaA and sau63 expression were performed (Fig. 7D). ZA-, miltefosine- and 5-DSA-treated infections showed significantly higher expression of rsaA and sau63 compared to untreated controls, suggesting that drug-mediated alterations in flotillin oligomerization occurs during in vivo infections.
Discussion
Research in the last decade has unraveled the importance of scaffold proteins in regulating the assembly of interacting protein components and the implication of these proteins in many diverse biological reactions has attracted much attention (Chapman and Asthagiri, 2009; Dueber et al., 2009; Good et al., 2011). Here we show that the scaffold protein FloA of S. aureus contributes to the development of infection by promoting more efficient interaction of protein complexes necessary for staphylococcal virulence, such as the degradosome. However, it is likely that the scaffold activity of FloA influences the interaction of other protein complexes related to virulence.
FloA scaffold activity appears to promote self-interaction of the RNase Rny, consistent with reports that Rny self-interacts and homo-oligomerizes (Roux et al., 2011). Our protein-protein interaction assays indicate that of all degradosome proteins, FloA interacts only with Rny. It is thus likely that FloA scaffold activity promotes efficient Rny oligomerization, necessary for degradosome function and is consistent with recent publications showing a focal subcellular localization of the degradosome in bacterial membranes (Strahl et al., 2015). The degradosome is a supramolecular protein complex comprised of four multi-enzymatic units interconnected via interaction of the ribonuclease transmembrane region (Bandyra et al., 2013; Callaghan et al., 2005; Koslover et al., 2008). FloA might thus promote efficient Rny tetramerization, which would facilitate degradosome subunit assembly (Fig. S7). Lack of FloA would therefore negatively affect Rny homo-oligomerization and perturb degradosome activity, thus reducing RNA decay. Although scaffold protein activity assists the interaction of protein partners, scaffold proteins are not essential for this activity, as reported in other models (Devi et al., 2015). Some Rny oligomerization thus remains detectable in the absence of FloA.
Here we used the FloA-mediated stability of Rny as a case study to evaluate FloA scaffold activity in S. aureus cells. It is nonetheless likely that FloA activity contributes to oligomerization of additional protein complexes in the DRM fraction that also have an important role in virulence, such as the adhesion membrane protein EbpS or protease PrsA (Fig. S1A). Although the FloA connection with these proteins remains to be explored, we predict that the attenuated virulence of the ΔfloA mutant is the result of an oligomerization defect in several virulence-related, FMM-associated protein complexes such as Rny. Targeting the activity of bacterial scaffold proteins could be an innovative antimicrobial strategy that could reduce the virulence potential of a pathogen without affecting its viability, and would thus not add biological pressure to acquire mutations that confer resistance to antimicrobial therapy. Here we show the potential antimicrobial effect of several small molecules that modify bacterial membrane organization and interfere with flotillin oligomerization, without compromising pathogen viability. Moreover, these molecules present no toxic activity against humans as they are conventionally used to treat hypercholesterolemia, parasitic infections and immune-mediated diseases (Baxter et al., 1992; Dorlo et al., 2012; Verhaar et al., 2014). However, addition of these molecules led to a significant reduction in S. aureus strain virulence both in vitro and in vivo. The antimicrobial activity of these compounds could be a useful strategy toward eliminating hard-to-treat infections caused including S. aureus.
Significance
Scaffold proteins are ubiquitous proteins that facilitate physical interaction of multi-enzyme complexes. Thus, targeting scaffold proteins of pathogenic bacteria may prevent the development of infections by simultaneously inhibiting diverse multienzyme complexes that play a role in virulence. Here we present an example on the scaffold protein flotillin of the multi-drug-resistant pathogen Staphylococcus aureus and show how flotillin assists in the assembly of the degradosome, a protein complex that upregulates the expression of virulence genes in S. aureus. We identify several small-molecule compounds that alter flotillin oligomerization, thus affect the degradosome activity and reduce S. aureus virulence during in vivo S. aureus infections that are resistant to conventional antibiotics. Flotillin could thus be an attractive target for the development of new antimicrobial therapies against multi-drug resistant bacteria.
STAR Methods
Contact for reagents and resource sharing
Further information and requests for resources and reagents should be directed the Lead Contact, Daniel Lopez (dlopez@cnb.csic.es).
Experimental model and subject details
Bacterial Cultures
All bacterial strains used in this study are listed in Key Resource Table (KRT) and table S2 of the supplementary material. Staphylococcus aureus Newman (Lipinski et al., 1967) was used throughout all experiments unless otherwise stated. For cloning purposes, Escherichia coli strain DH5α (Reusch et al., 1986) was used. S. aureus strain RN4220 (Kornblum, 1990) served as the recipient for S. aureus electroporation. S. aureus strains were propagated in TSB medium supplemented with chloramphenicol (5 μg/ml), erythromycin (2 μg/ml) or tetracycline (10 μg/ml) when appropriate. E. coli strains were cultivated in Luria-Bertani medium containing ampicillin (100 μg/ml), kanamycin (50 μg/ml) or gentamycin (5 μg/ml) when required.
Invertebrate infection model
The invertebrate greater wax moth Galleria mellonella was used as an in vivo model to examine the pathogenicity of diverse S. aureus strains. G. mellonella nowadays represents a powerful and reliable model for testing human pathogens (Fuchs et al., 2010; Koch et al., 2014a; Loh et al., 2013; Seed and Dennis, 2008). Infection studies were performed according to the protocol published by (Koch et al., 2014b). Larvae were challenged with 20 μl bacterial suspension (5x106 CFU) into the last left proleg of each caterpillar. Infections were allowed to progress in infected larvae for 48 h at 37°C. Three independent experiments were performed using cohorts of fifteen larvae per strain and experiment. Larvae injected with 20 μl 10 mM MgSO4 served as a control group. Larvae were considered dead when they did not react anymore to gentle tapping.
Vertebrate infection model
All animal studies were approved by the local government of Lower Franconia, Germany (license number 55.2-DMS-2532-2-57 and were performed in strict accordance with the guidelines for animal care and animal experimentation of the German animal protection law and directive 2010/63/EU of the European parliament on the protection of animals used for scientific purposes. Female BALB/c mice (16 to 19 g) were purchased from Charles River (Charles River Laboratories, Erkrath, Germany), housed in polypropylene cages and supplied with food and water ad libitum. The different S. aureus strains used were cultured for 18 h at 37 °C on BHI medium. Subsequently, cells were collected and washed three times with PBS and diluted to reach an OD600 nm = 0.05. Viable cell counts were determined by plating dilutions of the inoculum on TSB agar plates. Cohorts of 5 mice were infected with 150 µl of cultures of S. aureus, containing 1x107 cells via tail vein injection. Infections were allowed to progress until severe infection signs occurred or to the endpoints of 24 h, 48 h, 72 h and 96 h after mice challenging. Animals were sacrificed when they met the following criteria: 1) loss of at least 20% of body weight; 2) loss of at least 15% of body weight and ruffled fur; 3) loss of at least 10% of body weight and hunched posture; or 4) 24 h, 48 h, 72 h and 96 h time points after infection.
Method details
Construction of mutant and replacement strains
To replace rny with rny-flag, we used a two-step recombination process using the temperature-sensitive vector pMAD (Arnaud et al., 2004). The rny-flag fragment was generated by long-flanking homology PCR (Wach, 1996) using the primers GK311/GK259 for rny and GK259/GK312 for the flag-tag. Complete lists of plasmids and primers used in this study are shown in KRT and tables S2 and S3 of the supplementary material, respectively. To generate ΔrsaA::spc ΔrsaA::tet and Δsau63::cm deletions, the deletion cassettes were generated by long-flanking homology PCR (Wach, 1996) using the primers GK85/GK85 for floA, GK102/GK163-GK166/GK167 for rsaA and GK96/GK97-GK100/GK101 for sau63. The resulting constructs were verified using Sanger sequencing (Sanger and Coulson, 1975) and transformed into RN4220. The plasmids were integrated into the chromosome via a first recombination by growing the strains for 6 h at 42°C. Cultures were plated onto selective medium (erythromycin 2 μg/ml and X-Gal 50 μg/ml). Blue colonies were selected and the integration of the plasmid into the chromosome was verified by PCR. The genetic constructs obtained from the first recombination process were transferred to strain Newman using Phi11 phage transduction (Rudin et al., 1974). A second recombination was induced in the strain Newman. To do this, cultures of blue colonies were incubated for 6 h at 30°C, then shifted for 3 h to 42°C, and subsequently plated onto TSB with X-Gal. After 48 h of incubation at 42°C, the blue colonies were discarded as they still carry the plasmid. PCR analysis was used to validate whether the white colonies carried the corresponding genome modification.
Generation of labelled strains
Labelled strains were generated using pAmy and pLac plasmids (KRT) (Yepes et al., 2014). The coding sequence of floA was amplified using GK84/GK85 primers (Table S3) and cloned into the plasmid pSG1154 (KRT). From there, the promoter and floA were amplified using AYG165/AYG166 primers (Table S3) and cloned into pAmy. The coding sequence of rny was amplified using GK77/GK78. The SNAP-tag was amplified from pSNAPf (NEB) using GK79/GK83. Both fragments were joined together using GK77/GK83 and cloned into pSG1154. As before the promoter region including the insert was amplified using AYG165/AYG166 and cloned into pLac. Verified plasmids were transformed first in RN4220 and then transduced into strain Newman. All the strains used in our experiments contained the genetic constructs integrated into their chromosomes.
RNA extraction, Northern blot and qRT-PCR
S. aureus cells were grown overnight on TSB plates or in BHI medium. Cells were harvested and resuspended in RNA Protect (QIAGEN) followed by subsequent lysis in a Fast Prep Shaker (two times: 45 s; speed, 6.5) using glass beads. Total RNA was extracted using the hot-phenol method described previously (Blomberg et al., 1990). Northern blot analysis was performed as described by (Dugar et al., 2013). Then, 10 to 15 µg RNA per sample was separated on 6% polyacrylamide gels containing 7 M urea. RNA was then transferred to Hybond-XL membranes, which were hybridized with [γ32P]-ATP end-labeled oligodeoxyribonucleotide probes. 5S rRNA served as a loading control for all experiments. For qRT-PCR analysis, total RNA was transcribed into cDNA using hexameric random primers. Subsequently, cDNA served as a template for real-time PCR amplification using Sso-Advanced SYBR Green Supermix (BioRad) and the primers listed in table S3 of the supplementary material. The relative amounts of amplicons for each gene were determined using gyrA as an internal standard.
Cell fractionation
Cell fractionation was performed as described in (Yepes et al., 2014). Briefly, cells were harvested and lysed in SMM buffer (1 M sucrose, 0.04 M maleic acid, 0.04 M MgCl2 [pH 6.5]) supplemented with lysostaphin (10 μg/ml) followed by French press disruption at 10,000 psi. After a centrifugation step (11,000 x g for 10 min, 4°C), this sample was saved as the total cell fraction (referred to as T fraction in the body of the paper). We used the total cell fraction to isolate the membrane fraction (referred to as M fraction in the body of the paper) and the cytoplasm fraction (referred to as C fraction in the body of the paper) using ultracentrifugation (100,000 x g for 1 h, 4°C). Unless otherwise stated, membranes were solubilized in Tris buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% DDM [n-dodecyl β-d-maltoside], and 1 mM phenylmethylsulfonyl fluoride [PMSF]).
FMM isolation, pull down analysis and SNAP detection
For FMM isolation, the abovementioned M fractions were further processed using the CellLytic MEM protein extraction kit (Sigma), as described by (Bach and Bramkamp, 2013; Dempwolff et al., 2012; Donovan and Bramkamp, 2009; Lopez and Kolter, 2010; Mielich-Süss et al., 2013; Yepes et al., 2012). Detergent resistant (FMM) and sensitive fractions were separated according to the manufacturer’s protocol. Samples were analyzed on SDS-PAGE and immunoblotting. Pull-down experiments were performed using Ni-NTA resin (QIAGEN) and samples were kept at 4°C throughout the experiments. Membranes of three different S. aureus strains (Newman amy::Pxyl-sa1402His, Newman lac::Pxyl-rny-SNAP and Newman amy::Pxyl-sa1402His lac::Pxyl-rny-SNAP) were resuspended in binding buffer (50 mM Tris-HCl, 500 mM NaCl, 10% glycerol [vol/vol], 20 mM imidazole, 14 mM β-mercaptoethanol, 1% Tween [pH 8]). Binding of the proteins (100 mg membrane/strain) to the resin was carried out at 4°C for 1h. In order to remove unbound and unspecific protein, the resin was washed 2x with wash buffer A (50 mM Tris-HCl, 500 mM NaCl, 10% glycerol [vol/vol], 20 mM imidazole, 14 mM β-mercaptoethanol [pH 8]) and 2x with wash buffer B (same as wash buffer A but 50 mM imidazole). His-tagged proteins were eluted using elution buffer (50 mM Tris-HCl, 500 mM NaCl, 10% glycerol [vol/vol], 500 mM imidazole, 14 mM β-mercaptoethanol [pH 8]). Subsequently, eluents were prepared for SNAP-tag detection. For this, 5x SNAP buffer (250mM TRIS-HCl pH 7.5, 500mM NaCl, 0.5% Tween 20 [vol/vol], 5mM DTT) was added to the elution fraction together with a final concentration of 1 mM DTT and 0.8 μM SNAP-Cell TMR-Star dye (NEB). Samples were incubated for 30 min at 37°C in the dark before loading them on a SDS-PAGE. In-gel detection of the SNAP-tag was performed using Typhoon 8600 (GE Healthcare) with the following settings: 532 nm excitation; emission filter set = Rox 610 BP 30; voltage = 999 V; focal plane = + 3 mm.
Western blot analysis and Rny detection
Immunoblotting was carried out as previously described (Koch et al., 2014c). 80 μg of total protein was separated using 10% SDS polyacrylamide gel. Proteins were transferred from the gel to a nitrocellulose membrane using semi-dry blotting for 2 h. After blotting, the nitrocellulose membrane was blocked with 5% skim milk for 1h and probed with anti-flag tag antibody (Sigma) to detect the presence of Rny-Flag. Proteins were detected after incubation with the secondary antibody anti-rabbit IgG-HRP (BioRad), using the chemiluminescent substrate Kit (Thermo Scientific). Chemiluminescence was recorded with the Illumination System ImageQuant LAS4000 (General Electric).
Epifluorescence microscopy, confocal microscopy and Stimulated Emission Depletion microscopy (STED)
For epifluorescence microscopy, 1 ml of TSB culture was washed in PBS, resuspended in 0.5 ml of 4% paraformaldehyde solution and incubated at room temperature for 6 min. After two washing steps with PBS buffer, cells were resuspended in 0.5 ml of PBS buffer and mounted on a microscope slide. Microscopy images were taken on a Leica DMI6000B microscope equipped with a Leica CRT6000 illumination system (Leica). The microscope was equipped with a HCX PL APO oil immersion objective with 100x1.47 magnification and a color camera Leica DFC630FX. Linear image processing was done using Leica Application Suite Advance Fluorescence Software. The YFP fluorescence signal was detected using an excitation filter 489 nm and an emission filter 508 nm (excitation filter BP 470/40 and suppression filter BP 525/20). For confocal microscopy and STED microscopy, 1 ml of TSB culture was washed in PBS and fixed with 4% paraformaldehyde. After washing with PBS buffer, cells were transferred to coverslips pre-treated with poly-lysine and allowed them to adhere to the coverslips for 30 min. Coverslips were washed three times with PBS buffer. Prior immunofluorescence staining, nonspecific binding sites of the coverslips were blocked with 5% BSA solution for 1 hour at room temperature. The primary antibody (α-FLAG) was diluted in 5% BSA solution to a final concentration of 1:200. Incubation was carried out at room temperature for 2 h. Samples were washed three times with PBS buffer before 1 h incubation with the second antibody (conjugated Alexa Fluor 488) at room temperature. Samples were washed three times with PBS buffer and stabilized with 4 μl of ProLong mounting medium. The glass slide was placed together with the coverslip facing the side containing the cells and the ProLong facing and were allowed to dry during 48 h. Confocal and STED microscopy was performed using a Leica SP8 TCS STED system (Leica Microsystems) with LAS X v 2.0.1. software (Leica Microsystems) for acquisition control. Single optical slices were acquired with an HC PL APO CS2 100x/1.40 N.A. oil objective (Leica Microsystems), a scanning format of 1,024 × 1,024, 12-bit sampling, and 10x zoom, yielding a pixel dimension of 11.3 nm and 11.3 nm in the x and y dimensions, respectively. STED imaging was performed exciting Alexa 488 at 488 nm with a pulsed WLL. Alexa 488 was depleted at 592 nm (50% intensity). Fluorescence signal was detected with time-gated hybrid detector (0.8-8 ns), ranging from 496 nm to 557 nm. Linear image processing was done using Leica Application Suite Advance Fluorescence Software and FiJi software.
Sucrose gradient
Cultures were harvested and cells were cross-linked using 0.5 mM Dithiosuccinimidylproprionate (DSP) following the protocol that is described in (Lee et al., 2013). Next, cells were harvested and lysed in SMM buffer (1 M sucrose, 0.04 M maleic acid, 0.04 M MgCl2 [pH 6.5]) supplemented with lysostaphin (10 μg/ml) followed by French press disruption at 10,000 psi. Total cell fraction was obtained after centrifugation (11,000 x g for 10 min, 4°C). The purified membrane fraction was obtained using ultracentrifugation (100,000 x g for 1 h, 4°C). The pellet was solubilized in Tris buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% DDM [n-dodecyl β-d-maltoside], 1 mM phenylmethylsulfonyl fluoride [PMSF]). The solubilized membrane fraction (200 μl) was layered on top of a 5-40% linear sucrose gradient, which was generated using a Biocomp gradient maker. Centrifugation was performed in a SW40 Ti rotor (Beckman) at 100,000 x g for 16 hours at 4°C. A pool of fractions of 500 μl each was collected from the top of the sucrose gradient generated in the tube during centrifugation. The proteins associated with each of the fractions were precipitated by adding 10% trichloroacetic acid. The protein fractions were collected by centrifugation, resuspended in PBS buffer and analyzed via SDS-PAGE and immunoblotting.
Two-dimensional blue-native PAGE (2D BN-PGE)
Cultures were harvested and cells were cross-linked using 0.5 mM DSP (Lee et al., 2013) prior to cell lysis and cell fractionation. The membrane fraction (approx. 80 µg) was mixed with Coomassie dye G-250 and loaded on a Native gel with a polyacrylamide gradient concentration of 3-12% (Invitrogen). Blue-native electrophoresis was carried out according to the manufacturer’s protocol (Invitrogen) and according to the methodology that is published by Wittig et al. (Wittig et al., 2006). Blue-native PAGE (BN-PAGE) makes use of Coomassie G-250 to confer a negative charge to proteins and allows resolving the distinct oligomeric complexes according to their native state (Swamy et al., 2006; Wittig et al., 2006). Whole protein lanes form the BN-PAGE were excised and layered on top of an SDS-PAGE. This second electrophoresis denaturates proteins and resolves the proteins of each of the multimeric complexes according to their molecular weight. Immunoblotting was performed to detect Rny-Flag using polyclonal antibodies against Flag tag (Invitrogen).
Bacterial two-hybrid analysis
Bacterial two-hybrid analysis was used to quantitatively determine the interaction between FloA and Rny. Accordingly, the coding sequences were amplified from S. aureus Newman and cloned in frame into the bacterial two-hybrid expression vectors. floA was cloned into pKNT25, pKT25, pUT18, and pUT18C (EuroMedex), whereas rny was cloned in pKNT25 or pUT18 plasmids. Similarly, the coding sequences of eno (Enolase) and cshA (RNA helicase) were cloned into pKNT25 or pUT18 plasmids. Thereby, N- and C-terminal fusions to the catalytic domains T25 and T18 of Bordetella pertussis adenylate cyclase were generated. Subsequently, all combinations of plasmid pairs were co-transformed in E. coli BTH101 strain, which harbors a lacZ gene under the control of a cAMP inducible promoter. Upon interaction, the T25 and T18 catalytic domains of the adenylate cyclase form an active enzyme leading to the production of cAMP and hence to the expression of the reporter. Positive cells will turn blue in the presence of X-gal. Protein-interaction assays were performed following the protocol previously described by Karimova et al. (Karimova et al., 1998). Plates were incubated for 48h at 30°C. pKT25-zip and pUT18C-zip, as well as pKT25 and pUT18C, served as positive and negative controls, respectively. For quantitative measurements, β-galactosidase levels were determined. Therefore, transformants were grown for 48h at 30°C in LB medium supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), and 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG; 0.5 mM). Optical density at 600nm was determined before cells were permeabilized using chloroform and SDS. 200 μl o-nitrophenol-β-galactoside (ONPG; 4 mg/ml) was added and reactions were stopped after 10 min of incubation at 30°C by adding 500 μl 1 M Na2CO3. The absorbance was determined for 420 nm (cleavage of ONPG) and 550 nm (cellular debris). All tests were performed in triplicate and are shown in Miller units (Miller, 1972).
Bacterial three-hybrid analysis (B3H assay)
To semi-quantitatively determine the scaffold activity of FloA, a bacterial three-hybrid assay (B3H assay) was performed. Therefore, the abovementioned rny constructs were used in the plasmids pKNT25 and pUT18 (Karimova et al., 1998). A co-transformed strain was used to perform protein–protein interaction assays following the protocol of Karimova et al. (Karimova et al., 1998) to determine the interaction efficiency of Rny. Moreover, this strain was subsequently transformed with pSEVA modulable plasmids (Silva-Rocha et al., 2013). These plasmids contain pBR101 and pRO1600 replication origins, respectively, and propagate in E. coli at lower and higher copy numbers, respectively (Silva-Rocha et al., 2013). floA was cloned into the control of a lac promoter in the plasmids pSEVA631 and pSEVA641 (10 µg/ml gentamicin) yielding FloA levels at lower and higher concentrations, respectively. Experiments that required the propagation of pSEVA vectors were performed in LB medium with 100 µg/ml ampicillin, 50 µg/ml kanamycin and 10 µg/ml gentamicin. Miller Units were quantified to monitor the efficiency of protein interactions, as described in (Miller, 1972).
Hemolysis activity
Hemolysin activity was tested using 24-h culture supernatants from the different strains. Heparinized sheep blood was washed with PBS pH 7.2. A 2% concentration of red blood cells was added to two-fold serially diluted culture supernatants in a microtiter plate. After 24 h incubation at room temperature, well plates were checked for cell blood lysis. Samples were collected and centrifuged at room temperature to pellet red blood cells. The supernatant was collected and the absorbance at 404 nm was measured as indication of the hemoglobin released into the supernatant due to the red blood cell lysis. Positive control was 1% Triton x-100.
Quantification and statistical analysis
Statistical analyses were performed using the software Prism® 6 (version 6.0f, GraphPad). Graphs represent data from three independent experiments. Each experiment includes three technical replicates. Error bars represent the standard error of the mean (mean + SD). To determine statistically significant variations between two groups, we performed parametric unpaired two-tailed Student’s t-test with Welch’s correction or non-parametric unpaired Mann-Whitney test. Comparisons between three or more groups were analyzed using the parametric one-way ANOVA test. Post hoc analysis included multiple comparisons Tukey's test, Dunnett’s test or Dunn’s tests, depending on the data set. Differences were considered significant when p value was smaller than 0.05. Statistical significance: ns = not statistically significant, * p<0.05, ** p<0.01, *** p<0.001.
Data and software availability
Quantification of fluorescence signal in fluorescence microscopy images has been performed using LAS Leica Application Suite (from Leica microsystems) (http://www.leica-microsystems.com/products/microscope-software/details/product/leica-las-x-ls/) and FiJi (from SciJava) (https://fiji.sc).
Supplementary Material
Highlights.
The membrane scaffold protein flotillin promotes protein oligomerization in S. aureus
Flotillin scaffolds degradosome oligomerization to upregulate virulence in S. aureus
Flotillin mutants show attenuated virulence in vitro and in vivo in infection models
Flotillin-inhibiting compounds limit infections by multi-drug-resistant S. aureus
Acknowledgments
GK received a postdoctoral fellowship from the FWF (Austria). This work was funded by ERC Starting Grant ERC335568 (European Union) and BFU2014-55601-P (MINECO, Spain) to DL. We thank C. Mark for editorial assistance.
Footnotes
Author contribution
Conceptualization, DL; Methodology, DL; Investigation, GK, CW, ICA, LK, STS, AY and DL; Writing – Original Draft, DL – Review & Editing, DL; Funding Acquisition, DL; Resources, DL; Supervision, GK and DL.
References
- Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins KL, Burman JD, Chamberlain ES, Cooper JE, Poutrel B, Bagby S, Jenkins AT, Feil EJ, van den Elsen JM. S. aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol Immunol. 2008;45:1600–1611. doi: 10.1016/j.molimm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86:525–532. doi: 10.1016/j.ejcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Bach JN, Bramkamp M. Flotillins functionally organize the bacterial membrane. Mol Microbiol. 2013;88:1205–1217. doi: 10.1111/mmi.12252. [DOI] [PubMed] [Google Scholar]
- Bandeiras C, Serro AP, Luzyanin K, Fernandes A, Saramago B. Anesthetics interacting with lipid rafts. Eur J Pharm Sci. 2013;48:153–165. doi: 10.1016/j.ejps.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Bandyra KJ, Bouvier M, Carpousis AJ, Luisi BF. The social fabric of the RNA degradosome. Biochim Biophys Acta. 2013;1829:514–522. doi: 10.1016/j.bbagrm.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Pelkmans L. A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Baxter A, Fitzgerald BJ, Hutson JL, McCarthy AD, Motteram JM, Ross BC, Sapra M, Snowden MA, Watson NS, Williams RJ, et al. Squalestatin 1, a potent inhibitor of squalene synthase, which lowers serum cholesterol in vivo. J Biol Chem. 1992;267:11705–11708. [PubMed] [Google Scholar]
- Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, VanMiddlesworth FL, Hensens OD, et al. Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc Natl Acad Sci U S A. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley BD, Chapman AM, McNaughton BR. Split-superpositive GFP reassembly is a fast, efficient, and robust method for detecting protein-protein interactions in vivo. Mol Biosyst. 2012;8:2036–2040. doi: 10.1039/c2mb25130b. [DOI] [PubMed] [Google Scholar]
- Blomberg P, Wagner EG, Nordstrom K. Control of replication of plasmid R1: the duplex between the antisense RNA, CopA, and its target, CopT, is processed specifically in vivo and in vitro by RNase III. EMBO J. 1990;9:2331–2340. doi: 10.1002/j.1460-2075.1990.tb07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramkamp M, Lopez D. Exploring the Existence of Lipid Rafts in Bacteria. Microbiol Mol Biol Rev. 2015;79:81–100. doi: 10.1128/MMBR.00036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Isolation and use of rafts. Curr Protoc Immunol. 2002;Chapter 11:Unit 11 10. doi: 10.1002/0471142735.im1110s51. [DOI] [PubMed] [Google Scholar]
- Bruckner R, Wagner E, Gotz F. Characterization of a sucrase gene from Staphylococcus xylosus. J Bacteriol. 1993;175:851–857. doi: 10.1128/jb.175.3.851-857.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantous S, Terwilliger TC, Waldo GS. Protein tagging and detection with engineered self-assembling fragments of green fluorescent protein. Nat Biotechnol. 2005;23:102–107. doi: 10.1038/nbt1044. [DOI] [PubMed] [Google Scholar]
- Callaghan AJ, Marcaida MJ, Stead JA, McDowall KJ, Scott WG, Luisi BF. Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature. 2005;437:1187–1191. doi: 10.1038/nature04084. [DOI] [PubMed] [Google Scholar]
- Castro BM, Fedorov A, Hornillos V, Delgado J, Acuna AU, Mollinedo F, Prieto M. Edelfosine and miltefosine effects on lipid raft properties: membrane biophysics in cell death by antitumor lipids. J Phys Chem B. 2013;117:7929–7940. doi: 10.1021/jp401407d. [DOI] [PubMed] [Google Scholar]
- Chapman SA, Asthagiri AR. Quantitative effect of scaffold abundance on signal propagation. Mol Syst Biol. 2009;5:313. doi: 10.1038/msb.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB. Site-specific protein labeling with SNAP-tags. Curr Protoc Protein Sci. 2013;73:Unit 30 31. doi: 10.1002/0471140864.ps3001s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sa MM, Sresht V, Rangel-Yagui CO, Blankschtein D. Understanding Miltefosine-Membrane Interactions Using Molecular Dynamics Simulations. Langmuir. 2015;31:4503–4512. doi: 10.1021/acs.langmuir.5b00178. [DOI] [PubMed] [Google Scholar]
- DeLoache WC, Dueber JE. Compartmentalizing metabolic pathways in organelles. Nat Biotechnol. 2013;31:320–321. doi: 10.1038/nbt.2549. [DOI] [PubMed] [Google Scholar]
- Dempwolff F, Moller HM, Graumann PL. Synthetic motility and cell shape defects associated with deletions of flotillin/reggie paralogs in Bacillus subtilis and interplay of these proteins with NfeD proteins. J Bacteriol. 2012;194:4652–4661. doi: 10.1128/JB.00910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempwolff F, Schmidt FK, Hervas AB, Stroh A, Rosch TC, Riese CN, Dersch S, Heimerl T, Lucena D, Hulsbusch N, et al. Super Resolution Fluorescence Microscopy and Tracking of Bacterial Flotillin (Reggie) Paralogs Provide Evidence for Defined-Sized Protein Microdomains within the Bacterial Membrane but Absence of Clusters Containing Detergent-Resistant Proteins. PLoS Genet. 2016;12:e1006116. doi: 10.1371/journal.pgen.1006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi SN, Vishnoi M, Kiehler B, Haggett L, Fujita M. In vivo functional characterization of the transmembrane histidine kinase KinC in Bacillus subtilis. Microbiology. 2015 doi: 10.1099/mic.0.000054. [DOI] [PubMed] [Google Scholar]
- Diekmann Y, Pereira-Leal JB. Evolution of intracellular compartmentalization. Biochem J. 2013;449:319–331. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- Dinic J, Biverstahl H, Maler L, Parmryd I. Laurdan and di-4-ANEPPDHQ do not respond to membrane-inserted peptides and are good probes for lipid packing. Biochim Biophys Acta. 2011;1808:298–306. doi: 10.1016/j.bbamem.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Donovan C, Bramkamp M. Characterization and subcellular localization of a bacterial flotillin homologue. Microbiology. 2009;155:1786–1799. doi: 10.1099/mic.0.025312-0. [DOI] [PubMed] [Google Scholar]
- Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KL, Keasling JD. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009;27:753–759. doi: 10.1038/nbt.1557. [DOI] [PubMed] [Google Scholar]
- Dugar G, Herbig A, Forstner KU, Heidrich N, Reinhardt R, Nieselt K, Sharma CM. High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple Campylobacter jejuni Isolates. PLoS Genet. 2013;9:e1003495. doi: 10.1371/journal.pgen.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Gilet L, Bessieres P, Nicolas P, Condon C. Three essential ribonucleases-RNase Y, J1, and III-control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet. 2012;8:e1002520. doi: 10.1371/journal.pgen.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie ES. Variation in the antigenic composition of staphylococcal coagulase. J Gen Microbiol. 1952;7:320–326. doi: 10.1099/00221287-7-3-4-320. [DOI] [PubMed] [Google Scholar]
- Faller R. Molecular modeling of lipid probes and their influence on the membrane. Biochim Biophys Acta. 2016;1858:2353–2361. doi: 10.1016/j.bbamem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Feng X, Hu Y, Zheng Y, Zhu W, Li K, Huang CH, Ko TP, Ren F, Chan HC, Nega M, et al. Structural and functional analysis of Bacillus subtilis YisP reveals a role of its product in biofilm production. Chem Biol. 2014;21:1557–1563. doi: 10.1016/j.chembiol.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- Gaus K, Zech T, Harder T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol Membr Biol. 2006;23:41–48. doi: 10.1080/09687860500466857. [DOI] [PubMed] [Google Scholar]
- Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammenos A, Mouithys-Mickalad A, Guelluy PH, Lismont M, Piel G, Hoebeke M. ESR technique for noninvasive way to quantify cyclodextrins effect on cell membranes. Biochem Biophys Res Commun. 2010;398:350–354. doi: 10.1016/j.bbrc.2010.06.050. [DOI] [PubMed] [Google Scholar]
- Heermann R, Weber A, Mayer B, Ott M, Hauser E, Gabriel G, Pirch T, Jung K. The universal stress protein UspC scaffolds the KdpD/KdpE signaling cascade of Escherichia coli under salt stress. J Mol Biol. 2009;386:134–148. doi: 10.1016/j.jmb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Jezek P, Bauer M, Trommer WE. EPR spectroscopy of 5-DOXYL-stearic acid bound to the mitochondrial uncoupling protein reveals its competitive displacement by alkylsulfonates in the channel and allosteric displacement by ATP. FEBS Lett. 1995;361:303–307. doi: 10.1016/0014-5793(95)00201-j. [DOI] [PubMed] [Google Scholar]
- Kaito C, Kurokawa K, Matsumoto Y, Terao Y, Kawabata S, Hamada S, Sekimizu K. Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol Microbiol. 2005;56:934–944. doi: 10.1111/j.1365-2958.2005.04596.x. [DOI] [PubMed] [Google Scholar]
- Kang SO, Caparon MG, Cho KH. Virulence gene regulation by CvfA, a putative RNase: the CvfA-enolase complex in Streptococcus pyogenes links nutritional stress, growth-phase control, and virulence gene expression. Infect Immun. 2010;78:2754–2767. doi: 10.1128/IAI.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash ME, Dzuba SA. Communication: Orientational self-ordering of spin-labeled cholesterol analogs in lipid bilayers in diluted conditions. J Chem Phys. 2014;141:211101. doi: 10.1063/1.4902897. [DOI] [PubMed] [Google Scholar]
- Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Klijn A, Moine D, Delley M, Mercenier A, Arigoni F, Pridmore RD. Construction of a reporter vector for the analysis of Bifidobacterium longum promoters. Appl Environ Microbiol. 2006;72:7401–7405. doi: 10.1128/AEM.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Komatsu T, Kamiya M, Campos C, Gonzalez-Gaitan M, Terai T, Hanaoka K, Nagano T, Urano Y. Highly activatable and environment-insensitive optical highlighters for selective spatiotemporal imaging of target proteins. J Am Chem Soc. 2012;134:11153–11160. doi: 10.1021/ja212125w. [DOI] [PubMed] [Google Scholar]
- Koch G, Nadal-Jimenez P, Cool RH, Quax WJ. Assessing Pseudomonas virulence with nonmammalian host: Galleria mellonella. Methods Mol Biol. 2014a;1149:681–688. doi: 10.1007/978-1-4939-0473-0_52. [DOI] [PubMed] [Google Scholar]
- Koch G, Nadal-Jimenez P, Reis CR, Muntendam R, Bokhove M, Melillo E, Dijkstra BW, Cool RH, Quax WJ. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc Natl Acad Sci U S A. 2014b;111:1568–1573. doi: 10.1073/pnas.1311263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Yepes A, Forstner KU, Wermser C, Stengel ST, Modamio J, Ohlsen K, Foster KR, Lopez D. Evolution of resistance to a last-resort antibiotic in Staphylococcus aureus via bacterial competition. Cell. 2014c;158:1060–1071. doi: 10.1016/j.cell.2014.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Kornblum J, Kreiswirth B, Projan SJ, Ross H, Novick RP. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick RP, editor. Molecular biology of the staphylococci. New York: VCH Publishers; 1990. [Google Scholar]
- Koslover DJ, Callaghan AJ, Marcaida MJ, Garman EF, Martick M, Scott WG, Luisi BF. The crystal structure of the Escherichia coli RNase E apoprotein and a mechanism for RNA degradation. Structure. 2008;16:1238–1244. doi: 10.1016/j.str.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B, Kornblum J, Arbeit RD, Eisner W, Maslow JN, McGeer A, Low DE, Novick RP. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol. 2015;16:232–244. doi: 10.1038/nrm3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Pontes MH, Groisman EA. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium's own F1Fo ATP synthase. Cell. 2013;154:146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnik-Habrink M, Lewis RJ, Mader U, Stulke J. RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases. Mol Microbiol. 2012;84:1005–1017. doi: 10.1111/j.1365-2958.2012.08072.x. [DOI] [PubMed] [Google Scholar]
- Lewis PJ, Marston AL. GFP vectors for controlled expression and dual labelling of protein fusions in Bacillus subtilis. Gene. 1999;227:101–110. doi: 10.1016/s0378-1119(98)00580-0. [DOI] [PubMed] [Google Scholar]
- Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- Lipinski B, Hawiger J, Jeljaszewicz J. Staphylococcal clumping with soluble fibrin mmonomer complexes. J Exp Med. 1967;126:979–988. doi: 10.1084/jem.126.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh JM, Adenwalla N, Wiles S, Proft T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence. 2013;4:419–428. doi: 10.4161/viru.24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–1902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GA. RNase E: at the interface of bacterial RNA processing and decay. Nat Rev Microbiol. 2013;11:45–57. doi: 10.1038/nrmicro2930. [DOI] [PubMed] [Google Scholar]
- Magliery TJ, Wilson CG, Pan W, Mishler D, Ghosh I, Hamilton AD, Regan L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J Am Chem Soc. 2005;127:146–157. doi: 10.1021/ja046699g. [DOI] [PubMed] [Google Scholar]
- Marincola G, Schafer T, Behler J, Bernhardt J, Ohlsen K, Goerke C, Wolz C. RNase Y of Staphylococcus aureus and its role in the activation of virulence genes. Mol Microbiol. 2012;85:817–832. doi: 10.1111/j.1365-2958.2012.08144.x. [DOI] [PubMed] [Google Scholar]
- Mielich-Süss B, Schneider J, Lopez D. Overproduction of flotillin influences cell differentiation and shape in Bacillus subtilis. MBio. 2013;4:e00719–00713. doi: 10.1128/mBio.00719-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. edn. Cold Spring Harbor N.Y: Cold Spring Harbor: Cold Spring Harbor Laboratories; 1972. [Google Scholar]
- Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Nagata M, Kaito C, Sekimizu K. Phosphodiesterase activity of CvfA is required for virulence in Staphylococcus aureus. J Biol Chem. 2008;283:2176–2184. doi: 10.1074/jbc.M705309200. [DOI] [PubMed] [Google Scholar]
- Otto GP, Nichols BJ. The roles of flotillin microdomains--endocytosis and beyond. J Cell Sci. 2011;124:3933–3940. doi: 10.1242/jcs.092015. [DOI] [PubMed] [Google Scholar]
- Oun S, Redder P, Didier JP, Francois P, Corvaglia AR, Buttazzoni E, Giraud C, Girard M, Schrenzel J, Linder P. The CshA DEAD-box RNA helicase is important for quorum sensing control in Staphylococcus aureus. RNA Biol. 2013;10:157–165. doi: 10.4161/rna.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redder P, Linder P. DEAD-box RNA helicases in gram-positive RNA decay. Methods Enzymol. 2012;511:369–383. doi: 10.1016/B978-0-12-396546-2.00017-6. [DOI] [PubMed] [Google Scholar]
- Reusch RN, Hiske TW, Sadoff HL. Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986;168:553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Milla E, Stuermer CA, Malaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63:343–357. doi: 10.1007/s00018-005-5434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux CM, DeMuth JP, Dunman PM. Characterization of components of the Staphylococcus aureus mRNA degradosome holoenzyme-like complex. J Bacteriol. 2011;193:5520–5526. doi: 10.1128/JB.05485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin L, Sjostrom JE, Lindberg M, Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974;118:155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Kudo K. Supramolecular control of split-GFP reassembly by conjugation of beta-cyclodextrin and coumarin units. J Am Chem Soc. 2008;130:9574–9582. doi: 10.1021/ja802313a. [DOI] [PubMed] [Google Scholar]
- Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94:441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Sawant P, Eissenberger K, Karier L, Mascher T, Bramkamp M. A dynamin-like protein involved in bacterial cell membrane surveillance under environmental stress. Environ Microbiol. 2016;8:2705–20. doi: 10.1111/1462-2920.13110. [DOI] [PubMed] [Google Scholar]
- Schneider J, Klein T, Mielich-Suss B, Koch G, Franke C, Kuipers OP, Kovacs AT, Sauer M, Lopez D. Spatio-temporal remodeling of functional membrane microdomains organizes the signaling networks of a bacterium. PLoS Genet. 2015a;11:e1005140. doi: 10.1371/journal.pgen.1005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Mielich-Suss B, Bohme R, Lopez D. In vivo characterization of the scaffold activity of flotillin on the membrane kinase KinC of Bacillus subtilis. Microbiology. 2015b;161:1871–1887. doi: 10.1099/mic.0.000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed KD, Dennis JJ. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect Immun. 2008;76:1267–1275. doi: 10.1128/IAI.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MB, Sehgal PB. Nondetergent isolation of rafts. Methods Mol Biol. 2007;398:21–28. doi: 10.1007/978-1-59745-513-8_3. [DOI] [PubMed] [Google Scholar]
- Silva-Rocha R, Martinez-Garcia E, Calles B, Chavarria M, Arce-Rodriguez A, de Las Heras A, Paez-Espino AD, Durante-Rodriguez G, Kim J, Nikel PI, et al. The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 2013;41:D666–675. doi: 10.1093/nar/gks1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Strahl H, Burmann F, Hamoen LW. The actin homologue MreB organizes the bacterial cell membrane. Nat Commun. 2014;5:3442. doi: 10.1038/ncomms4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl H, Turlan C, Khalid S, Bond PJ, Kebalo JM, Peyron P, Poljak L, Bouvier M, Hamoen L, Luisi BF, et al. Membrane recognition and dynamics of the RNA degradosome. PLoS Genet. 2015;11:e1004961. doi: 10.1371/journal.pgen.1004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuermer CA. Reggie/flotillin and the targeted delivery of cargo. J Neurochem. 2011;116:708–713. doi: 10.1111/j.1471-4159.2010.07007.x. [DOI] [PubMed] [Google Scholar]
- Swamy M, Siegers GM, Minguet S, Wollscheid B, Schamel WW. Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci STKE. 2006;2006:pl4. doi: 10.1126/stke.3452006pl4. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24:425–427. doi: 10.1016/s0968-0004(99)01467-x. [DOI] [PubMed] [Google Scholar]
- Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide signaling in the staphylococci. Chem Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Luit AH, Budde M, Ruurs P, Verheij M, van Blitterswijk WJ. Alkyl-lysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J Biol Chem. 2002;277:39541–39547. doi: 10.1074/jbc.M203176200. [DOI] [PubMed] [Google Scholar]
- Verhaar AP, Wildenberg ME, Peppelenbosch MP, Hommes DW, van den Brink GR. Repurposing miltefosine for the treatment of immune-mediated disease? J Pharmacol Exp Ther. 2014;350:189–195. doi: 10.1124/jpet.113.212654. [DOI] [PubMed] [Google Scholar]
- Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Magliery TJ, Regan L. Detecting protein-protein interactions with GFP-fragment reassembly. Nat Methods. 2004;1:255–262. doi: 10.1038/nmeth1204-255. [DOI] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- Wu F, Gaffney BJ. Dynamic behavior of fatty acid spin labels within a binding site of soybean lipoxygenase-1. Biochemistry. 2006;45:12510–12518. doi: 10.1021/bi061415l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes A, Koch G, Waldvogel A, Garcia-Betancur JC, Lopez D. Reconstruction of mreB expression in Staphylococcus aureus via a collection of new integrative plasmids. Appl Environ Microbiol. 2014;80:3868–3878. doi: 10.1128/AEM.00759-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes A, Schneider J, Mielich B, Koch G, Garcia-Betancur JC, Ramamurthi KS, Vlamakis H, Lopez D. The biofilm formation defect of a Bacillus subtilis flotillin-defective mutant involves the protease FtsH. Mol Microbiol. 2012;86:457–471. doi: 10.1111/j.1365-2958.2012.08205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhang J, Liu YS, Li L, He YL. Research advances on flotillins. Virol J. 2011;8:479. doi: 10.1186/1743-422X-8-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.