Abstract
Angiogenesis involves the formation of new blood vessels and is critical for fundamental events such as development and repair after injury. Perturbances in angiogenesis contribute to the pathogenesis of diverse clinical conditions including cancer, complications of diabetes mellitus, ischemia/reperfusion injury of the heart and other organs, and preeclampsia, as well as a number of inflammatory disorders. Recent work has identified heme oxygenase-1 and its gaseous product, carbon monoxide, to possess potent proangiogenic properties in addition to well-recognized antiinflammatory, antioxidant, and antiapoptotic effects. Angiogenic factors, such as vascular endothelial growth factor and stromal cell–derived factor-1, mediate their proangiogenic effects through induction of heme oxygenase-1, making it an attractive target for therapeutic intervention. This review will provide an overview of the role of heme oxygenase-1 and carbon monoxide in angiogenesis.
Keywords: angiogenesis, antioxidants, endothelium, vasculature, growth substances, hypoxia, nitric oxide
Carbon monoxide (CO) is a colorless, tasteless, and odorless gas that, when inhaled, enters the bloodstream and replaces the oxygen on hemoglobin to form carboxyhemoglobin. Increasing levels of carboxyhemoglobin can result in a wide range of symptoms from mild cognitive impairment, including reduction in visual perception and driving performance, to more severe effects like headache, weakness, gastrointestinal symptoms, and finally progressive confusion, collapse, and coma. Indeed, thoughts that come to mind when CO is being discussed relate mostly to accidental deaths from malfunctioning home appliances, suicides in closed garages, and assisted suicides. Although the toxicity of CO has been studied extensively, it is now also being explored for its physiological effects and potential therapeutic benefits.1 Since the realization that the poisonous gas nitric oxide (NO) has a significant biological role in physiology and pathophysiology, CO, which is a structurally similar gas, has gained much attention as a molecule with many analogous chemical and biological properties (Table 1). Like NO, CO is produced endogenously during cellular metabolism, primarily from the degradation of heme by the heme oxygenase (HO) enzyme system.2 Endogenous CO formation has been measured in several biological systems, and normal human adults have been shown to exhale ≈12 mL of CO per day.3 The major exogenous source for CO is generated from the incomplete burning of carbon from solid, liquid, and gaseous fuels. The predominant endogenous source of CO is through the degradation of heme via the HO enzyme system (Table 2).
Table 1.
Similarities Between NO and CO
| Features |
|---|
| Gaseous modulators Bind to hemoglobin Formed by inducible and constitutive isoforms Regulate intracellular cGMP Neurotransmitter actions Dose-dependent effects Effects on leukocyte and platelet function Vasodilatory actions |
Table 2.
Sources of CO
| Exogenous Automobile exhaust Cigarette smoke Unvented kerosene and gas space heaters Leaking chimneys and furnaces Gas water heaters, wood stoves, and fireplaces Endogenous Heme degradation via heme oxygenase Lipid peroxidation Photo-oxidation of organic compounds Auto-oxidation of phenols, flavonoids, and halomethanes |
HO catalyzes the rate-limiting step in heme degradation, leading to the generation of equimolar amounts of iron, biliverdin, and CO (Figure 1).2 Biliverdin is then converted to bilirubin by biliverdin reductase. HO exists in 2 distinct isoforms, an inducible (HO-1) and a constitutive form (HO-2). HO-1 is a 32-kDa protein that is highly upregulated in mammalian tissues by a wide variety of stimuli including heme, heavy metals, growth factors (eg, transforming growth factor-β [TGF-β], platelet-derived growth factor [PDGF], vascular endothelial growth factor [VEGF]), stromal cell–derived factor-1 (SDF-1), NO, peroxynitrite, modified lipids, hypoxia, hyperoxia, cytokines, and others.4 HO-2 is a 36-kDa protein that is constitutively expressed in distinct locations including in the brain, endothelium, and testis.
Figure 1.
HO enzymatic reaction. Heme (iron protoporphyrin IX) released from heme proteins is cleaved by HO to yield equimolar quantities of iron, CO, and biliverdin. Biliverdin is then converted to bilirubin by biliverdin reductase.
Although the role of HO in heme degradation was recognized by Tenhunen and colleagues5 in 1968, the protective properties of HO-1 were first demonstrated in an animal model of heme protein–induced kidney injury by Nath and colleagues6 in 1992. Previous in vitro studies had shown the remarkable inducibility of HO-1 by a wide range of oxidant stimuli in assorted cell types,7 but the functional significance of such induction was not reported. Subsequent work demonstrated that the induction of ferritin along with HO-1 contributed to the protective effects of HO-1 in endothelial cells in vitro.8 These pivotal studies played a major role in fostering an enormous expansion of the HO field, attracting numerous investigators from different areas to the study of this enzyme system. The products of HO-mediated heme degradation (biliverdin, bilirubin, CO, and ferrous iron) regulate important biological processes including oxidative stress, inflammation, apoptosis, cell proliferation, fibrosis, and angiogenesis. Several recent reviews and editorials have highlighted the biological effects of the reaction product(s) and the importance of HO-1 as a potent cytoprotective enzyme in diverse conditions.1,9–11 A list of vascular diseases associated with HO-1 and CO is shown in Table 3. The purpose of this article is to provide an overview of the role of HO-1 and CO in vascular biology, specifically as it relates to angiogenesis.
Table 3.
Vascular Disorders Associated With HO/CO
| Aneurysms Arteriovenous fistula restenosis Atherosclerosis Cancer Hypertension Impaired wound healing Ischemia/reperfusion injury Peripheral vascular disease Preeclampsia Psoriasis Sickle cell disease Transplant-related arteriosclerosis Vascular restenosis |
Angiogenesis and Vasculogenesis
The sprouting of endothelial cells from preexisting vessels along with their subsequent migration and proliferation for the generation of tubelike structures is termed angiogenesis. The de novo formation of blood vessels from bone marrow–derived precursor cells, a population that possesses great plasticity, is the process of vasculogenesis.12 Aside from its critical role in fetal development, vasculogenesis also has been shown recently to play a major role in adult neovascularization. Angiogenesis is necessary for the development of several physiological and pathological processes, including endometrial proliferation and placental development, wound healing, cancer, and postischemic repair. Studies by Asahara and colleagues13 have elaborated the versatility of bone marrow–derived endothelial precursors in neovascularization in ischemia, wound healing, and cancer. The mobilization of these potentially therapeutic cell populations from the bone marrow into circulation and further colonization at angiogenic sites are influenced by modulators including VEGF, SDF-1, and other factors.14
Physiological Angiogenesis
Constant exchange of nutrients, respiratory gases, and waste products across the placenta is critical for proper fetal growth and development and is primarily dependent on the exchange of blood between maternal and fetal tissues. The rate of blood flow is determined by angiogenesis in the placenta and is necessary for the development of viable offspring. The role of HO in pregnancy has been reviewed recently.15 In preeclamptic patients, HO-1 protein levels in the placenta and exhaled CO (an indicator of HO activity) are significantly lower than in healthy pregnant women.16,17 It has also been reported that women who smoke during their pregnancies have significantly less incidence of developing preeclampsia,18,19 suggesting that exposure to cigarette smoke, an exogenous source for CO, may be protective in preeclampsia.15,18 Recent studies showing that HO-1 and CO block the release of antiangiogenic mediators of preeclampsia, soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin (sEng), provide further evidence for a protective role of HO-1/CO in pregnancy.20
Tumor Angiogenesis
Several human tumors, including renal cell and prostate cancer, express high levels of HO-1.21,22 HO-1 may promote tumor cell survival,23 hindering the effectiveness of anticancer therapies.24 In contrast, inhibition of HO has been shown to enhance tumor regression in animal models,25 suggesting that the HO-1 pathway may be a therapeutic target in carcinogenesis. The balance of endothelial cell proliferation and apoptosis is critical in mediating tumor angiogenesis and affects growth, invasion, and metastasis of tumors. Several angiogenic factors promote angiogenesis as well as survival of endothelial cells. In addition, HO-1 and CO enhance endothelial cell survival through antiapoptotic mechanisms.26 VEGF promotes endothelial cell survival not only in embryonic vasculogenesis but also in tumor angiogenesis.27 In the initial stages of tumor growth, a process resembling chemotaxis occurs toward the already existing host vasculature before tumor angiogenesis.28 These vessels then regress because of apoptosis of the resident endothelial cells, followed by induction of specific signals, nutrient gradients, growth factors, and chemokines that are secreted by the host vessels. Proangiogenic bone marrow cells including subsets of hematopoietic cells, which are known to provide vascular support as well as endothelial progenitor cells (EPC), are known to differentiate into functional vascular cells, which contribute to tumor vasculature.
Recent evidence indicates that the chemokine SDF-1 has a major role in the recruitment and retention of CXCR4+ bone marrow–derived cells to the neoangiogenic niches, supporting revascularization of not only ischemic tissue but also areas of tumor growth.29 SDF-1 also promotes tumor cell growth, migration, and invasion and has profound effects on the tumor microenvironment. CXCR4, the receptor for SDF-1, is implicated in the cross talk between tumor cells and the tumor microenvironment. Inhibition of the SDF-1/CXCR4 axis attenuates tumor growth in vivo by inhibiting angiogenesis in a VEGF-independent manner.30
Wound Healing
The replacement of damaged capillaries and reestablishment of a steady supply of oxygen to a wound are accomplished by neovascularization. The phases in wound healing including a coagulation phase (characterized by endothelial dysfunction and platelet activation), early extracellular matrix deposition, release of factors by platelets, inflammatory phase, and the resulting granulation are all events that rely on angiogenesis.31 Growth factors including VEGF, chemokines like SDF-1, and hypoxia-inducible factors (HIFs) also coordinate the multifaceted events involved in wound healing.32,33 Interestingly, compared with wild-type littermate mice, HO-1–deficient mice exhibit impaired wound healing due, in part, to reduced recruitment of EPC and capillary formation at the site of injury.34 It would be of interest to examine the effects of CO in reversing the defective wound repair in HO-1 knockout mice.
Mediators of Angiogenesis
Proangiogenic chemokines, such as SDF-1, and growth factors, such as VEGF, are essential elements in angiogenesis in the context of ischemic injury. Ischemia results in an increase in SDF-1 levels that leads to increased EPC number and formation of new blood vessels in the injured tissue.35 Overexpression of SDF-1 in ischemic tissues enhances EPC recruitment from peripheral blood to induce neovascularization. Although an influential role of VEGF and a potential synergy between VEGF and SDF-1 in therapeutic neovascularization have been suggested, VEGF-independent effects have also been clearly demonstrated.36 Pathological retinal neovascularization associated with proliferative diabetic retinopathy results from an imbalance in proangiogenic and antiangiogenic factors such as VEGF and SDF-1 as well as changes in other chemokines, cytokines, adhesion molecules, and immune cells. Notably, HO-1–deficient EPC are unable to reendothelialize the retinal vasculature after ischemic injury compared with wild-type EPC.34
There is considerable evidence to support the inverse correlation between the number of circulating EPC and risk factors for atherosclerosis.37 In patients with diabetes mellitus, the circulating EPC number and the ability to form tubules correlate with glycemic control. Cytoskeletal alterations dictate the impaired mobility of EPC for the purpose of vascular repair in the diabetic milieu.38 The presence of vasodilators, such as NO, seems to reverse these defects38 and mobilizes this population into the circulation for repair. Defects in EPC function and signaling and peripheral tissue responses to hypoxia have also been shown to be associated with diabetes. Smoking has also been linked to decreased EPC numbers, and cessation of smoking reverses this phenomenon.39 EPC number also correlates with the extent of ischemia in stroke or myocardial infarction.37,40
The therapeutic efficacy of EPC as a mode of cell therapy has drawn much attention. Reports from preclinical studies indicate that transplantation of EPC improved not only neovascularization and blood flow recovery but also reduced limb necrosis in models of hind limb ischemia, even with subtherapeutic doses of EPC.41–43 Initial clinical trials testing the efficacy of EPC in patients with coronary artery disease reported promising results.37,40 However, more recent, larger randomized controlled studies have shown only modest short-term benefits in the setting of coronary artery disease.44 Whether these results could be improved by engineering EPC with protective genes such as HO-1 would be of potential interest.
Role of HO-1 and CO in Angiogenesis
The first link of HO-1 in angiogenesis was suggested by Abraham and coworkers,45 who showed that overexpression of HO-1 in endothelial cells enhanced their proliferation. Subsequent experiments confirmed that HO-1 promotes endothelial cell cycle progression.46 Inhibition of HO-1 by antisense strategies decreased endothelial cell proliferation and capillary formation in vitro, an effect that was associated with increase in p21 and p27, inhibitors of the cell cycle.46 The studies supporting a role for HO-1 in angiogenesis have shown that proangiogenic factors such as VEGF47,48 and, more recently, SDF-134 activate HO-1 expression in endothelial cells. Furthermore, local HO inhibition with zinc protoporphyrin blocks angiogenesis and tumor growth in vivo.25 In parallel, in vivo studies have linked enhanced expression of HO-1 in tumor-infiltrating macrophages to accentuated angiogenesis in human gliomas.49 Recent studies performed in vitro have shown that the proangiogenic properties of HO-1 are attributable to CO.46 The well-recognized vasodilatory, antiinflammatory, and antiapoptotic effects of CO also contribute to the potentiation of angiogenesis.1,26
Cross Talk Between NO and CO in Angiogenesis
NO is recognized as a mediator of angiogenesis. It induces the synthesis of VEGF and also potentiates its effect on endothelial cells.50–52 Production of VEGF in response to NO occurs in vascular smooth muscle cells overexpressing inducible NO synthase,51 and the effect is mimicked by NO donors.52 A similar influence of NO has been observed in tumor cells, keratinocytes, and several other cell types.53
Endogenous NO and NO donors are potent inducers of HO-1.54 It can therefore be hypothesized that HO-1 and its by-products are also involved in NO-dependent angiogenic events. Indeed, enhancement of VEGF expression in vascular smooth muscle cells treated with interleukin-1β is partially dependent on HO activity.51 In these cells, induction of inducible NO synthase was accompanied by increased expression of HO-1, and inhibition of HO activity attenuated the effect of interleukin-1β.55 Similar interactions have been demonstrated in a rat model of adjuvant arthritis, in which tin protoporphyrin IX (SnPPIX) attenuated NO-dependent VEGF production.56 Also in tumor cells, treatment with NO donors enhanced VEGF expression in an HO-1–dependent manner.57 Thus, HO-1 is a mediator of NO-induced VEGF synthesis in various cells.
VEGF and HO-1
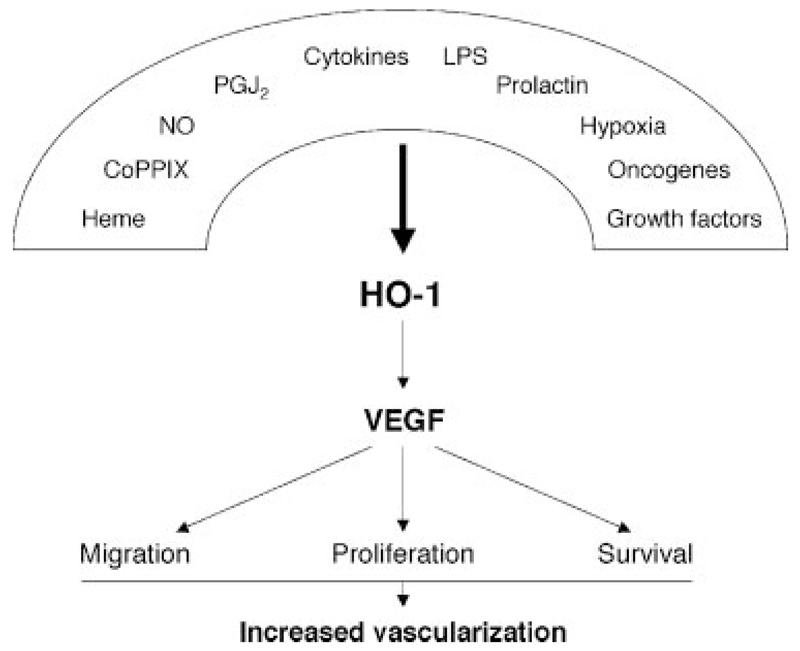
Treatment of cells by numerous activators of HO-1, such as heme, cobalt protoporphyrin, prostaglandin J2, hydrogen peroxide, NO, or hypoxia, induces VEGF expression in an HO-1–dependent manner in a variety of cell types (Figure 2). Treatment of macrophages with prolactin58 and endothelial cells with interleukin-659 also enhances VEGF expression in an HO-1–dependent manner. Accordingly, genetic overexpression of HO-1 leads to the stimulation of VEGF synthesis.55,59,60 The angiogenic effect of heme, the “classic” activator of HO-1 expression, can be considered in relation to conditions associated with release of large amounts of heme from damaged erythrocytes, eg, after tissue injury and in hemorrhagic tumors. In human HaCaT keratinocytes, short exposure to heme induces VEGF expression, but longer treatment attenuates its production, an effect probably related to the released iron.61 This observation may explain the discrepant results in some studies wherein no induction of VEGF on heme exposure has been reported.62,63
Figure 2.
Induction of VEGF synthesis and HO-1. Several mediators known to enhance VEGF expression exert their effects through HO-1. For hypoxia, the involvement of HO-1 can be cell-type dependent. LPS indicates lipopolysaccharide.
Potentially all 3 by-products of HO-1 activity can affect the synthesis of VEGF. In smooth muscle cells and human microvascular endothelial cells, CO, but not biliverdin or bilirubin, enhances VEGF synthesis.55 Treatment of vascular smooth muscle cells with 1% CO gas55 or endothelial cells with CORM-2,64 a CO-releasing molecule, induces VEGF production. Accordingly, inhibition of CO action, either by scavenging with oxyhemoglobin or by attenuation of soluble guanylyl cyclase activity, abolishes the stimulatory effect of prostaglandin J2 (PGJ2) on VEGF synthesis.60 HO-1 can exert its effect by stimulating transcription factors, such as nuclear factor-κB or activator protein-1,65 which regulate VEGF synthesis.
Induction of HO-1 expression by oxidative stress suggests that HO-1 can be particularly involved in the angiogenesis linked to inflammatory conditions. Indeed, the demonstration of enhanced vascularization in tumors overexpressing HO-1,23 the link between HO-1 and angiogenesis in rheumatoid arthritis,56 and the significance of HO-1 in wound healing34 confirm such a supposition. HO-1 induction can also be elicited by overexpression of other genes, particularly oncogenes. In an elegant study, Marinissen et al59 demonstrated that herpes virus-8–derived G-protein coupled receptor induces potent HO-1 upregulation and concomitant VEGF synthesis in fibroblast and endothelial cells. It would be worthwhile to investigate the involvement of HO-1 in mediating the angiogenic effects of other oncogenes.
HO-1 as a Downstream Mediator of Angiogenic Stimuli
The findings that NO is a mediator of both upstream VEGF synthesis and downstream response of endothelial cells to VEGF stimulation suggest that HO-1/CO can also be involved in a similar fashion. Indeed, stimulation of endothelial cells with VEGF in the presence of SnPPIX, a blocker of HO activity, attenuates their proliferation and differentiation in angiogenic assays in vitro.64 Endothelial cells from HO-1 knockout mice do not proliferate on VEGF stimulation.65 Conversely, overexpression of HO-1 enhances endothelial cell sprouting in response to VEGF exposure.64 The effect appears to be mediated by CO because cells treated with CORM-2 are more permissive to VEGF treatment.64
VEGF is capable of inducing HO-1 expression in endothelial cells.47,48 Compared with HO-1 inducers like hemin, the increase in HO-1 protein after exposure to VEGF is delayed and occurs after 24 to 48 hours in human umbilical vein endothelial cells and microvascular endothelial cells.47,48 In contrast, treatment with fibroblast growth factor-1 (acidic fibroblast growth factor) does not induce HO-1 expression in endothelial cells,47 although murine endothelial cells devoid of HO-1 are insensitive to fibroblast growth factor-2 (basic fibroblast growth factor) stimulation.65 Recent work by Siner and colleagues66 has shown that lung-specific overexpression of VEGF results in marked induction of HO-1 in the lung and is protective in an in vivo model of hyperoxia-induced lung injury through the HO-1 pathway. HO-1 has been also implicated in the regulation of synthesis and activity of other growth factors known to be involved in angiogenesis indirectly. For example, upregulation of HO-1 was observed in various cells on treatment with TGF-β,67 PDGF,68 hepatocyte growth factor,69 nerve growth factor,70 fibroblast growth factor-1, and fibroblast growth factor-2,71 although such an effect has not been demonstrated as yet in endothelial cells. It remains to be elucidated whether these growth factors, known to upregulate VEGF production, can do so via an HO-1–dependent mechanism. Induction of HO-1 in endothelial cells, resulting in increased proliferation, is observed on exposure to prolactin, which stimulates endothelial tube formation on Matrigel.72 Overexpression of thymidine phosphorylase or treatment with its catalytic product 2-deoxy-D-ribose-1 phosphate and downstream 2-deoxy-D-ribose promotes endothelial tubulogenesis in vitro in an HO-1–dependent manner.73
HO-1 in endothelial cells can also promote angiogenesis by attenuating the synthesis of antiangiogenic mediators. In a recent study, Cudmore et al20 demonstrated that adenoviral overexpression of HO-1 in endothelial cells diminished the production of antiangiogenic sFlt1 receptor and sEng in response to VEGF ligands. Mice deficient in HO-1 showed significantly higher levels of sFlt1 and sEng compared with wild-type mice.20 Both sFlt1 and sEng released from the placenta are key mediators in the pathogenesis of preeclampsia.74,75 The identification of HO-1 in suppressing the release of sFlt1 and sEng provides an exciting avenue for further investigation in pregnancy-related diseases.
SDF-1 and HO-1
Recent work has established a direct link between the proangiogenic effects of SDF-1 and HO-1.34 SDF-1 (also referred to as CXCL12) binds to a high-affinity receptor, CXCR4, and is the predominant chemokine that mobilizes hematopoietic stem cells and EPC to sites of injury and facilitates repair. The importance of SDF-1 in angiogenesis is highlighted by the fact that inactivation of SDF-1 or its receptor CXCR4 in mice leads to embryonic lethality due to abnormal vascular development.76,77 Exposure of human endothelial cells and EPC to nanomolar concentrations of SDF-1 results in a marked upregulation of HO-1 mRNA, protein, and enzyme activity.34 Pharmacological and genetic inhibition of HO-1 impairs SDF-1–mediated endothelial tube formation and capillary sprouting in aortic rings. A role for HO-1 in SDF-1–mediated angiogenesis was also confirmed in vivo with the use of Matrigel plug, wound healing, and retinal ischemia models. HO-1–deficient (HO-1–/–) endothelial cells and EPC show defective response in transwell migration assays toward an SDF-1 gradient. Because VEGF has been shown to induce HO-1 and SDF-1 can modulate VEGF levels, the role of VEGF in SDF-1–mediated HO-1 induction has also been explored. With the use of multiple lines of investigation, the results show that the induction of HO-1 and the proangiogenic effects of SDF-1 are VEGF independent.34 An in vivo retinal ischemia model was used to examine the role of HO-1 in SDF-1–mediated neovascularization. With the use of fluorescently labeled HO-1+/+ and HO-1–/– EPC, the homing and incorporation of EPC into acellular capillaries were investigated by intravitreal injection of EPC. In comparison to eyes injected with HO-1+/+ EPC, impaired migration as well as reduced incorporation of HO-1–/– EPC into the injured retinal vasculature was observed.34
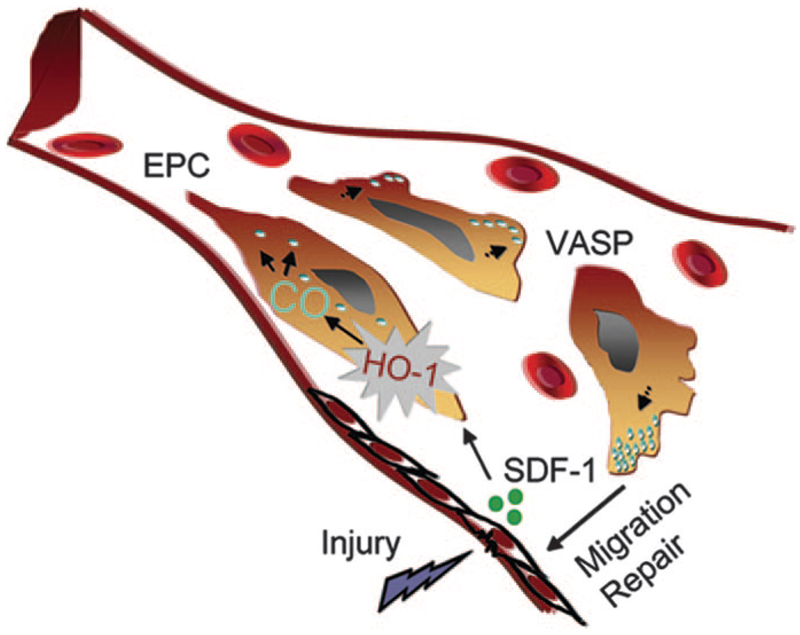
A mechanistic role for CO in promoting SDF-1–mediated postischemic neovascularization was also explored.34 The addition of CORM-2, but not bilirubin, restored responsiveness of HO-1–/– aortic rings to SDF-1, providing direct evidence for CO-dependent modulation of the effects of SDF-1. Because CO has been implicated to modestly activate soluble guanylate cyclase and affect downstream cellular targets, the phosphorylation status of vasodilator-stimulated phosphoprotein (VASP) at the protein kinase G preferred site at Ser 239 by SDF-1 and CORM-2 was investigated in HO-1+/+ and HO-1–/– endothelial cells. VASP is a cytoskeletal-associated protein that is abundant in microfilaments78 and is implicated in EPC migration.38 SDF-1–induced phosphorylation of VASP was dependent on HO-1 because no phosphorylation was observed in HO-1–/– cells.34 However, CORM-2 was able to phosphorylate VASP in the absence and presence of HO-1. Treatment of EPC with CORM-2 also resulted in a rapid redistribution of VASP to the filopodia (Figure 3). A hypothetical model for the mechanistic link between HO-1 and SDF-1 in vascular repair is shown in Figure 4. Other potential mechanisms including effects of CO on cell cycle regulatory proteins such as p21, cell proliferation, and recruitment of bone marrow–derived cells could also contribute to the SDF-1–mediated proangiogenic effects of HO-1 and need further exploration.
Figure 3.
Redistribution of VASP to filopodia in response to a CO donor. Human EPC isolated from peripheral blood were treated with vehicle (left) or tricarbonyl-dichlororuthenium (II) dimer (CORM-2, CO donor) (10 μmol/L) (right) for 15 minutes before fixation and immunocytochemistry. Green indicates VASP; blue, DAPI (nuclei). Scale bar=5 μm. We are grateful to Drs Maria Grant and Sergio Li Calzi, University of Florida, Gainesville, for providing this figure.
Figure 4.
Schematic of a blood vessel showing release of the chemokine SDF-1 at the site of injury. SDF-1 induces the heme-degrading enzyme HO-1 in EPC, resulting in the release of CO, which induces redistribution of VASP at the leading edge of EPC, promoting migration and vascular repair.
Cross Talk Between Hypoxia, Hypoxia-Inducible Factor-1, and HO-1 in Angiogenesis
HIF-1 is a transcriptional activator that is potently induced in hypoxia and drives the expression of >100 genes (reviewed in Pouyssegur et al79). Active HIF-1 consists of HIF-1α and HIF-1β, both of which are constitutively generated in cells; however, under normoxic conditions HIF-1α is immediately degraded. The process is dependent on hydroxylation of proline residues and is performed by a group of specific prolyl hydroxylases, oxygen, α-ketoglutarate, and iron-dependent enzymes.79 The capability to hydroxylate prolines disappears during low oxygen tension, resulting in the stabilization of the HIF-1α subunit. HIF-1 is a potent inducer of VEGF expression.79 Interestingly, the stability of HIF-1α can be increased in normoxia on exposure to NO80 or reactive oxygen species.81 HIF-1 is also linked to the upregulation of HO-1 gene expression. The hypoxia response element sequence has been found in the promoter of murine HO-1, but, interestingly, in many human cells, particularly endothelial, the expression of HO-1 is not induced by hypoxia, and even downregulation has been observed.82
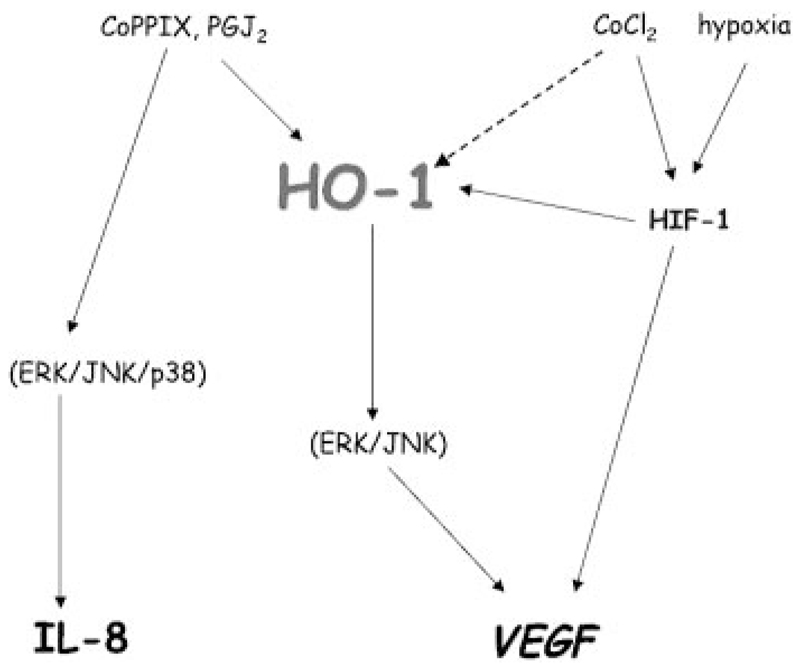
The stimulatory effect of hypoxia (1% oxygen) on VEGF in rat vascular smooth muscle cells can be reverted by inhibitors of HO activity.55 In human HaCaT keratinocytes, hypoxic induction of HIF upregulates VEGF in an HO-1–dependent manner, as the effect is blunted by inhibitors of HO-1 activity or HO-1 siRNA.61 The production of VEGF, induced by cobalt chloride, in human microvascular endothelial cells is dependent on reactive oxygen species–driven stabilization of HIF-1 but independent of HO-1, although cobalt chloride concomitantly induces HO-1 expression (Figure 5).83
Figure 5.
HO-1–dependent and –independent regulation of VEGF and IL-8 synthesis. In human microvascular endothelial cells, induction of VEGF synthesis by CoPPIX and PGJ2 is dependent on HO-1. However, the same stimuli enhance IL-8 expression independently of HO-1. Cobalt chloride (CoCl2), which increases HIF-1 stability, also strongly induces HO-1 and stimulates VEGF synthesis through HIF-1 but not HO-1. In these microvascular endothelial cells, hypoxia does not induce HO-1 but stimulates VEGF through HIF-1.
CO treatment can activate HIF-1 by inducing systemic hypoxia. However, in vitro studies show that hypoxic induction of HIF-1 is attenuated by CO.63 The same effect is exerted by NO,63 indicating that the influence of CO and NO on HIF can vary depending on the oxygen availability. Treatment with CO or NO in hypoxia attenuates VEGF expression in vascular smooth muscle cells,63 and CO also downregulates hypoxic induction of PDGF.84 Interestingly, induction of VEGF by hypoxia is also attenuated by PGJ2,85 which is a potent inducer of HO-1 expression via the Nrf2 transcription factor,86 and in normoxic conditions PGJ2 enhances VEGF expression in an HO-1–dependent manner.60 However, inhibition of VEGF production in hypoxia by PGJ2 is further attenuated by blocking of HO-1, suggesting that possibly other HO-1–independent pathways are responsible for the variable effects of PGJ2 under different oxygen tensions.
On exposure of human microvascular endothelial cells to cobalt protoporphyrin IX (CoPPIX), VEGF production is augmented in an HO-1–dependent manner, but HIF-1 transcription factor is not activated (Figure 5).83 In contrast, CO treatment stabilizes HIF-1 in murine macrophages.87 The effect is dependent on reactive oxygen species production by mitochondria and leads to increased synthesis of TGF-β. However, in another study, retroviral overexpression of HO-1 attenuates TGF-β production in rat endothelial cells, whereas it concomitantly upregulates VEGF,88 suggesting that the stimulatory effect of HO-1/CO on TGF-β may be cell-type specific.
Angiogenesis in HO-1 Deficiency
Animals lacking the functional HO-1 gene do not demonstrate any visible phenotype suggestive of defective angiogenesis. However, the impairment of pregnancy in HO-1–/– homozygotes and the significant mortality of HO-1–/– embryos89 suggest an effect of HO-1 on prenatal angiogenesis. It would be interesting to test whether CO could normalize pregnancy outcomes in HO-1–/– mice. The role of HO-1 in development is supported by observations showing that overexpression of HO-1 enhances VEGF in placenta, and an increased pup size is noted in such animals.90
The absence of HO-1 disrupts not only the production of VEGF on stimulation with H2O2, hemin, lysophosphatidylcholine, and PGJ291 but also the response of endothelial cells to angiogenic stimuli. The proliferation of murine endothelial cells stimulated with VEGF or fibroblast growth factor-2 is almost completely blunted by HO-1 inactivation.65 SDF-1–induced proliferation and migration of endothelial cells or EPC from HO-1–/– mice is also deranged.34 This defect is also seen in vivo because the formation of blood vessels during wound healing is impaired in HO-1 knockout mice.34 Diminished expression of HO-1 may also impede vascularization by increasing production of antiangiogenic mediators in vivo. In agreement with this are the recent findings that plasma of HO-1 knockout animals contains higher levels of sFlt1 and sEng than plasma of their wild-type counterparts.20
HO-1–Dependent and –Independent Regulation of Angiogenic Mediators
Data on the effect of HO-1 on the production of other angiogenic mediators are limited. Transcriptome analysis revealed several potential candidates in tumor cells overexpressing HO-1.23 The synthesis of interleukin-8 (IL-8), a member of the chemokine family with important roles in tumor growth, angiogenesis, and metastasis, can be regulated by HO-1. Accordingly, human umbilical vein endothelial cells treated with S-nitroso-penicillamine (SNAP), a NO donor, expressed HO-1 and produced more VEGF and IL-8.92 When HO-1 expression was attenuated by transfection of specific siRNA or antisense oligonucleotides, the production of VEGF and IL-8 was blunted. With the use of VEGF neutralizing antibodies, SNAP-induced IL-8 synthesis was attenuated, whereas IL-8 neutralizing antibodies had no effect on VEGF production. On the other hand, the production of IL-8 by human microvascular endothelial cells, induced by CoPPIX, a potent activator of HO-1, is independent of HO-1 induction.83 In addition, synthesis of VEGF induced by CoPPIX was abolished by SnPPIX, indicating the existence of HO-1–dependent and –independent pathways regulating the production of VEGF and IL-8, respectively (Figure 5). Furthermore, PGJ2 enhances VEGF synthesis in an HO-1–dependent manner,60 and the production of IL-8 is not blocked in the presence of inhibitors of HO activity (Figure 5).93
Monocyte chemotactic protein-1 is a mediator of inflammatory angiogenesis, stimulating macrophage recruitment and promoting inflammation in atherosclerosis, cancer, or rheumatoid arthritis.94 Interestingly, hemin induced monocyte chemotactic protein-1 expression in kidney proximal tubular epithelial cells through both HO-1–dependent and –independent pathways.95 Whether such a mechanism is relevant to the proangiogenic activity of monocyte chemotactic protein-1 remains to be established.
Dual Role of HO-1 in Angiogenesis
The role of HO-1 in angiogenesis may vary depending on the underlying conditions. Bussolati and coworkers47,96 demonstrated that VEGF-induced angiogenesis required HO-1 activity, whereas inflammation-induced blood vessel formation was attenuated by overexpression of HO-1. In lipopolysaccharide-induced angiogenesis, blood vessel formation is secondary to leukocyte infiltration, which can be attenuated by prior induction of HO-1 expression, which prevented leukocyte invasion into Matrigel plugs and subsequent angiogenesis. Conversely, in VEGF-induced noninflammatory angiogenesis, pharmacological inhibition of HO-1 induced marked leukocytic infiltration, which further enhanced VEGF-induced angiogenesis. However, blocking of HO-1 with interruption of leukocytic infiltration by anti-CD18 antibodies inhibited VEGF-induced angiogenesis.47,96 In addition, inhibition of HO enzyme activity with SnPPIX significantly decreased angiogenesis induced by agonistic antibodies against CD40. Thus, it is hypothesized that during chronic inflammation, HO-1 (1) inhibits leukocytic infiltration and (2) facilitates tissue repair by promoting VEGF-driven noninflammatory angiogenesis.96 Such an interaction may also operate in portal hypertensive rats, in which HO-1 attenuates oxidative stress and inflammation in the splanchnic circulation, whereas it concomitantly induces VEGF production.97 This complex interaction indicates that when HO-1 expression occurs in the environment free of the inflammatory reactions, the products of HO-1 activity can be proangiogenic. In contrast, when blood vessel formation is driven by lipopolysaccharide-induced inflammation, the expression of HO-1 may inhibit new vessel formation. However, in inflammatory conditions associated with cancer, rheumatoid arthritis, or wound healing, HO-1 may also be proangiogenic.
Pharmacological Modulation of HO-1 in Angiogenesis
HO-1 expression can be enhanced by numerous compounds, including several drugs that are in clinical use in cardiovascular diseases (eg, statins, rapamycin, erythropoietin, probucol).98–102 Upregulation of HO-1 by statins was observed in vascular smooth muscle cells and macrophages98,103 but not in endothelial cells.98,104 However, the physiological relevance of such an effect is not obvious because induction of HO-1 expression occurs particularly at high, micromolar concentrations of statins, which are not attained in patients (reviewed in Stocker and Perrella11 and Dulak and Jozkowicz105). Statins affect angiogenesis in a dual way, being proangiogenic at low, nanomolar concentrations and antiangiogenic at higher concentrations.106 However, higher doses of statins are also cytotoxic. This complicates the issue because pharmacological levels of these agents appear to not104 or modestly103 induce HO-1 in endothelial cells. Therefore, it remains to be established whether proangiogenic or antiangiogenic effects of statins via HO-1 operate in physiological conditions.
Neovascularization by HO-1 Gene and Cell Therapy
The beneficial effect of HO-1 gene transfer in vascular diseases in vivo has been linked to increased angiogenesis. In a rat hind limb ischemia model, adenoviral delivery of HO-1 enhances angiogenesis in the ischemic muscles through production of VEGF, an effect that is abrogated by inhibition of HO activity.107
Age-related and disease-linked impairment of EPC may be due to a loss of antioxidative defense. EPC express antioxidative enzymes, like catalase, glutathione peroxidase-1 (GPx-1), and manganese superoxide dismutase, which make them resistant to oxidative stress.108 Experimental overexpression of manganese superoxide dismutase109 enhances EPC protection, whereas knockout of GPx-1 gene diminishes viability and impairs vasculogenic potency of EPC.110 Our recent work has shown that the same may apply to HO-1 because the function of EPC in vitro and in vivo is impaired by the lack of HO-1.34 Therefore, it is possible that enhanced expression of HO-1 in EPC can improve neovascularization in postnatal vasculogenesis.
In a recent study, rabbit EPC, modified with retroviral vectors harboring either green fluorescent protein, endothelial NO synthase, or HO-1,111 were delivered to denuded arteries. As expected, the instillation of progenitor cells enhances the process of reendothelialization. Additionally, overexpression of endothelial NO synthase in EPC significantly improved endothelial regeneration in comparison to green fluorescent protein–transduced cells. HO-1 transduction, however, did not affect the capacity of EPC. The reason for such an insufficiency of HO-1 is not clear. Although HO enzyme activity and CO concentrations were not assessed in these studies, the authors suggest that the level of HO-1 expression may not have been sufficiently high to generate CO in amounts required to enhance endothelial cell proliferation.111 Hypoxia-regulated vectors harboring HO-1 have recently been used to modify murine mesenchymal stem cells. HO-1 overexpression enhances the tolerance of engrafted mesenchymal stem cells to hypoxia-reoxygenation injury in vitro and improves their viability in ischemic hearts.112 Further examination of engineered stem cells or EPC with HO-1 in models of angiogenesis in vivo would be of immense interest.
Perspectives and Therapeutic Implications
HO-1 is as an inducer of VEGF synthesis and is a downstream mediator of the activity of 2 major angiogenic growth factors, VEGF and SDF-1. HO-1 enhances endothelial cell and EPC proliferation, migration, and differentiation. The angiogenic response, in part, may also be dependent on the salutary effect of HO-1 on endothelial cell apoptosis. HO-1 induces the synthesis of VEGF, which in turn enhances HO-1 expression. Such a positive feedback can be of particular importance in EPC, which are known to be permissive not only to proangiogenic mediators but also are capable of generating large amounts of growth factors. It is possible that the presence of HO-1 in such cells can facilitate their survival in a noxious environment. Expression of HO-1 can protect EPC from oxidative injury, and it can also mediate EPC migration.34 Several of these HO-1–dependent processes are due, at least in part, to CO. The beneficial effects of CO as an inhaled gas or through the use of CO-releasing molecules have been demonstrated in cell culture and animal models of a number of diseases (reviewed by Wu and Wang,1 Durante et al,113 Kim et al,114 and Motterlini et al115). As the results of inhaled CO treatment from clinical trials116 become available, the utility of this therapy to facilitate angiogenesis in ischemia/reperfusion and wound healing will need to be established.
Interaction between HO-1 and angiogenesis, although crucial for proper wound healing and neovascularization of ischemic heart and peripheral muscles, will have obvious detrimental outcomes in diseases, in which new blood vessel formation is undesirable. Indeed, overexpression of HO-1 is linked to enhanced tumor neovascularization.23,57,59,117,118 Therefore, blocking HO activity can be considered an additional strategy to enhance the efficiency of antitumor therapy. Additionally, the role of HO-1 in lymphangiogenesis remains to be explored. These data are not as yet available, although 1 study demonstrated the lack of an effect of HO-1 on expression of VEGF-C,59 one of the VEGF receptors that regulates lymphatic vessel growth. Further studies should also elucidate the detailed mechanisms of induction of HO-1 expression by VEGF and SDF-1. More work is required to envision the role of HO-1 in the growth of atherosclerotic plaques. It remains to be established whether interactions between HO-1 and VEGF, and probably other growth factors, play any role in the progression of atherosclerosis.
Human HO-1 expression is dependent partially on a GT repeat length polymorphism in the proximal promoter.119 Epidemiological studies have linked long GT repeats (>30) with aggravation of human diseases, whereas short repeats (<25) provide a higher level of HO-1 expression in response to oxidative stress120 and have been claimed to protect against numerous pathologies, including restenosis, emphysema, and graft rejection (reviewed in Exner et al121). However, several recent studies in large populations have shown no effect of the GT repeat polymorphism in vascular restenosis and other disorders.122,123 It remains to be established whether such a polymorphism is of significance in terms of EPC function in patients with vascular diseases.
The role of HO-2, the constitutive isoform of HO, in angiogenesis is also emerging. A recent study demonstrated that in animals lacking HO-2, corneal wound closure was impaired, and this was characterized by enhanced neovascularization.124 Exaggerated inflammation, represented by an increased number of leukocytes, superoxide, cyclooxygenase-2 expression, and elevated levels of KC chemokine, was observed, whereas HO-1 was downregulated in the HO-2–deficient mice. It can be hypothesized that decreased expression of HO-1 in HO-2–/– animals is responsible for the aggravated inflammation-dependent angiogenesis, which appears to be downregulated by HO-1, supporting the dual role of HO-1 in angiogenesis.96
It is not known whether the lack of or a decrease in HO-1 expression can be linked to any human angiogenesis-related pathologies, although the natural silencing of HO-1 in the single human case involved endothelial cell injury.125 The real significance of the upregulation of HO-1 and CO in cardiovascular diseases remains to be assessed in human subjects. Is it merely a marker of an oxidative injury or a prerequisite mediator of endogenous protection against more severe insults? In the latter case, finding potent and safe pharmacological agent(s) may allow us to employ the HO-1/CO pathway for the prevention of disease initiation and progression and modulation of neovascularization in an effective way.
Acknowledgments
The authors regret that, because of space limitations, we have been unable to cite all the primary literature in the field. We are grateful to Maria Grant and Sergio Li Calzi for providing the data shown in Figure 3.
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL068157 and DK075532 and funds from the Juvenile Diabetes Research Foundation (to Dr Agarwal); by the Polish Ministry of Education and Science–Solicited research projects 107/P04/2004, 096/P05/2004, and N301 08032/3156; and by the European Vascular Genomics Network contract LSHM-CT 2003-503254 (to Dr Dulak). Dr Jozkowicz is an International Senior Research Fellow of the Wellcome Trust.
Footnotes
Disclosures
None.
Contributor Information
Jozef Dulak, Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics, and Biotechnology, Jagiellonian University, Krakow, Poland.
Jessy Deshane, Department of Medicine, Nephrology Research and Training Center, Center for Free Radical Biology, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham.
Alicja Jozkowicz, Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics, and Biotechnology, Jagiellonian University, Krakow, Poland.
Anupam Agarwal, Department of Medicine, Nephrology Research and Training Center, Center for Free Radical Biology, Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham.
References
- 1.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 2.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Coburn RF, Williams WJ, Forster RE. Effect of erythrocyte destruction on carbon monoxide production in man. J Clin Invest. 1964;43:1098–1103. doi: 10.1172/JCI104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 5.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 9.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 11.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 12.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90:315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge SA, Smith GN. HO in pregnancy. Free Radic Biol Med. 2005;38:979–988. doi: 10.1016/j.freeradbiomed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, Bussolati B, John J. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 17.Baum M, Schiff E, Kreiser D, Dennery PA, Stevenson DK, Rosenthal T, Seidman DS. End-tidal carbon monoxide measurements in women with pregnancy-induced hypertension and preeclampsia. Am J Obstet Gynecol. 2000;183:900–903. doi: 10.1067/mob.2000.109047. [DOI] [PubMed] [Google Scholar]
- 18.Bainbridge SA, Sidle EH, Smith GN. Direct placental effects of cigarette smoke protect women from pre-eclampsia: the specific roles of carbon monoxide and antioxidant systems in the placenta. Med Hypotheses. 2005;64:17–27. doi: 10.1016/j.mehy.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181:1026–1035. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 20.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 21.Goodman AI, Choudhury M, da Silva JL, Schwartzman ML, Abraham NG. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc Soc Exp Biol Med. 1997;214:54–61. doi: 10.3181/00379727-214-44069. [DOI] [PubMed] [Google Scholar]
- 22.Maines MD, Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: normal, hyperplastic, and tumor tissue distribution. Urology. 1996;47:727–733. doi: 10.1016/s0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 23.Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K, Ratajska A, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, Wilczynski GM, Glodkowska E, Mrowka P, Issat T, Dulak J, Jozkowicz A, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang J, Sawa T, Akaike T, Akuta T, Sahoo SK, Khaled G, Hamada A, Maeda H. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–3574. [PubMed] [Google Scholar]
- 26.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 28.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 29.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, Yamori T, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factorindependent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 31.Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg. 2005;39:293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 33.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 34.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 36.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 38.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 39.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 40.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease, part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 41.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi J-I, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell–derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 43.Iwaguro H, Yamaguchi J-I, Kalka C, Murasawa S, Masuda H, Hayashi S-I, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 44.Rosenzweig A. Cardiac cell therapy: mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 45.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 46.Volti GL, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, Abraham NG. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antiox Redox Signal. 2005;7:704–710. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 47.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 48.Dulak J, Loboda A, Zagorska A, Jozkowicz A. Complex role of heme oxygenase-1 in angiogenesis. Antiox Redox Signal. 2004;6:858–866. doi: 10.1089/ars.2004.6.858. [DOI] [PubMed] [Google Scholar]
- 49.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 50.Dulak J, Jozkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antiox Redox Signal. 2003;5:123–132. doi: 10.1089/152308603321223612. [DOI] [PubMed] [Google Scholar]
- 51.Dulak J, Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659–666. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- 52.Jozkowicz A, Cooke JP, Guevara I, Huk I, Funovics P, Pachinger O, Weidinger F, Dulak J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001;51:773–783. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 53.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50:49–59. [PubMed] [Google Scholar]
- 54.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 55.Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, Pachinger O, Weidinger F, Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antiox Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 56.Devesa I, Ferrandiz ML, Guillen I, Cerda JM, Alcaraz MJ. Potential role of heme oxygenase-1 in the progression of rat adjuvant arthritis. Lab Invest. 2005;85:34–44. doi: 10.1038/labinvest.3700205. [DOI] [PubMed] [Google Scholar]
- 57.Sasaki T, Yoshida K, Kondo H, Ohmori H, Kuniyasu H. Heme oxygenase-1 accelerates protumoral effects of nitric oxide in cancer cells. Virchows Arch. 2005;446:525–531. doi: 10.1007/s00428-005-1247-x. [DOI] [PubMed] [Google Scholar]
- 58.Malaguarnera L, Imbesi RM, Scuto A, D’Amico F, Licata F, Messina A, Sanfilippo S. Prolactin increases HO-1 expression and induces VEGF production in human macrophages. J Cell Biochem. 2004;93:197–206. doi: 10.1002/jcb.20167. [DOI] [PubMed] [Google Scholar]
- 59.Marinissen MJ, Tanos T, Bolos M, de Sagarra MR, Coso OA, Cuadrado A. Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor. J Biol Chem. 2006;281:11332–11346. doi: 10.1074/jbc.M512199200. [DOI] [PubMed] [Google Scholar]
- 60.Jozkowicz A, Huk I, Nigisch A, Weigel G, Weidinger F, Dulak J. Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antiox Redox Signal. 2002;4:577–585. doi: 10.1089/15230860260220076. [DOI] [PubMed] [Google Scholar]
- 61.Jazwa A, Loboda A, Golda S, Cisowski J, Szelag M, Zagorska A, Sroczynska P, Drukala J, Jozkowicz A, Dulak J. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006;40:1250–1263. doi: 10.1016/j.freeradbiomed.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eyssen-Hernandez R, Ladoux A, Frelin C. Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Lett. 1996;382:229–233. doi: 10.1016/0014-5793(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J Biol Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 64.Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antiox Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 65.Dulak J, Wegrzyn J, Cisowski J, Loboda A, Agarwal A, Jozkowicz A. Heme oxygenase-1 and angiogenesis: therapeutic implications. In: Otterbein LE, Zuckerbraun BS, editors. Heme Oxygenase: The Elegant Orchestration of its Products in Medicine. New York, NY: Nova Biomedicals Books; 2005. pp. 245–270. [Google Scholar]
- 66.Siner JM, Jiang G, Cohen ZI, Shan P, Zhang X, Lee CG, Elias JA, Lee PJ. VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 2007;21:1422–1432. doi: 10.1096/fj.06-6661com. [DOI] [PubMed] [Google Scholar]
- 67.Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem. 2000;275:40904–40909. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- 68.Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2666–2672. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 69.Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- 70.Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 71.Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–25579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- 72.Malaguarnera L, Pilastro MR, Quan S, Ghattas MH, Yang L, Mezentsev AV, Kushida T, Abraham NG, Kappas A. Significance of heme oxygenase in prolactin-mediated cell proliferation and angiogenesis in human endothelial cells. Int J Mol Med. 2002;10:433–440. [PubMed] [Google Scholar]
- 73.Sengupta S, Sellers LA, Matheson HB, Fan TP. Thymidine phosphorylase induces angiogenesis in vivo and in vitro: an evaluation of possible mechanisms. Br J Pharmacol. 2003;139:219–231. doi: 10.1038/sj.bjp.0705216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 76.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 77.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 78.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 79.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 80.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 81.Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-kappaB and HIF-1, two extremes. Free Radic Biol Med. 2002;33:1231–1242. doi: 10.1016/s0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- 82.Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, Hattori T, et al. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 83.Loboda A, Jazwa A, Wegiel B, Jozkowicz A, Dulak J. Heme oxygenase-1-dependent and -independent regulation of angiogenic genes expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol Biol (Noisy-le-grand) 2005;51:347–355. [PMC free article] [PubMed] [Google Scholar]
- 84.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jozkowicz A, Nigisch A, Wegrzyn J, Weigel G, Huk I, Dulak J. Opposite effects of prostaglandin-J2 on VEGF in normoxia and hypoxia: role of HIF-1. Biochem Biophys Res Commun. 2004;314:31–38. doi: 10.1016/j.bbrc.2003.12.059. [DOI] [PubMed] [Google Scholar]
- 86.Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antiox Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 87.Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdel-Aziz MT, el-Asmar MF, el-Miligy D, Atta H, Shaker O, Ghattas MH, Hosni H, Kamal N. Retrovirus-mediated human heme oxygenase-1 (HO-1) gene transfer into rat endothelial cells: the effect of HO-1 inducers on the expression of cytokines. Int J Biochem Cell Biol. 2003;35:324–332. doi: 10.1016/s1357-2725(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 89.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, Abraham N, Dennery PA. Heme oxygenase-1 modulates fetal growth in the rat. Lab Invest. 2002;82:687–692. doi: 10.1097/01.lab.0000017167.26718.f2. [DOI] [PubMed] [Google Scholar]
- 91.Cisowski J, Loboda A, Jozkowicz A, Chen S, Agarwal A, Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem Biophys Res Commun. 2005;326:670–676. doi: 10.1016/j.bbrc.2004.11.083. [DOI] [PubMed] [Google Scholar]
- 92.Pae HO, Oh GS, Choi BM, Kim YM, Chung HT. A molecular cascade showing nitric oxide-heme oxygenase-1-vascular endothelial growth factor-interleukin-8 sequence in human endothelial cells. Endocrinology. 2005;146:2229–2238. doi: 10.1210/en.2004-1431. [DOI] [PubMed] [Google Scholar]
- 93.Jozkowicz A, Dulak J, Prager M, Nanobashvili J, Nigisch A, Winter B, Weigel G, Huk I. Prostaglandin-J2 induces synthesis of interleukin-8 by endothelial cells in a PPAR-gamma-independent manner. Prostaglandins Other Lipid Mediat. 2001;66:165–177. doi: 10.1016/s0090-6980(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 94.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 95.Kanakiriya SK, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol. 2003;284:F546–F554. doi: 10.1152/ajprenal.00298.2002. [DOI] [PubMed] [Google Scholar]
- 96.Bussolati B, Mason JC. Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antiox Redox Signal. 2006;8:1153–1163. doi: 10.1089/ars.2006.8.1153. [DOI] [PubMed] [Google Scholar]
- 97.Angermayr B, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J, Fernandez M. Heme oxygenase attenuates oxidative stress and inflammation, and increases VEGF expression in portal hypertensive rats. J Hepatol. 2006;44:1033–1039. doi: 10.1016/j.jhep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 98.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 99.Visner GA, Lu F, Zhou H, Liu J, Kazemfar K, Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 100.Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Katavetin P, Inagi R, Miyata T, Shao J, Sassa R, Adler S, Eto N, Kato H, Fujita T, Nangaku M. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochem Biophys Res Commun. 2007;359:928–934. doi: 10.1016/j.bbrc.2007.05.207. [DOI] [PubMed] [Google Scholar]
- 102.Deng YM, Wu BJ, Witting PK, Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- 103.Chen JC, Huang KC, Lin WW. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell Signal. 2006;18:32–39. doi: 10.1016/j.cellsig.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 104.Loboda A, Jazwa A, Jozkowicz A, Dorosz J, Balla J, Molema G, Dulak J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis. 2006;187:26–30. doi: 10.1016/j.atherosclerosis.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dulak J, Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki M, Iso-o N, Takeshita S, Tsukamoto K, Mori I, Sato T, Ohno M, Nagai R, Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem Biophys Res Commun. 2003;302:138–143. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 108.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 109.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 110.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 112.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 113.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 115.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 116.Scott JR, Chin BY, Bilban MH, Otterbein LE. Restoring homeostasis: is heme oxygenase-1 ready for the clinic? Trends Pharmacol Sci. 2007;28:200–205. doi: 10.1016/j.tips.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 117.Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J Cancer. 2007;120:500–505. doi: 10.1002/ijc.22287. [DOI] [PubMed] [Google Scholar]
- 118.Sunamura M, Duda DG, Ghattas MH, Lozonschi L, Motoi F, Yamauchi J, Matsuno S, Shibahara S, Abraham NG. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6:15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 119.Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, Sasaki H, Higuchi S, Okita N, Takase S, et al. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet. 1997;100:145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- 120.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 121.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP. Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant. 2007;7:908–913. doi: 10.1111/j.1600-6143.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 123.Tiroch K, Koch W, von Beckerath N, Kastrati A, Schomig A. Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting. Eur Heart J. 2007;28:968–973. doi: 10.1093/eurheartj/ehm036. [DOI] [PubMed] [Google Scholar]
- 124.Seta F, Bellner L, Rezzani R, Regan RF, Dunn MW, Abraham NG, Gronert K, Laniado-Schwartzman M. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169:1612–1623. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]