Abstract
Biliverdin reductase (BVR) was known for a long time solely as an enzyme converting biliverdin to bilirubin, the major physiological antioxidant. Recent years revealed unique features of this protein which are not related to its reductase activity. The most intriguing and surprising finding is its dual-specificity kinase character. As such serine/threonine/tyrosine kinase BVR is involved in regulation of glucose metabolism or in control of cell growth and apoptosis. In consequence, it may play a role in pathogenesis of many diseases, such as diabetes or cancers. Moreover, in the nucleus BVR, being a leucine zipper-like DNA binding protein, can act as a transcription factor for activator protein 1 (AP-1)-regulated genes. It has been shown that BVR modulates ATF-2 and HO-1 expression, what suggests its potential role in control of AP-1 and cAMP-regulated genes. In conclusion, BVR together with its substrate, biliverdin, and product, bilirubin, are revealed to be important players in cellular signal transduction pathways, gene expression and oxidative response. These features make BVR unusually interesting and unique among all enzymes characterized to date.
Keywords: heme oxygenase 1, biliverdin reductase, antioxidant, dual-specificity kinase
Introduction
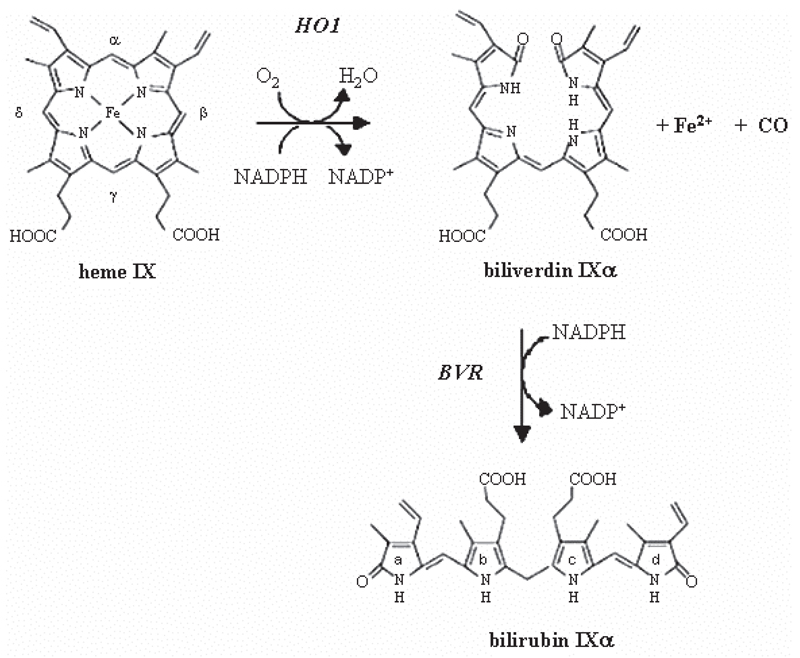
Synthesis of biliverdin is a prominent dimension of heme oxygenase (HO) system function in cellular defense mechanisms. HO catalyzes the rate-limiting step in heme (Fe-protoporphyrin IX) degradation resulting in the release of equimolar quantities of ferrous ion (Fe2+), carbon monoxide (CO), and biliverdin. The latter is reduced by biliverdin reductase (BVR) to bilirubin, the major physiological antioxidant [42, 62, 63] (Fig. 1). BVR was known for a long time solely as an enzyme converting biliverdin to bilirubin. However, recent years revealed new important features of this protein which are not related to its reductase activity. Amongst them dual-specificity kinase [40] and leucine zipper-like DNA binding protein [3] activities seem to be the most intriguing and significant. Owing to this BVR may take part in cellular processes of major importance: cell growth, apoptosis, oxidative response or gene expression.
Fig. 1.
Heme degradation by heme oxygenase system. α-Meso carbon bridge of the heme molecule is broken by heme oxygenase, forming biliverdin and liberating CO and iron. Biliverdin is further reduced to bilirubin by biliverdin reductase
Heme oxygenase
Three isoforms of HO have been reported so far [42, 46]. Amongst them HO-1 is highly inducible by heme itself and various other stimuli including nitric oxide (NO) or oxidative stress [2, 4, 6, 20, 65]. On the contrary, HO-2 is constitutively expressed [42]. Moreover, they are differentially regulated and expressed in tissues. HO-1 is ubiquitously induced in mammalian tissues and is localized to the endoplasmic reticulum, caveoli, and mitochondria, whereas HO-2 is expressed in the brain, endothelium, testes, distal nephron segments, and liver with subcellular localization in the mitochondria [1]. A third isoform HO-3 has been also described [46], but it has been later uncovered to be a pseudogene [25]. The most widely studied is HO-1, which has been reported as an important cytoprotective enzyme modulating tissue response to injury, while HO-2 regulates normal physiological cell activities. The anti-oxidant, anti-inflammatory and cytoprotective functions associated with HO-1 are attributable to removal of prooxidant heme and to its degradation products, which will be discussed below [15].
Iron
Iron is liberated during the breakdown of heme. Free iron is a prooxidant, mostly owing to its role in the Fenton reaction [24]. However, it has been shown that the induction of HO-1 is accompanied by upregulation of ferritin. Accordingly, ferritin limits pro-oxidative capacity of free iron and is hypothesized to be as advantageous as HO-1 induction [7, 32, 49].
Carbon monoxide (CO)
Carbon monoxide is a colorless, odorless gas that is liberated through incomplete combustion of organic compounds or by natural sources. The enzymatic activity of heme oxygenase accounts for > 85% of endogenous carbon monoxide, while the remaining amount arises during cellular metabolism. For a long time CO was solely considered to be a toxic air pollutant. Its harmfulness lies in strong affinity to hemoglobin and displacement of oxygen what results in tissue hypoxia. Although it is the major cause of CO-induced mortality, the cellular targets might be of importance as well [32, 57]. Noteworthy, augmented levels of exhaled CO are connected with several disorders such as asthma and diabetes [55, 71]. One may assume that increase in CO would be deleterious, however, there are experimental evidences which suggest cytoprotective effects of this gas in response to cellular stress. Although the toxicity that occurs following prolonged exposure to high concentrations of CO is undeniable, it is apparent that in physiological doses it exerts vasodilatory, anti-apoptotic, and antiinflammatory effects [32]. Such functions of CO are known to be partially mediated by several intracellular signal transduction pathways with guanylate cyclase and the mitogen activated protein kinases (MAPK) signaling being most prominent [57]. Firstly, it has been revealed that vasodilatory action of CO is mediated by binding to the moiety of soluble guanylyl cyclase (sGC), which in turn causes elevation of cyclic guanosine monophosphate (cGMP) and leads to vascular relaxation [56]. Cytoprotective action of CO has been reported to involve MAPK signaling pathways, namely p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) [37]. The important anti-inflammatory and anti-apoptotic effects of CO appear to be mediated through p38-dependent mechanisms [53, 73]. Thus, it is evident that CO is not merely an injurious byproduct of heme catabolism but that it serves a clear physiological function in cellular defense.
Biliverdin and bilirubin
The third product formed from heme by heme oxygenase is biliverdin-α (BVα), the linear tetrapyrrole molecule, which is one of four possible BV isoforms α, β, γ and δ [70]. Water-soluble and readily excreted biliverdin is reduced by biliverdin reductase, in energetically expensive process, to form bilirubin (BR). The latter is known as a toxic and insoluble pigment, which must be glucuronidated before being excreted in the bile. In spite of this, bilirubin performs important cellular functions being the major physiologic cytoprotectant [61]. Thus, BVR owing to production of BR seems to be one of the essential enzymes involved in response to oxidative stress.
Taken together, there are several processes, where the role of HO-1 is crucial and indispensable. Reasonably, most studies have considered it as a beneficial player in cardiovascular diseases thanks to its anti-oxidative, anti-inflammatory and pro-angiogenic activities [19, 69, 72]. Many reports, including ours, have demonstrated that increased HO-1 expression may upregulate synthesis of vascular endothelial growth factor (VEGF), the major angiogenic mediator [12, 18, 29]. Moreover, recent studies have shown that HO-1 is a mediator of the pro-angiogenic and pro-vasculogenic effects of stromal derived factor (SDF-1) [14]. The latter induced HO-1 in endothelial cells through a protein kinase (PKC) zeta-dependent mechanism. Involvement of HO-1 in SDF-1-induced processes has been confirmed in wound healing and retinal ischemia models in vivo [14]. These findings suggest new therapeutic possibilities in vascular repair.
On the other hand, HO-1 activation may play a role in carcinogenesis and influence the growth and metastasis of tumors [66]. The expression of HO-1 is often increased in tumors and can be further enhanced in response to therapies [30]. Accordingly, HO-1, possessing cytoprotective activities, has been reported to protect tumor cells against photodynamic, radio- and chemo-therapy-mediated cytotoxicity [11, 22, 51]. Thus, HO-1 may be considered as an enzyme facilitating tumor progression and its inhibition could be potential therapeutic approach sensitizing tumors to chemotherapy or radiation [30]. However, although the role of HO-1 seems to be pivotal for these processes, the enzyme tightly connected to HO, namely BVR, may be involved here as well.
Biliverdin reductase
BVR drives, in a powerful redox cycle, the conversion of BV to BR [8]. Thereby, it enables continous protection of cells against oxidative stress. But this is not a sole function of BVR. Recently the other fascinating features of the protein have been discovered and a wide spectrum of its diverse potential functions in cell signaling pathways have been suggested.
Structure
Biliverdin reductase has two forms of different molecular weight: A and B (BVR IXα and BVR IXβ), each of them with two isoforms. BVRA reacts most effectively with biliverdin IXα, whereas BVRB does not reduce BV-IXα at all. BVRB has been reported to be predominant during fetal development, while BVRA dominates in adult life [70].
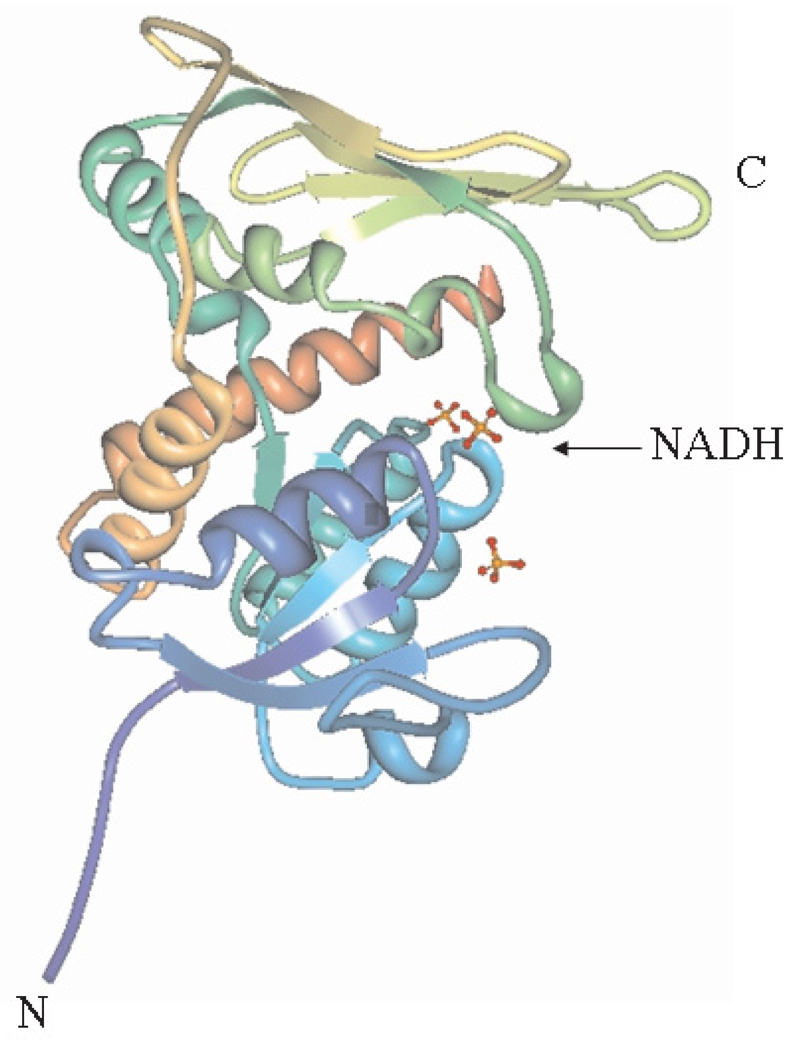
BVR is a monomeric protein, which consists of two structural domains: an N-terminal dinucleotide-binding domain (Rossmann-fold) and a C-terminal domain which possesses a six-stranded beta-sheet that is flanked on one face by alpha-helices (Fig. 2). Both domains take part in the formation of the active site cleft at their interface. To this sole substrate binding site, an inhibitor may be bound as well as a substrate [67].
Fig. 2.
Rat BVR-NADH enzyme-cofactor complex. Stereoview ribbon diagram (adapted from Protein Database). The chain termini are indicated by N and C. NADH is depicted as a ball-and-stick model
Human BVRA is encoded by a single copy gene consisting of five exons and four introns. The enzyme is composed of 296 amino acids what gives a mass of 33.5 kDa, while smaller BVRB found in fetus and in adult erytrocytes has 206 amino acids and a mass of 21 kDa [45]. Furthermore, BVRA is posttranslationally modified, what is reflected by several variants of BVR with different isoelectric points. They are heterogenous in molecular mass and appear to have two pH optima [27]. BVR is evolutionary conserved and, contrarywise to the common perception, not only found across metazoa, but a homolog of mammalian form is present in red algae and cyanobacteria as well [9, 59]. Considering mammalian species, the average sequence identity is greater than 80%. In such sequences several conserved key features are found. Among those are the leucine zipper (bzip) motif, adenine dinucleotide-binding motif, serine/threonine kinase domain, Src homology (SH2) binding domains (YMXM and YSLF), and Zn/metal-binding motif [21, 33, 41, 44]. These features are probably fundamental in the activities of BVR connected with signal transduction pathways.
Regulation of the activity
The reduction of biliverdin by the biliverdin reductase is coupled to the oxidation of pyridine nucleotide cofactors, NADH and NADPH, which are used at different pH optima: 6.7 and 8.7, respectively [35]. Thus, the reductase activity is NADH-dependent at acidic pH, whereas NADPH is used in the basic range [35]. The most likely NADH/NADPH binding site is the N-terminal Rossmann fold [67]. Having dual pH/dual cofactor requirement makes this oxidoreductase unique among all enzymes characterized to date.
It has been identified that BVR is a phosphoprotein and phosphorylation is essential to the reduction of biliverdin to bilirubin [58]. Furthermore, it has been reported that the enzyme needs to be autophosphorylated for its activity and that phosphorylation is reversible [58], what is another rare feature. This reversible phosphorylation is pH-dependent. The enzyme seems to be best adapted for both autokinase and reductase activity in an alkaline pH range (7.4 and 8.7). The phosphotransferase activity is nearly undetectable at pH 6.7, which corresponds to the strongest reductase activity in the acidic range. The biological significance of this finding is not fully understood to date. However, the reason may be the fact that in the acidic range the affinity of BVR for adenine nucleotides is decreased [58].
Role in oxidative stress response
Oxidative stress is a condition in the cell, where the balance between anti- and pro-oxidative compounds is disordered with the overbalance of the latter. Amongst pro-oxidative agents are mainly reactive oxygen species (ROS), including free radicals. The excessive production of reactive oxygen species may lead to cell damage (e.g. lipid peroxidation, mutagenesis) and in turn to cell death. Oxidative stress is responsible for development of many disorders such as cardiovascular diseases, diabetic complications, or cancers. There are many mechanisms defending cells from oxidative injury, e.g. antioxidants such as ascorbic acid and α-tocopherol, chelators of heavy metal ions (ferritin, transferrin), and enzymes such as superoxide dismutase, catalase, glutathione peroxidase. One of important pathway is also activity of BVR and production of BR [8].
BR is regarded as a major physiologic cytoprotectant. Stocker and coworkers, in a landmark study, revealed that BR possesses strong antioxidant potential against peroxyl radicals [61]. In fact, at physiological oxygen concentration, micromolar amounts of BR were able to scavenge peroxyl radicals more effectively than α-tocopherol, which had previously been considered the most powerful serum antioxidant [61]. Importantly, recent studies revealed that cellular depletion of BR by RNA interference against BVR markedly augments tissue levels of ROS and causes apoptotic cell death [8]. In spite of the low concentrations of BR in tissues [< 0.1% the levels of antioxidants such as glutathione (GSH)], it has been proven that this pigment protects neuronal cells from oxidative injury [16]. As little as 10 nM BR was shown to protect cells against 10,000-fold higher concentrations of hydrogen peroxide. Such enormous efficacy is possible because BR, oxidized to BV, is then recycled back to BR by BVR [8, 16]. Thus, BVR driving a powerful redox cycle, enables continuous protection of cells against oxidative stress [8], Furthermore, BR could protect the nuclear components against oxidative injury thanks to being an effective chain-breaking antioxidant [60] and an inhibitor of superoxide-producing NADPH oxidase [36].
BVR action is tightly connected to HO-1 and the direct link between oxidative stress responses mediated by these two enzymes have been convincingly confirmed. In 293A cells treated with small interference RNA (siRNA) for BVR or with antisense BVR, HO-1 protection against superoxide anion and arsenite was attenuated [47]. It proves that BVR is a key component in the HO-1 stress response pathway advancing the role of the latter in cytoprotection independent of heme degradation. Here, the activities of BVR as a kinase and transcription factor are probably involved [47].
Role of BVR in cell signaling: serine/threonine/tyrosine kinase activity
Several protein kinases are known as dual-specificity kinases and owing to that they are able to autophosphorylate on, or transfer phosphate to serine/threonine and tyrosine residues. They are called protein tyrosine kinases (PTKs) and are a multigenic family [28]. Interestingly, it has been uncovered that BVR has such a capacity [40] and it is unrelated to its reductase activity. The majority of tyrosine kinases are membrane bound, but there are also a few, including BVR, which are known to be soluble. Interestingly, it has been recently shown that BVR localized in part to plasma membrane caveolae in endothelial cells [31].
BVR in insulin/IGF signaling
One of the pathways modulated by BVR is cellular response to insulin or IFG-1 [41]. The function of insulin as a metabolic regulator and a growth factor is known to be PTK-dependent. Insulin/insulin like growth factor (IGF) actions are mediated through the activation of the insulin receptor (IR/IGFR). Activation of IR/IGFR is involved in many metabolic processes such as glucose uptake, and regulation of lipid or protein metabolism. It is also an important factor influencing proliferation and differentiation of cells [41].
Many insulin responses are mediated through the induction of insulin receptor substrates, IRS-1 and IRS-2 complexes. Such activation is achieved after insulin binding to the extracellular domain of the receptor and subsequent autophosphorylation on tyrosine residues of the intracellular kinase domain (insulin receptor tyrosine kinase-IRK). The coupling of receptor to its substrate is crucial for the initiation of the signaling cascade [48, 68]. It has been reported that IRS-1/2 undergoes multisite tyrosine phosphorylation and mediates downstream signals by docking various effector proteins that contain Src homology 2 (SH2) domains [48]. BVR possesses the same Y198MKM sequence as reported in IRS-1, which functions as a binding site for SH2 domain and this motif is predicted to be binding site for the phosphatidylinositol 3-kinase (PI3K) [48]. The latter most prominently acts in cell signaling, where it mediates the responses of Toll-like membrane glycoproteins and cytoplasmic 3-phosphoinositide-dependent kinases [41]. Furthermore, BVR has been shown to be a substrate for insulin receptor tyrosine kinase. The tyrosine phosphorylation sites for IRK are Y198MKM and Y228LSF motifs and tyrosine at position 291, while BVR autophosphorylates tyrosine residues at positions 72 and 83 [40].
In spite of being a substrate for IRK phosphorylation, BVR can also phosphorylates serine residues in IRS-1. Whereas tyrosine phosphorylation activates insulin signaling, serine phosphorylation blocks it. Serine phosphorylation of the IRS proteins diminishes the ability to interact with the receptor and to influence effector proteins. Thus, it has been considered as a mechanism for insulin resistance. Importantly, when expression of BVR is knocked down by siRNA, an increase in glucose uptake occurs in response to insulin [40]. Thus, cascade inhibition allows us to consider BVR as a negative regulator of glucose uptake.
In conclusion, BVR has been clearly defined as one of the dual-specificity (serine/threonine/tyrosine) kinases. As such it may play a role in the insulin-signaling pathway.
BVR in MAPK signaling
Two pathways of IR signaling are considered to be the most important: the mitogen activated protein kinases (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K) pathway. MAPK family consists of three important groups: extracellular signal-regulated kinases (ERK), JNK and p38. The first subfamily is known to be activated by phorbol esters and extracellular growth factors and play important role in cell differentiation and proliferation. The other two groups of kinases are predominantly affected by extracellular stress and cytokines. Thus, if MAPK signaling is influenced by BVR, it apparently plays a regulatory role in the stress conditions occurring in the cell [34, 37, 41, 47].
Role in gene expression: BVR as a transcription factor
Localization of BVR in the cell
Amongst the whole tyrosine kinase family, the majority of the members is connected with receptors in cell membranes. BVR is known to be one of the others, which are soluble and not receptor-associated. However, it has been recently found in the caveoli [31] and in the mitochondrial membrane as well [13]. Moreover, under some circumstances (activation/hyperphosphorylation) it can be translocated to the nucleus [43]. Such a relocalization enables BVR to influence gene expression, especially that it has been reported to be a member of the leucine zipper family of transcription factors [3].
Nuclear transport of cytosolic proteins across the nuclear membranes is a process which requires an intact nuclear localization signal, with basic residues (Gly-Leu-Lys-Arg-Asn-Arg) contained within the transported protein [23]. Importantly, such characteristic clusters are present in BVR within the carboxy terminal end of the reductase domain [43].
Moreover, it has been shown that BVR is induced and localizes into the nucleus in rat kidney cells in response to renal toxins: bacterial lipopolisaccharide (LPS) and bromobenzene, known as heme oxygenase-1 inducers [43]. However, the increase in nuclear fraction of BVR protein and activity was not accompanied by increased mRNA expression. That finding suggests post-transcriptional regulation of BVR by LPS and bromobenzene. That study has also revealed that nuclear localization of BVR is not exclusive to the rat kidney cells, but extends also to human cell lines such as HeLa [43].
Interestingly, it has been recently demonstrated in hepatic cells that biliverdin reductase is present in the inner mitochondrial membrane [13], where it might colocalize with HO-1. The targeting of HO-1 to the inner mitochondrial membrane is increased after hemin or LPS stimulation, suggesting that tightly controlled HO-1 import may be limited by the intrinsic translocon activity [50]. Owing to HO-1 localization in mitochondria, it may influence the expression of mitochondrial heme proteins such as cytochrome oxidase (COX) subunit I and nitric oxide synthase (NOS) resulting in a limitation of NO-dependent mitochondrial oxidants production. Thus HO-1 operates here on redox components by regulating heme availability, and in turn modulates mitochondrial O2 uptake and ROS production [13].
Biliverdin reductase DNA binding
BVR is a transcription factor of leucine zipper protein family, with typical dimerization domain in its primary structure. It contains the repeat of five leucines (L1-L5), which are separated by six other amino acids [38]. In some proteins the leucines may be substituted at different positions with other residues. For example, in BVR leucine L3 is replaced with lysine. The second element of dimerization domain is an invariant basic domain, which starts seven residues N-terminally to L1 and is flanked by alanine residues. The basic region is actually considered as the DNA binding domain [3, 38, 64]. Dimerization domain forms helix-turn-helix in its secondary structure [52, 64]. The presence of leucine zipper motif (bZip), makes proteins able to form functional homo- or heterodimers, and availability of the dimeric partners determines their preference for DNA binding sites.
BVR has been reported to form homodimer that binds to DNA with the involvement of the leucine repeat motif [3]. It appears that in the nucleus BVR, being a leucine zipper-like DNA-binding protein, can act as a transcription factor and may influence expression of several genes.
Role in HO-1 expression
DNA binding sites in BVR have been identified as two activator protein 1 (AP-1) recognition sequences [3]. Thus, BVR may play a role in the AP-1 pathway of cellular signaling. It is known that in stress conditions, AP-1 binds to multiple copies of consensus sequence (TGACTCA) in the HO-1 promoter [5]. Indeed, it has been reported that mutations in AP-1 binding sites inhibit HO-1 gene activation by oxidative stimuli [5, 39].
BVR could specifically bind to DNA of HO-1 promoter being a mediator of HO-1 upregulation in response to oxidative stress [3]. Furthermore, it has been shown that BVR binds not only to AP-1 but also to cyclic adenosine monophosphate (cAMP) response element (CRE) sites. Owing to such activity it increases the level of activating transcription factor (ATF)-2 and HO-1 expression, what suggests its potential role in regulation of AP-1 as well as cAMP-regulated genes [34].
AP-1 is the family of proteins which form homo- or heterodimers. Besides HO-1, AP-1 sites are found in the promoters of genes coding for many growth factors, cytokines or chemokines. Among known dimers of AP-1 proteins are: ATF-2 homodimer, which binds to CRE (TGACNTCA, N=any nucleotide); c-Jun/ATF-2 heterodimer, which binds to AP-1 site rather than the usual site of ATF-2 [10]; or c-Jun/c-Fos heterodimer, which affinity for AP-1 is lower and complex with the DNA is less stable comparing to c-Jun/ATF-2 [26]. c-Jun/c-Fos DNA binding is a key mechanism of HO-1 induction and occurs in turn of the activation of MAPK pathway. Also cAMP can be an inducer of HO-1 gene [20]. Accordingly, an increase in ATF-2 activity is suspected to have a direct effect on HO-1 expression. When ATF-2 level is increased it competes with c-Fos, the dimer partner of c-Jun and binds more effectively to AP-1 sites in HO-1 promoter [34]. ATF-2 also forms a dimer with nuclear factor κB (NFκB) transcription factor, which is involved in the activation of many proinflammatory mediators like growth factors, cytokines, and adhesion molecules [17]. Thus NFκB functions may be modulated by BVR activation of ATF-2 and this could be a therapeutic approach to the regulation of inflammatory processes [34].
Conclusions
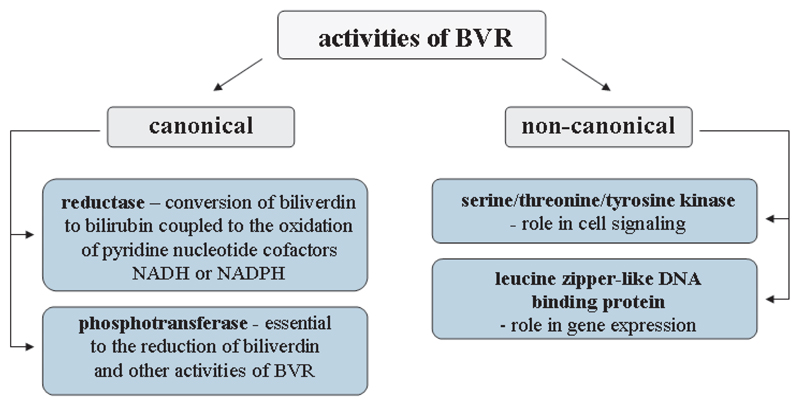
Although biliverdin reductase was considered for a long time solely as an enzyme converting biliverdin to BR, recent studies revealed different features of this enzyme, which are not related to its reductase activity (Fig. 3). Looking merely in its structure one could notice BVR uniqueness. Different, and highly conserved key sequences having more than 80% identity across mammalian species are probably fundamental for intensively investigated but still unclear role of BVR in signal transduction pathways. Possessing the leucine zipper (bzip) motif, adenine dinucleotide-binding motif, serine/threonine kinase domain, Src homology (SH2) binding domains, and Zn/metal-binding motif [21, 33, 41, 44] BVR is able to link heme metabolism, gene expression and cell signaling. Being a member of dual-specifity kinase family BVR could control glucose metabolism, cell growth and apoptosis, but also development of some diseases such as cancer and diabetes [41]. Moreover, in the nucleus BVR, being a leucine zipper-like DNA binding protein, can act as a transcription factor for activator protein 1 (AP-1) and cAMP-regulated genes modulating, among others, ATF-2 and HO-1 expression [3, 34]. Furthermore, BVR together with its product, BR, play an important role in response to oxidative stress [8] and their cytoprotective potential is tightly connected to HO-1 [47].
Fig. 3.
The major activities of biliverdin reductase
BVR is a constitutive enzyme and so far the data concerning its expression in tumor cells was limited. There is, however, a report that BVR is upregulated in kidney cancer cells [47]. If it would be a general mechanism in various tumors then the inhibition of BVR expression might be considered as an additional anti-cancer therapy. On the other hand overexpression of BVR in normal cells, obtained by gene transfer, may provide protection against the undesirable effects of anti-cancer drugs. For example, since chemotherapeutics like doxorubicin are known to induce cardiotoxicity [54], BVR overexpression may be considered as preventive strategy against heart injuries.
In conclusion, BVR together with its substrate biliverdin and product bilirubin, are important players in cellular signal transduction pathways, gene expression and oxidative response, which makes this protein not only attractive for basic research but also indicates for BVR as a potential therapeutic target.
Acknowledgments
We thank Mr Krzysztof Szade for help with graphic work. The work has been supported by the grant PBZ KBN 107/P04/2004 (to JD) and PBZ KBN 106 P05 07 (to AJ) from the Ministry of Science and Higher Education. Alicja Józkowicz is the International Senior Research Fellow of The Wellcome Trust.
Abbreviations
- AP-1
activator protein-1
- ATF-2
activating transcription factor 2
- BVR
biliverdin reductase
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CRE
cAMP response element
- ERK
extracellular signal-regulated kinase
- GSH
glutathione
- HO-1
heme oxygenase-1
- HO-2
heme oxygenase-2
- HO-3
heme oxygenase-3
- IGF
insulin-like growth factor
- IR/IGFR
insulin/insulin-like growth factor receptor
- IRK
insulin receptor tyrosine kinase
- IRS-1(2)
insulin receptor substrate 1(2)
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen activated protein kinase
- NADH
nicotinamide adenine dinucleotide
- NADP(H)
(reduced) nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PTK
protein tyrosine kinase
- ROS
reactive oxygen species
- SDF-1
stromal cell-derived factor 1
- sGC
guanylyl cyclase
- siRNA
small interference RNA
- VEGF
vascular endothelial growth factor
References
- 1.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Shiraishi F, Visner GA, Nick HS. Linoleyl hydroperoxide transcriptionally upregulates heme oxygenase-1 gene expression in human renal epithelial and aortic endothelial cells. J Am Soc Nephrol. 1998;9:1990–1997. doi: 10.1681/ASN.V9111990. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 4.Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. [PubMed] [Google Scholar]
- 5.Alam J, Zhining D. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- 6.Alcaraz MJ, Habib A, Creminon C, Vicente AM, Lebret M, Levy-Toledano S, Maclouf J. Heme oxygenase-1 induction by nitric oxide in raw 264.7 macrophages is upregulated by a cyclo-oxygenase-2 inhibitor. Biochim Biophys Acta. 2001;1526:13–16. doi: 10.1016/s0304-4165(01)00112-x. [DOI] [PubMed] [Google Scholar]
- 7.Balla G, Jacob HS, Balla J, Rosenburg M, Nath K, Apple F, Eaton JW, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 8.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: A major physiologic cytoprotectant. PNAS. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beale SI, Cornejo J. Enzymatic heme oxygenase activity in soluble extracts of the unicellular red alga, Cyanidium caldarium. Arch Biochem Biophys. 1984;235:371–384. doi: 10.1016/0003-9861(84)90210-8. [DOI] [PubMed] [Google Scholar]
- 10.Benbrook DM, Jones NC. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990;5:295–302. [PubMed] [Google Scholar]
- 11.Berberat PO, Dambrauskas Z, Gulbinas A, Giese T, Giese N, Kunzli B, Autschbach F, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11:3790–3798. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 12.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di-Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 13.Converso DP, Taille C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 14.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshane J, Wright M, Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 16.Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin formed by activation of heme oxygenase-2, protects neuron against oxidative stress injury. Proc Natl Acad Sci. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du W, Thanos D, Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 18.Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, et al. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 19.Dulak J, Loboda A, Zagorska A, Jozkowicz A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid Redox Signal. 2004;6:858–866. doi: 10.1089/ars.2004.6.858. [DOI] [PubMed] [Google Scholar]
- 20.Durante W, Christodoulides N, Cheng K, Peyton KJ, Sunahara RK, Schafer AI. cAMP induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Am J Physiol. 1997;273:317–323. doi: 10.1152/ajpheart.1997.273.1.H317. [DOI] [PubMed] [Google Scholar]
- 21.Fakhrai H, Maines MD. Expression and characterization of a cDNA for rat kidney biliverdin reductase. Evidence suggesting the liver and kidney enzymes are the same transcript product. J Biol Chem. 1992;267:4023–4029. [PubMed] [Google Scholar]
- 22.Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1–8. doi: 10.1002/ijc.11644. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Bustos J, Heitman J, Hall MN. Nuclear protein localization. Biochem Biophys Acta. 1991;1071:83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Chemistry of biologically important radicals. 3rd edn. Oxford Univ Press; New York: 1999. [Google Scholar]
- 25.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Herr I, van Dam H, Angel P. Binding of promoter-associated AP-1 is not altered during induction and subsequent repression of the c-jun promoter by TPA and UV irradiation. Carcinogenesis. 1994;15:1195–1113. doi: 10.1093/carcin/15.6.1105. [DOI] [PubMed] [Google Scholar]
- 27.Huang TJ, Trakshel GM, Maines MD. Detection of ten variants of biliverdin reductase in rat liver by two-dimensional gel electrophoresis. J Biol Chem. 1989;264:7844–7849. [PubMed] [Google Scholar]
- 28.Hunter T, Cooper JA. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- 29.Jozkowicz A, Huk I, Nigisch A, Weigel G, Weidinger F, Dulak J. Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxid Redox Signal. 2002;4:577–585. doi: 10.1089/15230860260220076. [DOI] [PubMed] [Google Scholar]
- 30.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase 1 in endothelial cells. FASEB J. 2004;19:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 32.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol. 2006;290:563–571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 33.Komuro A, Tobe T, Nakano Y, Yamaguchi T, Tomita M. Cloning and characterization of the cDNA encoding human biliverdin-IXα reductase. Biochim Biophys Acta. 1996;1309:89–99. doi: 10.1016/s0167-4781(96)00099-1. [DOI] [PubMed] [Google Scholar]
- 34.Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- 35.Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956–3962. [PubMed] [Google Scholar]
- 36.Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys Acta. 1991;1076:369–373. doi: 10.1016/0167-4838(91)90478-i. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 38.Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 39.Lee PJ, Camhi SL, Chin BY, Alam J, Choi AM. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:175–182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- 40.Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc Natl Acad Sci USA. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maines MD. New insights into biliverdin reductase functions: linking cell metabolism, to cell signaling. Physiology. 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 42.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 43.Maines MD, Ewing JF, Huang TJ, Panahian N. Nuclear localization of biliverdin reductase in the rat kidney: response to nephrotoxins that induce heme oxygenase-1. J Pharmacol ExpTher. 2001;296:1091–1097. [PubMed] [Google Scholar]
- 44.Maines MD, Polevoda BV, Huang TJ, McCoubrey WK., Jr Human biliverdin IXα reductase is a zinc-metalloprotein. Characterization of purified and Escherichia Coli expressed enzymes. Eur J Biochem. 1996;235:372–381. doi: 10.1111/j.1432-1033.1996.00372.x. [DOI] [PubMed] [Google Scholar]
- 45.McCoubrey WK, Jr, Cooklis MA, Maines MD. The structure, organization and differential expression of the rat gene encoding biliverdin reductase. Gene. 1995;160:235–240. doi: 10.1016/0378-1119(95)00112-j. [DOI] [PubMed] [Google Scholar]
- 46.McCoubrey WK, Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 47.Miralem T, Hu Z, Torno MD, Lelli KM, Maines MD. Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J Biol Chem. 2005;280:17084–17092. doi: 10.1074/jbc.M413121200. [DOI] [PubMed] [Google Scholar]
- 48.Myers MG, Jr, Sun XJ, White MF. The IRS-1 signaling system. Trends Biochem Sci. 1994;19:289–293. doi: 10.1016/0968-0004(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 49.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenburg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neupert W, Brunner M. The protein import motor of mitochondria. Nat Rev Mol Cell Biol. 2002;8:555–565. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- 51.Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, Wilczynski GM, Glodkowska E, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Shea E, Rutkowski R, Kim PS. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- 53.Otterbein LE, Otterbein SL, Ifedigbo E, Lui F, Morse DE, Fearns C, Ulevitch RJ. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxideinduced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Outomuro D, Grana DR, Azzato F, Milei J. Adriamycin-induced myocardial toxicity: New solutions for an old problem. Int J Cardiol. 2007;117:6–15. doi: 10.1016/j.ijcard.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Paredi P, Biernacki W, Invernizzi G, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest. 1999;116:1007–1111. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- 56.Ramos KS, Lin H, McGrath JJ. Modulation of cyclin guanosine monophosphate levels in cultured aortic smooth muscle cells by carbon monoxide. Biochem Pharmacol. 1989;38:1368–1370. doi: 10.1016/0006-2952(89)90347-x. [DOI] [PubMed] [Google Scholar]
- 57.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 58.Salim M, Brown-Kipphut BA, Maines MD. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem. 2001;276:10929–10934. doi: 10.1074/jbc.M010753200. [DOI] [PubMed] [Google Scholar]
- 59.Schluchter WM, Glazer AN. Characterization of cyanobacterial biliverdin reductase. Conversion of biliverdin to bilirubin is important for normal phycobiliprotein biosynthesis. J Biol Chem. 1997;272:13562–13569. doi: 10.1074/jbc.272.21.13562. [DOI] [PubMed] [Google Scholar]
- 60.Stocker R, Ames BN. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci, USA. 1987;84:8130–8134. doi: 10.1073/pnas.84.22.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 62.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci, USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenhunen R, Ross ME, Marver HS, Schmidt R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970;9:298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- 64.Vinson CR, Sigler PB, McKnight SL. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 65.Wagener, Fieldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1 and E-selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 66.Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitby FG, Philips JD, Hill CP, McCoubrey W, Maines MD. Crystal structure of a biliverdin-IX alpha reductase enzyme-cofactor complex. J Mol Biol. 2002;319:1199–1210. doi: 10.1016/S0022-2836(02)00383-2. [DOI] [PubMed] [Google Scholar]
- 68.White MF, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 2004;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 69.Wunder C, Potter RF. The heme oxygenase system:its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:199–208. doi: 10.2174/1568006033481410. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi T, Komoda Y, Nakajima H. Biliverdin-IX alpha reductase and biliverdin-IX beta reductase from human liver. Purification and characterization. J Biol Chem. 1994;269:24343–24348. [PubMed] [Google Scholar]
- 71.Yamara M, Sekizawa K, Ishizuka A, Monma M, Sasaki H. Exhaled carbon monoxide levels during treatment of acute asthma. Eur Respir J. 1999;12:757–760. doi: 10.1034/j.1399-3003.1999.13d10.x. [DOI] [PubMed] [Google Scholar]
- 72.Yao P, Li K, Song F, Zhou S, Sun X, Zhang X, Nussler AK, et al. Heme oxygenase-1 upregulated by Ginkgo biloba extract: potential protection against ethanol-iduced oxidative liver damage. Food Chem Toxicol. 2007;45:1333–1342. doi: 10.1016/j.fct.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AMK, et al. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 MAPK pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]