Abstract
Chronic hepatitis C infection is a systemic disease that leads to a high risk of cirrhosis and hepatic carcinoma, as well as extrahepatic related disorders, immune–related and metabolic alterations such as glucose metabolism impairment and steatosis, thus being a new cardio-metabolic risk factor. It has been shown that, due to chronic inflammation, HCV infection has a direct effect on the arterial wall, initiating endothelial dysfunction which is the first step in atherosclerotic processes with proatherogenic effects and numerous cardiovascular events. The recent data emphasize that HCV infection can induce insulin resistance in the liver and peripheral tissues through multiple mechanisms which interfere with insulin signaling, inducing the production of several proinflammatory cytokines, and modify the lipid metabolism with the result of hepatic steatosis, which is more pronounced in patients with HCV. The emergence of new direct acting, interferon-free antiviral treatment, leading to HCV cure in most cases with a satisfactory safety profile is, according to numerous studies, improving the glucose metabolism disorders and lowering the number of cardiovascular events in patients who obtained sustained viral response, thiugh further studies are needed to clarify definitively the role of HCV infection in cardiovascular and metabolic alterations, as well as the impact of viral eradication on cardiovascular outcomes.
Keywords: hepatitis C virus, cardiometabolic risk, insulin resistance
Chronic hepatitis caused by hepatitis C virus (HCV) is a major health problem, affecting about 170 million people globally. This condition causes over 100,000 deaths annually and is associated with high social costs related to the management of liver disease and treatment of extra hepatic manifestations [1].
So far, about 30 diseases secondary to the extra hepatic manifestations or its consequences have been documented. The mechanisms by which the virus has the ability to act outside the liver parenchyma are either immune-mediated, by producing cryoglobulins with multiple pathological implications (vascular, glomerulonephritis, B-cell non-Hodgkin lymphoma), or through indirect mechanisms that cause insulin resistance (IR), diabetes and cerebrovascular and cardiovascular disease (stroke, myocardial infarction). Due to the increased efficacy of direct-acting antiviral and high rate of viral eradication, researchers’ attention has been lately focused on HCV-related extra hepatic complications. The metabolic dysfunctions and damage to the cardiovascular system caused by chronic hepatitis C are still a priority for research. The reversibility of established lesions after viral eradication is still unknown, the cardiovascular risk resulting from the summed pathological processes at the molecular level [2].
Besides the classic cardiovascular risk factors (age, sex, lipid metabolism disorders, smoking, sedentary lifestyle, obesity, diabetes and hypertension), chronic HCV infection is considered in the literature an independent risk factor for cardiovascular disorders, both through the systemic inflammatory state and a series of indirect mechanisms.
Currently it is known that chronic HCV infection is a chronic systemic inflammatory state and a possible first step of lipid accumulation in the arterial wall that triggers the process of atherosclerosis after endothelial dysfunction has been initiated. Starting from this premise, many researchers studied the close relationship between HCV and cardiovascular system damage, the possibly accelerated atherosclerosis in the entire arterial system and mortality associated with liver disease [1]. A number of studies have shown a high mortality rate among these patients compared to uninfected population due to the high prevalence of type II diabetes mellitus, increased peripheral IR and association with hepatic steatosis [3].
The aim of this review is to present some particular aspects of cerebrovascular and metabolic damage in patients with chronic hepatitis C.
Chronic HCV infection: endothelial dysfunction and atherosclerosis
Endothelial dysfunction is the first step in the process of atherosclerosis, which consists of ischemia, insufficient vasodilator response, activation of procoagulant factors and increased proinflammatory activity.
Chronic viral activity causes significant changes in arterial endothelium by the increased oxidative stress and decreased nitric oxide activity, the most important mediator in endothelial function [4].
The early HCV-induced changes in the arterial endothelium currently investigated by different imaging methods have been shown to be present before the morphological lesions of atherosclerosis, thus representing an early predictor of cardiovascular disorder [5].
Atherosclerosis is a progressive process that involves monocyte infiltration in the artery wall with the formation of atherosclerotic plaques. The monocytes involved in plaque formation are differentiated into macrophages and foam cells, and the proteases and inflammatory molecules released from dying macrophages in the necrotic core contribute to plaque destabilization and occurrence of coronary events. It seems that the macrophages involved in the process of atherosclerosis can adopt various phenotypes, M1 macrophages being thought to be mainly associated with inflammatory processes, while M2 macrophages form fibrous plaques with a lower risk of rupture [6].
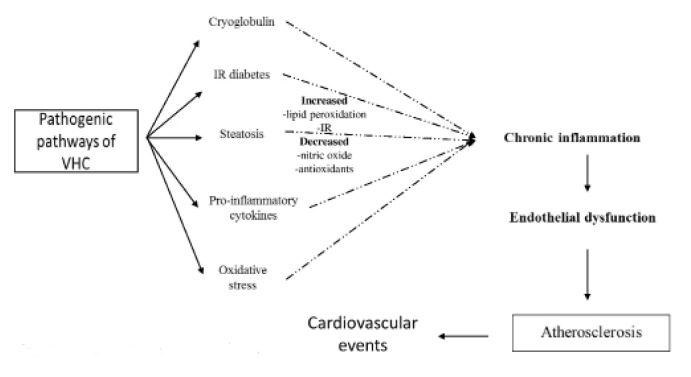
Current pathogenic hypotheses implicate the inflammation caused by persistent HCV infection in the genesis of atherosclerotic plaque, which shows the characteristics of a chronic inflammatory reaction: monocyte infiltration, proliferation of mesenchymal cells, connective tissue sclerosis, neoformation vessels [7]. Among inflammatory changes other processes including steatosis, IR, oxidative stress, intracellular adhesion molecules contribute to endothelial dysfunction and formation of atherosclerotic plaques (Figure 1).
Figure 1.
Pathogenic pathways of HCV in atherosclerosis.
In the process of atherosclerosis the macrophage phenotype is involved in increasing the number of coronary events among patients with chronic HCV infection. To highlight this association a recent study used macrophage markers (sCD163, sCD14, Gal-3BP) with specificity in the aggregation and activation of these cells; the immunohistochemical analyses demonstrated that patients with chronic HCV infection had higher sCD163 levels, indicating the presence of subclinical atherosclerosis and a higher proatherogenic risk in these patients [6].
Chronic inflammation caused by HCV plays an essential role in the proatherogenic effect attributed to the activity of virus, the circulating proinflammatory cytokines and adhesion molecules being directly involved in the genesis of atherosclerotic plaques. Numerous studies have supported the hypothesis of a direct action of HCV on the process of atherosclerosis, reason why it is thought to be an independent factor of cardiovascular disease [7].
The atherosclerotic process may be also accelerated by the association of noninsulin dependent diabetes mellitus, a new concept in numerous studies, which in close association with the change in lipid profile and presence of hypobetalipoproteinemia lead to arterial plaque occurrence.
According to recent studies, about 33% of patients with chronic HCV infection develop insulin-resistant diabetes, IR being the result of disturbances in carbohydrate metabolism and change in lipid profile. HCV interacts with numerous intracellular signaling proteins disturbing a series of processes at this level that lead to the modification of gene transcription, stimulation of apoptosis and immune system. Through its action HCV suppresses the activity of peroxisome proliferator-activated receptor (PPAR) considered responsible for the activity of genes involved in lipid degradation. Following the molecular analyses in patients chronically infected with HCV a lower level of PPAR and microsomal triglyceride transfer proteins was demonstrated, the biological outcome being their accumulation in the liver cells and an increased atherogenic risk [8].
HCV also uses other indirect immune mechanisms involved in cardiovascular disease through serum cryoglobulins responsible for the development of morphological lesions revealed by modern imaging methods.
Today, numerous non-invasive tests are used in research but also in practice to document and demonstrate the proatherogenic effect of HCV. Measurement of intima-media thickness is currently considered a true predictor of subclinical atherosclerosis and an independent predictor of stroke. By the use of other tests of greater complexity, such as computed tomography, brachial artery flow-mediated dilation, pulse wave velocity, the early atherosclerotic changes could be demonstrated, being frequently used in calculating the cardiovascular risk in patients with chronic HCV infection [9–12].
Many researchers, among which Ishizaki et al., have assessed the cardiovascular risk by calculating the carotid intima-media thickness in the absence of morphological lesions and concluded in the presence of the same risk factors that patients with chronic HCV infection were more predisposed to arterial atherosclerosis and thus to a greater number coronary events compared to the general population (Table I).
Table I.
Studies with a positive association between HCV and atherosclerosis.
| Author, year, country | Study design | Study cohort | Method of assessing atherosclerosis |
|---|---|---|---|
| Petta et al, 2012 Italy [13] | Case control | 174 infected/174 controls | IMT measurement ultrasonography |
| Adinolfi et al, 2012 Italy [14] | Case control | 326 HCV infected/477 matched controls | IMT measurement |
| Boddi et al, 2007 Italy [15] | Cohort study | 31 HCV infected/120 matched controls | IMT measurement Ultrasonography ARN HCV in carotid plaques |
| Barone et al, 2015 Italy [4] | Cohort study | 380/45 HCV infected | IMT measurement Ultrasonography |
| Ishizaka et al, 2012 Japan [16] | Case control | 4784/104 HCV infected | IMT measurement Ultrasonography |
Chronic HCV infection and stroke
Stroke is the second leading cause of death globally with approximately 11.13% of deaths annually. Investigation of multiple risk factors for stroke, chronic HCV infection included, led to a better understanding of the changes induced by the chronic viral infection in cerebral arteries.
The results of recent studies confirm the involvement of chronic HCV infection in the pathophysiology of stroke both through endothelial dysfunction and a series of mechanisms not fully understood yet. In a study conducted in Taiwan 4094 patients with chronic HCV infection were comparatively analyzed with 16,376 age-matched controls without this disease. It was found that the risk of atherosclerosis among the HCV-infected patients was 1.27 times higher, thus suggesting that this viral infection may be considered a risk factor for stroke. Data from other studies indicate that antiviral treatment reduces by up to 60% the incidence of stroke in the treated patients, thus a new correlation between HCV and stroke occurrence among these patients being detected. The cumulative aggressive effect of HCV infection on carotid system is revealed by studies on carotid intima-media thickness, considered as a negative predictor of cardiovascular events. Vascular Doppler measurements showed a higher intima-media index among HCV-infected patients compared to the uninfected ones, the studies aiming to establish these correlations among patients with the same global cardiovascular risk [17].
The presence of anticardiolipin antibodies, atherosclerotic lesions and type 2 diabetes mellitus in close association with the HCV-induced inflammatory and immunological state made that the incidence of acute cerebrovascular events to be higher among these patients, and viral load seems to influence the morphology and extent of ischemic lesions [18–20]. Comparative assessment of infected/uninfected population showed that HCV-infected patients with a higher HCV RNA levels were more severely affected, the ischemic lesions being smaller but the area of ischemic parenchymal destruction was wider. Thus, HCV RNA level can be considered a modern predictor for ischemic stroke severity and an independent risk factor [21].
HCV – associated metabolic dysfunctions
HCV-induced glucose metabolic disorders are a major cause of cardiovascular injury, insulin resistance being closely related to the presence of liver steatosis and acceleration of the atherosclerotic process. Numerous prospective and retrospective case-control studies that included HCV-infected patients have shown a close association between chronic hepatitis C and the development of type 2 diabetes mellitus. This new possible cause of developing diabetes has been rigorously evaluated in an extensive transversal study conducted by Mehta et al. in 9841 subjects. The prevalence of type 2 diabetes was 8.4% of which 2.1% were anti-HCV positive. After adjusting the data according to the study variables it was concluded that HCV-infected patients aged over 40 years are at a 4-fold increased risk of developing diabetes. This finding was further supported by a second prospective study conducted by the same researchers in a cohort of 1084 patients aged 44 to 65 years without a history of diabetes but with chronic HCV infection. After adjusting for the parameters used in the study, it was concluded that patients with chronic HCV infection followed-up for 9 years had an 11-fold higher risk of developing diabetes compared with the control group. Based on these results, further studies have demonstrated a decreased risk of diabetes among HCV-infected patients following HCV eradication. In another study, Giordanino assessed 202 patients with chronic HCV infection and with no changes in the glycemic profile followed over an 8-year period. In contrast to the results obtained by Mehta et al., Giordanino found that the patients who achieved sustained viral response showed no significant changes in glycemic profile compared with the non-responders [22].
Despite the fact that some studies have produced conflicting results, a significant number of studies have demonstrated a strong association between chronic HCV infection and the changes in the glycemic profile with the development of diabetes by the increase in the threshold for IR.
Insulin resistance is the initial stage in the development of type 2 diabetes in these patients. To demonstrate the direct involvement of HCV in the development of IR, some researchers used the homeostasis model assessment of IR (IR HOMA-) to measure IR in the infected population compared to the general population matched for the same risk factors for developing diabetes (body mass index, abdominal obesity, age, sex). The results were conflicting depending on the method used for the assessment of IR, but by using the hyperinsulinemic- euglycemic clamp as a routine method it was demonstrated that IR has a higher prevalence among people with chronic HCV infection without metabolic syndrome. Also, the study concluded that the different HCV genotypes have comparable levels of both hepatic and extrahepatic IR [17].
Different components of the viral structure have proven their ability to interact with the lipoprotein metabolism, being responsible for dyslipidemia detected in patients with chronic HCV infection. Some authors suggest that viral structures associate with lipoproteins and use the low-density lipoprotein receptors to gain access to hepatocytes [23].
Recent data show that the direct effects of the hepatitis C virus on lipid metabolism include diminished cholesterol synthesis and accelerated lipogenesis. Butt et al. bring new insights into understanding the lipid metabolism and the changes occurring during viral aggression. The results reported after a 10-year observation period revealed that over time triglycerides, total cholesterol, LDL-c, and HDL-c decrease, independently of body mass index or liver fibrosis [24].
In the era in which quality of life of the patient with chronic HCV is not just to obtain viral eradication, many researchers have studied the positive effects of antiviral therapy on extrahepatic manifestations, immune–related and metabolic alterations such as glucose metabolism impairment and cardiovascular atherosclerosis. Numerous antiviral regimens using interferon and direct acting antiviral agents have been used to ascertain the regression of metabolic and cardiovascular disorders induced by the chronic persistence of HCV.
The recent data showed us a metabolic improvement by lowering the IR levels and decreasing the incidence of stroke and myocardial infraction in patients treated with antiviral therapy. A study in Taiwan conducted over a period of 8 years, which included 3957 patients with type 2 diabetes and chronic HCV hepatitis analyzed an improvement in cardiovascular outcome after the patients obtained viral eradication. The control group consisted of 20,239 patients with type 2 diabetes, but no history of cardiovascular and liver disease was documented. The statistical analysis results showed the incidence of stroke and coronary events was significantly reduced in the group who obtained sustained viral response in comparison with the control group [25].
Conclusion
A rigorous review of the extrahepatic manifestations of HCV brought new evidence on viral activity that can be now considered a new global cerebrovascular and cardiovascular risk factor both through the induced metabolic disorders and its proatherogenic effect. The data presented in this review emphasize the fact that further studies are required in view of assessing the role of viral eradication, with the new interferon-free therapies, on the metabolic and atherosclerotic changes in patients with HCV infection.
References
- 1.Ampuero J, Romero-Gómez M. Assessing cardiovascular risk in hepatitis C: An unmet need. World J Hepatol. 2015;7:2214–2219. doi: 10.4254/wjh.v7.i19.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedimo R, Abodunde O. Metabolic and Cardiovascular Complications in HIV/HCV-Co-infected Patients. Curr HIV/AIDS Rep. 2016;13:328–339. doi: 10.1007/s11904-016-0333-9. [DOI] [PubMed] [Google Scholar]
- 3.Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2016;150:145–155.e4. doi: 10.1053/j.gastro.2015.09.007. quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 4.Barone M, Viggiani MT, Amoruso A, Schiraldi S, Zito A, Devito F, et al. Endothelial dysfunction correlates with liver fibrosis in chronic HCV infection. Gastroenterol Res Pract. 2015;2015:682174. doi: 10.1155/2015/682174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Rönnemaa T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 6.Shaked I, Hanna DB, Gleißner C, Marsh B, Plants J, Tracy D, et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol. 2014;34:1085–1092. doi: 10.1161/ATVBAHA.113.303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5:927–946. [PMC free article] [PubMed] [Google Scholar]
- 8.Dai CY, Yeh ML, Huang CF, Hou CH, Hsieh MY, Huang JF, et al. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol. 2015;30:879–884. doi: 10.1111/jgh.12313. [DOI] [PubMed] [Google Scholar]
- 9.Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Bos D, Ikram MA, Elias-Smale SE, Krestin GP, Hofman A, Witteman JC, et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol. 2011;31:2331–2337. doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 11.Lind L. Flow-mediated vasodilation over five years in the general elderly population and its relation to cardiovascular risk factors. Atherosclerosis. 2014;237:666–670. doi: 10.1016/j.atherosclerosis.2014.10.092. [DOI] [PubMed] [Google Scholar]
- 12.Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound. 2014 Aug 23;12:34. doi: 10.1186/1476-7120-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, et al. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317–1323. doi: 10.1002/hep.25508. [DOI] [PubMed] [Google Scholar]
- 14.Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, et al. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, et al. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39( Suppl 1):S55–S60. doi: 10.1016/s1590-8658(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, Hashimoto H, et al. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26–30. doi: 10.1253/circj.67.26. [DOI] [PubMed] [Google Scholar]
- 17.Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol. 2014;61(1 Suppl):S69–S78. doi: 10.1016/j.jhep.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Cojocaru IM, Cojocaru M, Iacob SA, Burcin C. Cerebral ischemic attack secondary to hepatitis C virus infection. Rom J Intern Med. 2005;43:255–260. [PubMed] [Google Scholar]
- 19.Fuckar K, Lakusic N, Mahovic D, Hirs I. Recurrent strokes as a leading presentation of chronic hepatitis C infection. Arch Med Res. 2008;39:358–359. doi: 10.1016/j.arcmed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, et al. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]
- 22.Wong RJ, Gish RG. Metabolic manifestations and complications associated with chronic hepatitis C virus infection. Gastroenterol Hepatol (N Y) 2016;12:293–299. [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer EA, Chung RT. HCV and host lipids: an intimate connection. Semin Liver Dis. 2013;33:358–368. doi: 10.1055/s-0033-1358524. [DOI] [PubMed] [Google Scholar]
- 24.Butt AA, Yan P, Simon TG, Chung RT, Abou-Samra AB ERCHIVES study team. Changes in circulating lipids level over time after acquiring HCV infection: results from ERCHIVES. BMC Infect Dis. 2015 Nov 11;15:510. doi: 10.1186/s12879-015-1268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]