Abstract
Thyroid cancer is a disease with a good prognosis and high survival rates, but having a marked growth of incidence all over the world in the last years. This fact requires special attention of researchers for understanding the behavior of this disease and to establish a correct therapy. Analysis of circulating tumor cells in patients with different malignancies is nowadays a new and exciting research tool, which can improve the diagnosis and prevent the metastatic disease. In the case of thyroid carcinoma there are few studies which explore these biomarkers and investigate the prognostic significance of circulating tumor cells. With this review we seek to emphasize the role of these cells to better understand the mechanisms of invasion or metastasis and to establish a new research base to treat aggressive forms of this type of cancer. Most of the included studies demonstrate the efficacy of these markers for diagnosis and follow up.
Keywords: circulating tumor cells, thyroid carcinoma, prognosis
Introduction
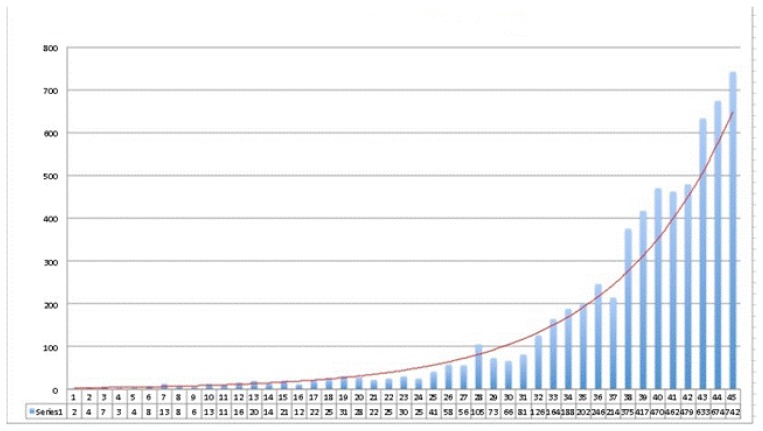
Thyroid cancer is the most frequent endocrine cancer having an impressive increase of the incidence in the past years, especially in young adults. It is estimated that by 2019, thyroid cancer will be the third most frequent type of women’s cancer [1]. According to Globocan records, a prediction for 2015 is that 804 new thyroid cancers will occur in Romania, accounting for about 0.004% of the country’s population, with a 5:1 F/B ratio [2]. Unfortunately these data are discordant with the institutional data, due to the lack of reports and to the absence of a national registry for thyroid cancer. Thyroid cancer may take several forms, among them differentiated thyroid carcinomas (DTC), with a very good prognosis and survival rate, with more than 90% complete remission; poorly differentiated carcinomas, with aggressive development and low survival rates [3]. The increased incidence of thyroid cancer might be due to higher ionizing radiation exposure, thyroid ultrasound screening with improved technical performance of the equipments, better access to medical examination and genetic testing. Figure 1 shows the thyroid cancer incidence between 1970–2014, in a Romanian tertiary institute [1].
Figure 1.
Thyroid cancer incidence at the “Ion Chiricuta” Oncology Institute (IOCN), 1970–2014 [1].
The diagnosis of this disease is determined by clinical examination, thyroid ultrasound, fine needle aspiration biopsy (FNAB), serological examinations, scintigraphy, CT (computed tomography)/RMN (magnetic resonance)/PET-CT (hybrid imaging positron emission tomography and CT), imaging being reserved to special conditions. In managing thyroid cancer, everything revolves around two methods currently regarded as landmarks: serum thyroglobulin concentration and FNAB, the latter being considered a “gold standard” in thyroid cancer screening.
This method has a good positive predictive value (90–100%), but it may be imprecise in 15–20% of cases, especially in follicular lesions [4]. FNA specimens cannot give information on vascular invasion and also they do not have the ability to distinguish between follicular adenoma and follicular carcinoma [3].
Due to the molecular biology development and the appearance of various biomarkers, many centers attempted to analyze various molecules for a precocious and minimally invasive diagnosis of thyroid cancer or to assess the prognosis of this disease. Serum markers were examined, as were micro molecular markers, such as the expression of various micro ARN molecules (mRNA) in thyroid cancer. The latest studies focus on the metastatic capacity of thyroid carcinomas, analyzing detection and quantification of circulating tumor cells (CTC) as a prognostic factor. CTCs are malignant cells which originate from the primary tumor, penetrate the vascular wall or the lymph vessel walls adjacent to the tumor into the bloodstream or lymphatic ducts. CTCs are most likely accountable for the metastasis of various types of carcinomas [5]. There is an epithelial specific antigen - EpCAM (Epithelial Cell Adhesion Molecule) on the membrane of these cells, which is often over-expressed in malignant cells [6–10]. Numerous studies performed on various types of cancer have shown a connection between the CTCs level and the development of the disease in the absence of treatment [11,12]. There are few thyroid cancer studies that assess these types of circulating cells or investigate the prognostic value or the variations of these cells depending on treatment [13,14]. The majority of these studies determine the CTCs in the patients’ venous blood, through centrifugation, by marking them with various antibodies, which enables their subsequent visualization and quantification under a fluorescence microscopy, a technique also described by Pachmann et al. in breast cancer [8,9]. Other researchers determine the level of these cells by quantification of various ARN messenger molecules (mRNA) in the bloodstream [15,16].
This review is intended to analyze, from the point of view of various research centers, the prognostic value and the feasibility of CTCs level determination in patients with thyroid carcinoma, especially in connection to the forms with a higher risk of metastasis. These studies have just begun, so there is no consensus and many research teams wish to further analyze whether the determination of these cells is relevant in elaborating a treat-to-target strategy.
This study includes pieces in which the CTCs identification was made by various methods, to assess some characteristics of thyroid carcinoma or even to diagnose this disease. The search was conducted online, in the PubMed database, using the keywords: ”circulating tumor cells in thyroid carcinoma” or ”circulating tumor cells in thyroid cancer,” and produced 158 studies containing each of these keywords or key phrases. We selected those studies which determined the level of circulating cells of thyroid cancer for various purposes. We disregarded the studies that assessed the circulating cells in other types of carcinoma as well as those dealing only with other serum markers, besides the CTCs, in thyroid carcinoma. The majority of the selected studies referred to differentiated thyroid carcinoma (DTC), and only one study dealt with the medullar thyroid carcinoma. Therefore, based on the inclusion and exclusion criteria, this study comprises 9 studies published from 1999 until 2014, as described in table I, with some details included along with their main characteristics.
Table I.
Details of included studies.
| Authors, country, year of publication | Number of patients | Subject studied | Conclusion/Results |
|---|---|---|---|
| Ghossein RA et al., USA, 1999 [21] | N/A* | Detection of circulating tumor cells and micrometastasis with PCR technique | Utility in melanoma. Discrepancies in thyroid carcinoma |
| Theresia W et al., Germany, 2003 [20] | 19 | Detection of CK20-, GRP-mRNA in circulating tumor cells from medullary thyroid carcinoma by RT-PCR technique | CK20-mRNA, GRP-mRNA useful for diagnosis of hematogenic and lymphogenic dissemintion |
| Takashi S et al., Japan, 2005 [19] | 121 | Detection of circulating tumor cells by RT-PCR for CEA-mRNA with the purpose to distinguishing follicular carcinoma by adenoma | CEA-mRNA determination is useful for distinguishing follicular carcinoma by adenoma. Sp=100%, Se=46% |
| Su-Ynn C et al., USA, 2006 [4] | 258 | TSHR-mRNA measurement role in the preoperative diagnosis of thyroid cancer | Se=93% in recurrence of thyroid carcinoma, a good alternative to serumTg |
| Gupta M, Chia SY, USA, 2007 [22] | N/A* | Circulating thyroid cancer markers evaluation | TSHR-mRNA count in patients with indeterminate FNAB can improve cancer detection |
| Tomislav N et al., USA, 2009 [18] | 510 | Determination of circulating cancer cells in patients with thyroid microcarcinoma | TSHR-mRNA presence in the CTC’s a high susceptibility of metastasis or aggressiveness |
| Winkens T et al., Germany, 2013 [14] | 162 | CEC determination helped by EpCAM, as a potential marker of thyroid malignant cancer dissemination | CEC’s were found at a high level in patients with DTC than in healthy volunteers |
| Winkens T et al., Germany, 2014 [13] | 28 | RIT influence on the CEC’s number in patients with differentiated thyroid carcinoma | It was observed a low number of CTC’s after RIT and also a high level of sTg |
| Sorg S et al., Germany, 2014 [17] | 5 | Determining tissue origin of CEC in patients with thyroid carcinoma by RT-PCR technique | 33% of CEC originate from thyroid tumor |
N/A* - not available
Winkens et al. studied circulating epithelial cells (CEC) in patients with thyroid carcinoma. They compare blood samples of different groups of patients: one with DTC before surgery, patients with DTC after surgery, patients with DTC after radioiodine-therapy (RIT), patients with benign thyroid disease and healthy volunteers. CEC were found in all groups, but the highest level of cells was described to be in patients with DTC post surgery. This can be explained by the mobilization of the tumor during surgery with detachment of cells into circulation. Anyway, the number of circulating cells count was significantly higher in all DTC groups compared to the healthy group. Within the DTC group, there was also a correlation between number of CEC and the serum levels of thyroglobulin (sTg) [14].
One year later, the same team attempted to determine the RIT (radioactive iodine therapy) impact on the number of circulating cells in patients with differentiated thyroid cancer. For this purpose, they enrolled a number of 28 patients suffering from this disease and divided them into 2 groups: 13 patients operated on, who had recurrent benign tissue and completed the RIT, and 15 patients operated on, who had recurrent malignant thyroid tissue and completed the RIT. The number of CECs was evaluated after surgery, at specific time intervals: basic check, one day before the first dose of I-131, 2nd day, 14th day, and 3 months after administration. The assessment of RIT effectiveness relied not on the absolute number of CECs, but on their modifications, in percentages, compared to the base worth calculated before the treatment, because the number of these cells may vary from one individual to another. The researchers found that the CEC levels decreased after the RIT, simultaneously with the transient increase in the serum thyroglobulin levels. However, no significant link was found between the CECs modifications and the clinical response revealed by scintigraphy, laboratory tests or morphologic examinations [13].
The two studies previously described showed a more elevated level of CECs in patients with differentiated thyroid carcinoma than in healthy subjects, as well as their decrease after the I-131 treatment. The origin of these circulating epithelial cells was impossible to determine. It was not until the end of 2014 that the members of this team attempted to determine, through the RT-PCR technique, the original tissue of the discovered circulating cells; 5 patients were included in the study from which 48 CECs were collected by identification of some mRNAs at their level: Tg, TSHR - TSH receptor, TPO – thyroperoxidase, NIS – sodium-iodide symporter. It was determined that the cells in which were detected over-expressions of at least 3 mRNA types originated from the thyroid, and it was found that the simultaneous expression of Tg-,TPO-,NIS-mRNA was up to 1200 times higher in the malignant tissue of the thyroid compared to the healthy tissue. The researchers singled out 16 out of 48 cells (33%) with this mRNA sequence, 7 of which came from patients with metastatic thyroid follicular carcinoma. Although this method may seem feasible, it has the shortcoming of a small number of enrolled patients [17].
A study conducted by Tomislav et al. assesses the presence of circulating tumor cells in the blood stream with the help of TSHR-mRNA, in patients with thyroid microcarcinoma. After selecting 510 patients for blood sampling, 37 patients with papillary carcinoma were singled out to be the subjects of the study. In 59% of the cases, the TSHR-mRNA was positive. 16% of the 37 patients had preoperative ganglion metastases, while 67% had positive TSHR-mRNA. Although the study targeted microcarcinoma patients, it was found that the percentage of the positivity of this marker, as studied at the level of circulating cells, was also maintained with tumors larger than 1 cm. This study concluded that, besides the known prognostic factors (tumor size, multifocality), the TSHR-mRNA presence in the circulating tumor cells was associated with the likelihood of metastasis development as well as a more aggressive histopathological form [18].
Another study conducted in 2005 in Japan by Takashi et al. attempted to differentiate the follicular thyroid cancer from the follicular adenoma by using another marker: CEA-mRNA (carcinoembryonic antigen - mRNA). It is a prospective study conducted on 121 patients with thyroid tumors. Among them, 77 were malignant (61 papillary, 3 follicular version of the papillary, 13 follicular - 9 minimally invasive follicular carcinoma and 4 widely invasive follicular carcinoma). In the study were also included 7 patients with thyroiditis and 7 healthy individuals for comparison purposes. The findings of this study revealed that CEA-mRNAs were present in the circulating tumor cells only in patients with follicular thyroid carcinoma, preoperatively as well as postoperatively. 44% of MIFTC patients and 50% of WIFTC patients had positive postoperative CEA-mRNA. The research revealed that this method had a specificity - Sp=100% and a sensitivity - Se=46% for the diagnosis of follicular thyroid carcinoma. To conclude, the authors claim that the postoperative CTC detection by using this marker may often single out malignant follicular tumors from benign ones, while also having an advantage in patient selection for surgery [19].
The role of tumor cells in the postoperative diagnosis of thyroid cancer was also studied by Su-Ynn Chia and her team; they calculated a 62% Se in the diagnosis of new TC cases and 93% in diagnosing the recurrences by using the TSHR-mRNA determination. They conducted the study on 258 patients, 51 - healthy and 207 - with thyroid condition, selected between 2002–2005, at Cleveland Clinic. Venous blood sampling was made before, and 7 days after, the FNB, and 89 patients were sampled right after surgery. The authors described 88 DTC cases of which 63 (71%) were TSHR-mRNA positive. The average value of this marker had the biggest growth in the patients with recurrent thyroid cancer, followed by the newly diagnosed DTC, those with benign thyroid condition and the healthy patients (p<0.001). However, the TSHR-mRNA level became normal one day post thyroidectomy, suggesting that positive circulating cells for this marker have a short lifespan in the bloodstream. This technique, together with FNB, increases detection of preoperative cancer in patients with thyroid nodules and reduces the number of unnecessary surgical operations, while the level of this marker after surgery may predict recurrence or metastasis. Regarding the 93% Se, the authors suggest the determination of TSHR-mRNA in the circulating cells as being a viable alternative to sTg determination in managing the DTC recurrences [4].
Regarding the poorly differentiated thyroid carcinomas, there are even fewer studies on circulating tumor cells. In the case of more than 40–50% of the patients with medullar thyroid carcinoma (MTC), the therapeutic failure is most likely caused by the precocious dissemination of tumor cells in the lymphatic or the bloodstream. Theresia et al. conducted a study on 19 MTC patients in which she evaluated with the help of the RT-PCR technique, the Cytokeratin 20 (CK20) and Gastrine-Releasing Peptide (GRP) presence rate in the tumour circulating cells. In a previous study, the same team noticed an over-expression of CK20 in the malignant thyroid tissue, especially in the medullar subtype, which is why the researchers chose to determine this circulating molecule. They noticed there were no CK20 molecules in the surgically removed thyroid tissue of patients with benign tumor, but the GRP was amplified. In the case of MTC patients, the CK20-mRNA and the GRP-mRNA were over-expressed at the primary tumor level. Based on these findings, they tried to determine the circulating cells leaving from the primary tumor into the bloodstream or the lymphatic system, by determining the 2 markers previously mentioned. Looking at the dissemination of these cells, the authors described an 87% Se for the detection of CK20 and 93% Se for GRP, while the Sp of both markers was 100%. Regarding the cells disseminated into the bloodstream, the detection by CK20 and GRP accounted to 28%. This study assessed the level of circulating cells in the bone marrow and employed the same techniques to demonstrate 50% detection. According to this study published in 2003, the 2 markers proved useful in detecting tumor cells with lymphatic or hematogenous dissemination [20].
In 1999 a team led by Ghossein R.A. conducted a review about the detection of micrometastases and circulating tumor cells in diseases such as melanoma, prostate and thyroid carcinoma. mRNA detection by RT-PCR technique was that they tried to evaluate it by including several pathologies. They described studies in which thyroid markers were positive in the blood of control subjects. Also there was a variation of the PCR positivity rates in melanoma and prostate cancer. Even if there were discrepancies, some authors have demonstrated the utility of this technique especially in melanoma. In thyroid cancer the results were unsatisfactory, caused by the small number of subjects [21].
Another review made by Gupta et al. seeks to assess circulating markers of thyroid cancer. Likewise they aim to observe changes of different thyroid-specific mRNA in the circulating cells of patients with this pathology. Most of the included studies were focused on determining Tg-mRNA. Although sTg is an important marker for recurrent or residual disease, the studies included showed a significant variability of the Tg-mRNA level, questioning the validity of this marker. Instead, when studying TSHR-mRNA, a high sensitivity and specificity in detecting recurrent and residual disease were found. For patients with indeterminate FNAB this technique can bring more information and save unnecessary surgery [22].
Research in malignant pathology started at the molecular level seems to return the most favorable results among patients worldwide [23–27]. This is the reason why many authors focus on the discovery of new molecular markers that can provide benefits concerning early diagnosis, or in follow up, and even in discovering new therapeutic strategies [28–30].
Even if, by its nature, thyroid carcinoma has a good prognosis, researchers are forced to do a thorough study in this pathology due to its increasing frequency. The latest studies of CTCs have become a new and useful tool of research for the biology of cancer cells and for their metastatic feature.
Many studies on circulating tumor cells have been performed, especially in patients with breast cancer, malignant melanoma and prostate adenocarcinoma; however, in thyroid carcinoma, these steps are still incipient [31,32]. This review reflects the concerns and interests in understanding the processes of metastasis in thyroid carcinoma, pathology with an increasing incidence worldwide [33,34]. Studying the behavior of thyroid cancer, by determining the role of these cells, can bring us benefits in controlling this pathology post-operatively, in follow-up and, why not, in the change of the therapeutic strategy for some histological subtypes. Moreover, our study shows the usefulness and effectiveness of the technique of CTCs determination using different molecular markers. The authors have tried to establish a more precise preoperative diagnosis, on the one hand, in order to improve the targeted therapeutic strategies and to avoid unnecessary treatment, and, on the other hand, to apply optimal and timely treatment in patients with a high risk of metastasis or of developing a recurrent disease. For example, if in most European and American guidelines, in case of minimally invasive follicular thyroid carcinoma, diagnosed postoperatively, without other negative prognostic factors, no other treatment is indicated, a presence in blood of CTCs can impose a therapy with I-131 for destruction of these cells and decrease the risk of metastasis by approximately 100%. On the other hand, in some cases that require postoperative radioiodine treatment, according to guidelines, and in blood are not detected circulating tumor cells, the patient can be followed in time using the values of TG and CTCs, reducing in this way the side effects of irradiation.
Most of the studies included show a high specificity and sensitivity as prognostic value of CTC levels in patients with thyroid carcinoma [35]. In conclusion, taking into consideration the recent findings [36–40], further prospective studies with many more patients are necessary, especially for selected cases with a high risk of premature metastasis.
The research on new possible prognostic factors in thyroid cancer may improve, or even change some recommendations from oncological guidelines, worldwide, bringing malignant thyroid pathology closer to the “ideal treatment”.
References
- 1.Piciu D. Imagistica de fuziune PET/CT in oncologie. [Fusion PET/CT imaging in oncology]. Cluj-Napoca: Editura Medicala Universitara „Iuliu Hatieganu”; 2016. pp. 207–211. [Google Scholar]
- 2.International Agency for Research in Cancer. GLOBOCAN 2012. Estimated Cancer Incidence, Mortality and Prevalence in 2012. Online Analysis. Available from: http://globocan.iarc.fr/Pages/online.aspx.
- 3.Zane M, Agostini M, Enzo MV, Casal Ide E, Del Bianco P, Torresan F, et al. Circulating cell-free DNA, SLC5A8 and SLC26A4 hypermethylation, BRAF(V600E): A non-invasive tool panel for early detection of thyroid cancer. Biomed Pharmacother. 2013;67(8):723–730. doi: 10.1016/j.biopha.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Chia SY, Milas M, Reddy SK, Siperstein A, Skugor M, Brainard J, et al. Thyroid-stimulating hormone receptor messenger ribonucleic acid measurement in blood as a marker for circulating thyroid cancer cells and its role in the preoperative diagnosis of thyroid cancer. J Clin Endocrinol Metab. 2007;92(2):468–475. doi: 10.1210/jc.2006-2088. [DOI] [PubMed] [Google Scholar]
- 5.Cebotaru CL, Olteanu ED, Antone NZ, Buiga R, Nagy V. Circulating tumour cells in germ cells tumours: are those biomarkers of real prognostic value? A review. Clujul Med. 2016;89(2):203–211. doi: 10.15386/cjmed-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96(3):417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pachmann K, Camara O, Kavallaris A, Schneider U, Schünemann S, Höffken K. Quantification of the response of circulating epithelial cells to neodadjuvant treatment for breast cancer: a new tool for therapy monitoring. Breast Cancer Res. 2005;7(6):R975–R979. doi: 10.1186/bcr1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachmann K, Clement JH, Schneider CP, Willen B, Camara O, Pachmann U, et al. Standardized quantification of circulating peripheral tumour cells from lung and breast cancer. Clin Chem Lab Med. 2005;43(6):617–627. doi: 10.1515/CCLM.2005.107. [DOI] [PubMed] [Google Scholar]
- 9.Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, et al. Monitoring the response of circulating epithelial tumour cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26(8):1208–1215. doi: 10.1200/JCO.2007.13.6523. [DOI] [PubMed] [Google Scholar]
- 10.Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA, Jr, et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumours. Int J Oncol. 2005;27(1):49–57. [PubMed] [Google Scholar]
- 11.Antolovic D, Galindo L, Carstens A, Rahbari N, Büchler MW, Weitz J, et al. Heterogeneous detection of circulating tumour cells in patients with colorectal cancer by immunomagnetic enrichment using different EpCAM-specific antibodies. BMC Biotechnol. 2010 Apr 28;10:35. doi: 10.1186/1472-6750-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumour cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkens T, Pachmann K, Freesmeyer M. The influence of radioiodine therapy on the number of circulating epithelial cells (CEC) in patients with differentiated thyroid carcinoma – a pilot study. Exp Clin Endocrinol Diabetes. 2014;122(4):246–253. doi: 10.1055/s-0034-1370921. [DOI] [PubMed] [Google Scholar]
- 14.Winkens T, Pachmann K, Freesmeyer M. Circulating epithelial cells in patients with thyroid carcinoma. Can they be identified in the blood? Nuklearmedizin. 2013;52(1):7–13. doi: 10.3413/Nukmed-0524-12-08. [DOI] [PubMed] [Google Scholar]
- 15.Chinnappa P, Taguba L, Arciaga R, Faiman C, Siperstein A, Mehta AE, et al. Detection of thyrotropin-receptor messenger ribonucleic acid (mRNA) and thyroglobulin mRNA transcripts in peripheral blood of patients with thyroid disease: sensitive and specific markers for thyroid cancer. J Clin Endocrinol Metab. 2004;89(8):3705–3709. doi: 10.1210/jc.2003-031967. [DOI] [PubMed] [Google Scholar]
- 16.Milas M, Barbosa GF, Mitchell J, Berber E, Siperstein A, Gupta M. Effectiveness of peripheral thyrotropin receptor mRNA in follow-up of differentiated thyroid cancer. Ann Surg Oncol. 2009 Feb;16(2):473–480. doi: 10.1245/s10434-008-0211-9. [DOI] [PubMed] [Google Scholar]
- 17.Sorg S, Pachmann K, Brede-Hekimian K, Freesmeyer M, Winkens T. Determining tissue origin of circulating epithelial cells (CEC) in patients with differentiated thyroid cancer by real-time PCR using thyroid mRNA probes. Cancer Lett. 2015;356(2 Pt B):491–495. doi: 10.1016/j.canlet.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Novosel T, Ritter HE, Gupta M, Harvey A, Mitchell J, Berber E, et al. Detection of circulating thyroid cancer cells in patients with thyroid microcarcinomas. Surgery. 2009;146(6):1081–1089. doi: 10.1016/j.surg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Harao M, Nakano S, Jotsuka T, Suda N, Yamashita J. Circulating tumour cells detected by reverse transcription-polymerase chain reaction for carcinoembryonic antigen mRNA: distinguishing follicular thyroid carcinoma from adenoma. Surgery. 2005;137(5):552–558. doi: 10.1016/j.surg.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Weber T, Lacroix J, Worner S, Weckauf H, Winkler S, Hinz U, et al. Detection of hematogenic and lymphogenic tumour cell dissemination in patients with medullary thyroid carcinoma by cytokeratin 20 and preprogastrin-releasing peptide RT-PCR. Int J Cancer. 2003;103(1):126–131. doi: 10.1002/ijc.10804. [DOI] [PubMed] [Google Scholar]
- 21.Ghossein RA, Carusone L, Bhattacharya S. Review: polymerase chain reaction detection of micrometastases and circulating tumour cells: application to melanoma, prostate, and thyroid carcinomas. Diagn Mol Pathol. 1999;8(4):165–175. doi: 10.1097/00019606-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Gupta M, Chia SY. Circulating thyroid cancer markers. Curr Opin Endocrinol Diabetes Obes. 2007;14(5):383–388. doi: 10.1097/MED.0b013e3282eeb2f4. [DOI] [PubMed] [Google Scholar]
- 23.Nikiforov YE. Molecular diagnostics of thyroid tumours. Arch Pathol Lab Med. 2011;135(5):569–577. doi: 10.5858/2010-0664-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 24.Agostini M, Pucciarelli S, Enzo MV, Del Bianco P, Briarava M, Bedin C, et al. Circulating cell-free DNA: a promising marker of pathologic tumor response in rectal cancer patients receiving preoperative chemoradiotherapy. Ann Surg Oncol. 2011;18(9):2461–2468. doi: 10.1245/s10434-011-1638-y. [DOI] [PubMed] [Google Scholar]
- 25.De Mattos-Arruda L, Olmos D, Tabernero J. Prognostic and predictive roles for circulating biomarkers in gastrointestinal cancer. Future Oncol. 2011;7(12):1385–1397. doi: 10.2217/fon.11.122. [DOI] [PubMed] [Google Scholar]
- 26.Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 27.Taback B, Hoon DS. Circulating nucleic acids in plasma and serum: past, present and future. Curr Opin Mol Ther. 2004;6(3):273–278. [PubMed] [Google Scholar]
- 28.Umetani N, Giuliano AE, Hiramatsu SH, Amersi F, Nakagawa T, Martino S, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol. 2006;24(26):4270–4276. doi: 10.1200/JCO.2006.05.9493. [DOI] [PubMed] [Google Scholar]
- 29.Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005;33(Pt 1):237–240. doi: 10.1042/BST0330237. [DOI] [PubMed] [Google Scholar]
- 30.Catalano MG, Fortunati N, Boccuzzi G. Epigenetics modifications and therapeutic prospects in human thyroid cancer. Front Endocrinol (Lausanne) 2012 Mar 19;3:40. doi: 10.3389/fendo.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta MK, Taguba L, Arciaga R, Siperstein A, Faiman C, Mehta A, et al. Detection of circulating thyroid cancer cells by reverse transcription-PCR for thyroid-stimulating hormone receptor and thyroglobulin: the importance of primer selection. Clin Chem. 2002;48(10):1862–1865. [Google Scholar]
- 32.Paterlini-Brechot P, Benali NL. Circulating tumour cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Min HS, Choe G, Kim SW, Park YJ, Park DJ, Youn YK, et al. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod Pathol. 2008;21(6):748–755. doi: 10.1038/modpathol.2008.51. [DOI] [PubMed] [Google Scholar]
- 34.Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. doi: 10.1097/01.SLA.0000055273.58908.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ditkoff BA, Marvin MR, Yemul S, Shi YJ, Chabot J, Feind C, et al. Detection of circulating thyroid cells in peripheral blood. Surgery. 1996;120(6):959–964. doi: 10.1016/s0039-6060(96)80041-9. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PW, Burchill SA, Selby PJ. The molecular detection of circulating tumour cells. Br J Cancer. 1995;72(2):268–276. doi: 10.1038/bjc.1995.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biscolla RP, Cerutti JM, Maciel RM. Detection of recurrent thyroid cancer by sensitive nested reverse transcription-polymerase chain reaction of thyroglobulin and sodium/iodide symporter messenger ribonucleic acid transcripts in peripheral blood. J Clin Endocrinol Metab. 2000;85(10):3623–3627. doi: 10.1210/jcem.85.10.6876. [DOI] [PubMed] [Google Scholar]
- 38.Ramskold D, Luo S, Wang YC, Li R, Deng Q, Faridani OR, et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumour cells. Nat Biotechnol. 2012;30(8):777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roddiger SJ, Bojunga J, Klee V, Stanisch M, Renneberg H, Lindhorst E, et al. Detection of thyroid peroxidase mRNA in peripheral blood of patients with malignant and benign thyroid diseases. J Mol Endocrinol. 2002;29(3):287–295. doi: 10.1677/jme.0.0290287. [DOI] [PubMed] [Google Scholar]
- 40.Mateo J, Gerlinger M, Rodrigues DN, de Bono JS. The promise of circulating tumor cell analysis in cancer management. Genome Biol. 2014 Aug 30;15(8):448. doi: 10.1186/s13059-014-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]