Abstract
Introduction
The aim of this study was to evaluate the impact of the interval between surgery and adjuvant treatments regarding the overall survival and recurrence-free survival in patients from a developing country. For stages II and III rectal cancer, international guidelines recommend neoadjuvant chemoradiotherapy (CRT) regardless of the tumor location. In the developing countries there is a shortage of radiotherapy centers, specialists, which lead to long waiting lists for radiotherapy. These problems might lead to protocol deviations.
Methods
We conducted a retrospective study on 161 patients with rectal cancer treated with surgery, postoperative CRT and with or without chemotherapy for a total of 6 months, at The Oncology Institute Cluj-Napoca between 2006–2010. All patients had 5 years of follow-up.
Results
A total of 161 patients were enrolled in this study. The majority of patients were locally advanced stages (89.44%). The well known prognostic factors, such as TNM stage, performance status, CEA serum level, perineural, vascular and lymphatic invasion, and node capsular effraction had a statistically significant influence on overall survival. In 21.12% of patients the first adjuvant treatment was started in the first 4 weeks after surgery. Only 13.04% of patients started the concomitant CRT within the limit of 6 weeks after surgery. Concerning the time between surgery and CRT, we did not observe a statistically significantly difference in OS if the radiotherapy started after the first 6 weeks (p=0.701). The OS rate for locally advanced rectal cancer patients was 69.44%.
Conclusions
In rectal cancer, the importance of the first therapeutic act is crucial. Following international guidelines provides a survival advantage and a better quality of life. In case of adjuvant treatment, it is recommended to start this treatment as soon as the local infrastructure allows it.
Keywords: rectal neoplasms, adjuvant therapy, chemoradiotherapy, prognosis
Introduction
Colorectal cancer is the second cause of cancer death in both genders in Europe, being the third most common cancer in men, and the second in women [1]. Rectal cancer represents almost one-third of the colorectal cancer diagnosed each year [2]. Incidence of rectal cancer has a close correlation with the western lifestyle and dietary factors. The prognosis of colorectal cancer was improved due to multidisciplinary approach [3,4] and to early detection by screening programs. The role of a multidisciplinary team (MDT) in rectal cancer treatment is best shown in locally advanced rectal cancer (LARC). For the stages II and III rectal cancer the current standard of care is neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME), with or without adjuvant chemotherapy [3,5–7]. This approach is worldwide accepted for the mid and lower rectal tumors. For the upper rectal cancer, due to its peritoneal coverage, the American Society of Colon and Rectal Surgeons recommends either preoperative or postoperative CRT [8]. However, the overall rates of acute and long-term toxicities are lower with the preoperative approach [5], and this is one reason why the National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant CRT regardless of the tumor location [9]. Although these guidelines are accepted in European countries the clinical approach depends on local infrastructure. The developing countries have a shortage of radiotherapy centers, specialists, and a long waiting list for radiotherapy. These problems might lead to protocol deviations.
Therefore, the aim of this study was to evaluate overall survival and prognostic factors, including the interval between surgery and adjuvant treatment in rectal cancer patients treated with surgery followed by chemoradiotherapy, in a developing country.
Patients and methods
Patient selection
After the approval of the institution’s research ethics board, we conducted a retrospective study of 161 patients with rectal cancer treated with surgery, postoperative CRT and with or without chemotherapy for a total of 6 months, at The Oncology Institute Prof. Dr. Ion Chiricuta, Cluj-Napoca from January, 2006 to December, 2010. All patients were newly diagnosed with rectal adenocarcinoma based on pathological examination and underwent surgical treatment as the first therapeutic act. Patients considered eligible had to be above the age of 18 years, with a tumor located between 0 and 15 cm from the anal verge, without extra-pelvic disease. Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1, with adequate complete blood count, good hepatic and renal function tests, and with carcinoembryonic antigen (CEA) measurement. The tumor stage was established using the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Involved resection margins were defined as tumor cell involvement within 2 mm of the circumferential resection margin and within 5 mm of the distal resection margin. Patients who had metastasic disease, who had been treated with cytotoxic drugs for other tumors, or who had interfering medical problems, were excluded. Also, patients who did not receive regular follow-up after completion of treatment were excluded from this study.
Surgery
Total mesorectal excision was performed as the first therapeutic act. Either an anterior resection of rectum (a sphincter-preserving surgery) or an abdominoperineal resection were performed, according to patients’ characteristics and tumor location.
Adjuvant chemoradiotherapy
Radiotherapy consisted either in 45 Gy in 25 fractions with a 1.8 Gy/fraction or in 50 Gy in 25 fractions with a 2 Gy/fraction. Concomitant chemotherapy with fluoropyrimidines from the first to the last day of radiotherapy was used as radiosensitizer: protracted infusional 5-FU (225 mg/m2/day) or capecitabine (1650 mg/m2/day orally).
Adjuvant chemotherapy
Do to a delay of chemoradiotherapy, some patients started with adjuvant chemotherapy FOLFOX-4 (oxaliplatin 85 mg/m2 on day 1 followed by 5-FU-LV every 2 weeks) or XELOX (oxaliplatin 130 mg/m2 on day 1 followed by capecitabine 1000 mg/m2 by mouth 2 times a day for days 1–14 every 3 weeks). Subsequently patients received the previously described radiosensitizing fluoropyrimidine-based chemotherapy concomitant with radiation.
Outcomes
The primary end point was overall survival (OS) defined as the time from the date of diagnosis to the date of death from any cause, and recurrence-free survival (RFS), defined as the time from the date of diagnosis of rectal cancer to the detection of recurrent disease or death. Data were censored on the date of the last contact or after the completion of the 5-years follow-up. Time until the first adjuvant treatment was measured from the date of surgical resection of the tumor. A value less or equal than 4 weeks was considered normal. Time between surgery and radiotherapy was measured from the date of surgical resection to the date of chemoradiotherapy. A value less or equal than 6 weeks was considered normal.
Statistical considerations
All analyses were carried out with the statistical program R version 3.2.1. The Kaplan-Meier method was used to estimate the rate of OS and RFS. Categorical variables were analyzed by means of the Chi squared test or Fisher’s exact test, and the t-test or Mann–Whitney U test were used for continuous data. A log-rank test was used in performance of univariate analysis for evaluation of possible prognostic factors associated with OS and RFS. Cox regression analyses were used to develop univariable and multivariable models. Multivariable models were adjusted for potentially confounding factors including performance status, age, sex, tumor grade, and T and N stages. Hazard ratios and confidence intervals were obtained at 95% significance. A P value < 0.05 was considered statistically significant.
Results
Between January, 2006 to December, 2010 a total of 161 patients with rectal cancer underwent surgery for curative intent, followed by CRT and were evaluable for this study. With 56.52 %, there were more male patients included. Regarding the performance status, there was a small difference between the two categories, ECOG 1 being more frequent (50.31%). More than half of the patients had lower rectum tumors (54.66%). A preoperative high CEA level was seen in only 14 patients (12.5%). Almost two thirds of the patients had T3 primary tumor (65.66%) followed by T2 (18.1%) and T4 with 14.91%. Nearly half of the patients were nodal negative (46.58 %). The most frequent nodal positive status encountered was N1 (29.81%). The majority of patients had TNM stage III and II (53.42% and 36.02%) while TNM stage I was less frequent (10.56%). Regarding the differentiation grade, more than half of the tumors were moderately differentiated (56.62%). In 55.9% of patients the lymph node sampling was done according to the international guidelines. Lymphatic invasion was seen in more than one third of cases (43.75%), followed by perineural invasion (21.66%) and vascular invasion (17.53%). Node capsular effraction was encountered in 9.94%. Patients were followed for 5 years. The recurrence occurred in one third of patients, with 27.95% death cases. Patient demographics and tumor characteristics are shown in Table I.
Table I.
Patient and tumor characteristics.
| Variables | Value (%) (n=161) |
|---|---|
| Gender (M vs. F) | 91/161 (56.52) |
| ECOG | 1: 81/161 (50.31) 0: 80/161 (49.69) |
| Anatomic location (lower rectum vs. middle/upper rectum) | 88/161 (54.66) |
| CEA ≥ 5 ng/mL | 14/112 (12.5) |
| Primary tumor (T) | 4: 24/161 (14.91) 3: 105/161 (65.22) 2: 29/161 (18.01) 1: 3/161 (1.86) |
| Regional lymph nodes (N) | 2: 38/161 (23.6) 1: 48/161 (29.81) 0: 75/161 (46.58) |
| TNM Stage | III: 86/161 (53.42) II: 58/161 (36.02) I: 17/161 (10.56) |
| Histological grade | 3: 38/157 (24.2) 2: 89/157 (56.69) 1: 30/157 (19.11) |
| Lymph nodes sampling ≥12 | 90/161 (55.9) |
| Node capsular effraction | 16/161 (9.94) |
| Vascular invasion | 27/154 (17.53) |
| Lymphatic invasion | 70/160 (43.75) |
| Perineural invasion | 34/157 (21.66) |
| Recurrence | 54/161 (33.54) |
| Local recurrence | 27/161 (16.77) |
| Death | 45/161 (27.95) |
ECOG: Eastern Cooperative Oncology Group performance status; CEA: carcinoembryonic antigen
Treatment characteristics
Almost half of the patients had an anterior resection of rectum, a sphincter-preserving surgery (45.96%). Most cases were with negative resection margin (85.71%). In 21.12% of patients the first adjuvant treatment was started in the first 4 weeks after surgery and in more than half of patients the first adjuvant treatment was CRT. Only 13.04% of patients started the concomitant CRT in the limit of 6 weeks after surgery. The majority of patients received concomitant CRT according to the protocol for radiotherapy of 45 Gy in 25 fractions (73.91%). The most frequent concurrent chemotherapy used was infusional 5FU (69.23%) followed by oral capecitabine (25.87%). One chemotherapy line was used more frequently (81.99%) but for some recurrent cases it went up to 5 lines (Table II).
Table II.
Treatment details.
| Characteristic | Number (%) (n=161) |
|---|---|
| Surgery (anterior resection of rectum vs. abdominoperineal resection) | 74/161 (45.96) |
| Resection margin (R0 vs. R1) | 138/161 (85.71) |
| Type of first adjuvant treatment (CRT vs. CT) | 84/161 (52.17) |
| Time until first adjuvant treatment ≤28 days | 34/161 (21.12) |
| Time between surgery and RT ≤42 days | 21/161 (13.04) |
| Adjuvant therapy (C RT 45Gy vs. CRT 50Gy) | 119/161 (73.91) |
|
| |
| Chemotherapy type | 5-FU/LV: 99/143 (69.23) CAPECITABINE: 37/143 (25.87) FOLFOX: 4/143 (2.8) XELOX: 3/143 (2.1) |
|
| |
| Chemotherapy line | 1: 132/161 (81.99) 2: 12/161 (7.45) 3: 14/161 (8.7) 4: 1/161 (0.62) 5: 2/161 (1.24) |
CRT: chemoradiotherapy; RT: radiotherapy; 5-FU/LV: 5-fluorouracil with leucovorin
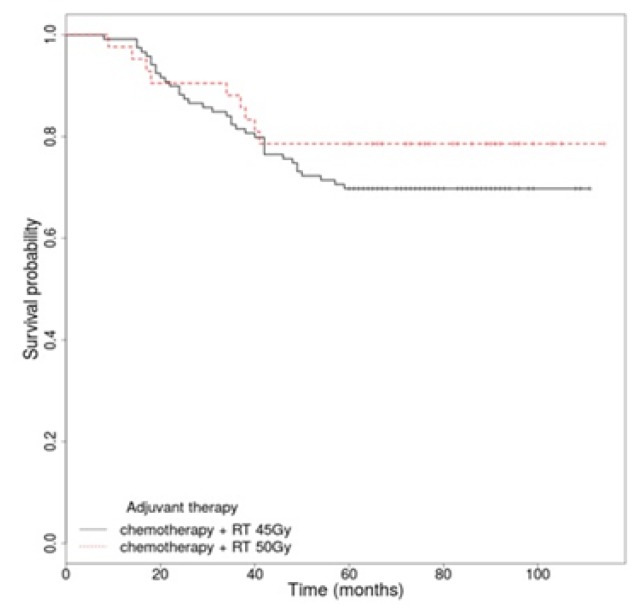
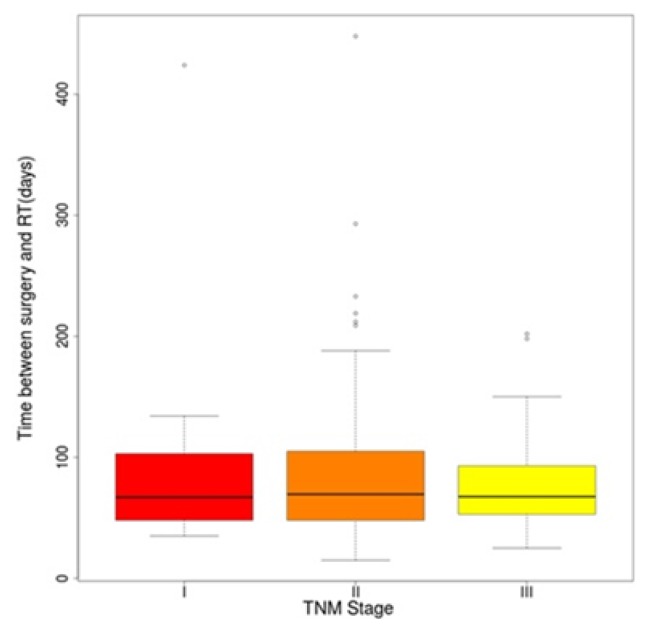
According to the place of residence the patients from rural places received adjuvant treatment sooner than the urban ones (Table III). There was no statistically significant difference between the TNM stages and the timing of radiotherapy (Figure 1). There was no significant difference for OS regarding the radiotherapy protocol used (45 Gy vs. 50 Gy), p=0.325 [HR, 1.44 (95% CI 0.69–2.99)]. There were small advantages in cases of radiotherapy of 50 Gy in 25 fractions (Figure 2).
Table III.
Time until first adjuvant treatment regarding patient’s place of residence.
| Place of residence: | R (n=58) | U (n=99) | P |
|---|---|---|---|
| Time until first adjuvant treatment (days), median (IQR) | 42.5 (29.25 – 68.5) | 50 (34 – 67.5) | 0.603 |
R-rural area; U-urban area
Figure 1.
Time between surgery and radiotherapy according to the TNM Stage.
Figure 2.
Overall survival rate by adjuvant radiotherapy schedule.
Prognostic factors for long-term survival
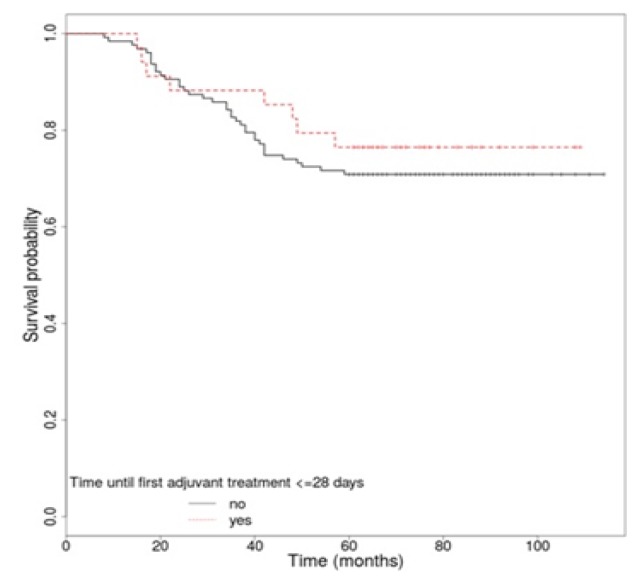
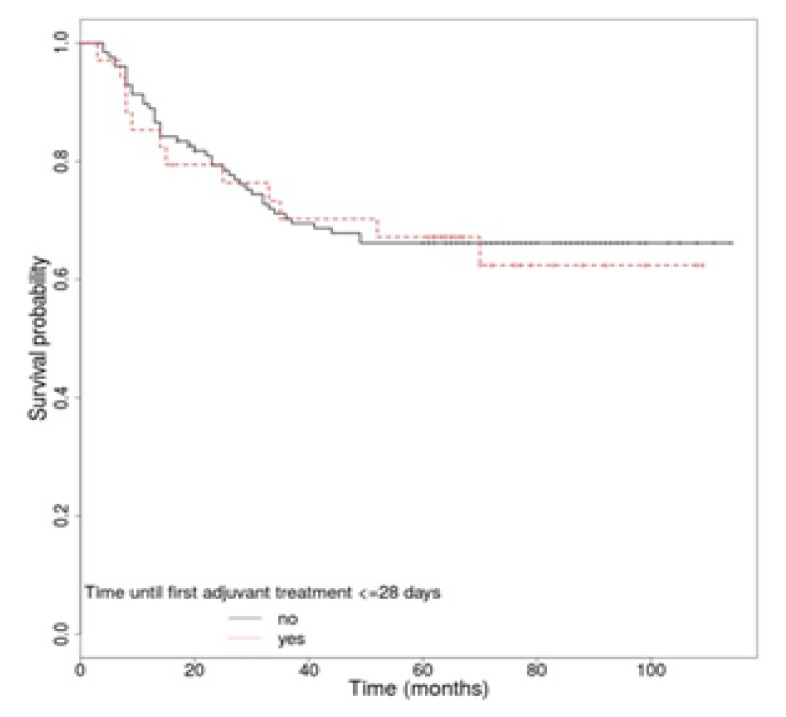
The 5-year overall survival rate of all patients was 72.05%. The OS rate for locally advanced rectal cancer patients (stages II and III) was 69.44%. We analyzed several factors that may affect OS, including CEA level, anatomic location, TNM stage, performance status, pathologic characteristics, surgical margin status, the interval between surgery and first adjuvant treatment, and the interval between surgery and radiation. For some of these we identified a statistically significantly higher rate of survival. The OS was better for patients with a performance status of 0 vs. 1 (p<0.001), when serum CEA level was normal (p=0.015), and also in patients without vascular (p=0.027), lymphatic (p=0.018) or perineural invasion (p<0.001). Likewise, the univariate analysis identified a superior OS in patients with stage II vs. stage III (p<0.001), with negative resection margins (p=0.04) and in patients without capsular effraction (p<0.001) or recurrence (p<0.001) (Table IV). The anatomic location (p=0.843), a correct lymph node sampling (p=0.322), histological grade (p=0.054) and the type of first adjuvant treatment (p=0.878) were not associated in a significant manner with long-term survival. Regarding the time until the first adjuvant treatment, there was no statistically significant difference in patients’ survival if the treatment started in the first 4 weeks (p=0.508), but however the OS was a bit higher for this group of patients [HR, 1.29 (95% CI 0.6 – 2.78)] (Figure 3). Concerning the time between surgery and CRT, we did not observe a statistically significant difference in OS if the radiotherapy started after the first 6 weeks (p=0.701) [HR, 1.49 (95% CI 0.19 – 11.68)], 8 weeks (p=0.486) [HR, 1.24 (95% CI 0.67 – 2.29)], 10 weeks (p=0.711) [HR, 1.12 (95% CI 0.62 – 2.01)] or 12 weeks (p=0.28) [HR, 1.43 (95% CI 0.75 – 2.72)]. In univariate analysis for recurrence free-survival according to the time until the first adjuvant treatment, there was no statistically significant difference if the treatment started in the first 4 weeks (p=0.81) [HR, 1.08 (95% CI 0.57 – 2.06)] (Figure 4). Likewise, in the case of the time between surgery and CRT, no statistically significant difference in RFS was observed if the radiotherapy started after the first 6 weeks (p=0.48) [HR, 1.31 (95% CI 0.62 – 2.78)], 8 weeks (p=0.425) [HR, 1.37 (95% CI 0.63 – 3.01)], 10 weeks (p=0.739) [HR, 1.14 (95% CI 0.53 – 2.43)] or 12 weeks (p=0.4) [HR, 1.43 (95% CI 0.62 – 3.26)].
Table IV.
Univariate analysis for OS predictors.
| Overall survival | P-value | Hazard ratio (95 % CI) |
|---|---|---|
|
| ||
| ECOG (1 vs. 0) | p< 0.001 | HR=4.24 (95% CI 2.1 – 8.57) |
|
| ||
| CEA≥5 ng/mL | p=0.015 | HR=1.02 (95% CI 1.003 – 1.04) |
| Vascular invasion | p=0.027 | HR=2.14 (95% CI 1.07 – 4.25) |
|
| ||
| Lymphatic invasion | p=0.018 | HR=2 (95% CI 1.11 – 3.62) |
|
| ||
| Perineural invasion | p< 0.001 | HR=3.52 (95% CI 1.93 – 6.42) |
|
| ||
| Node capsular effraction | p< 0.001 | HR=3.38 (95% CI 1.67 – 6.86) |
|
| ||
| TNM stage (III vs. II) | p<0.001 | HR=3.62 (95% CI 1.68 – 7.79) |
|
| ||
| Distant recurrence | p<0.001 | HR=18.05 (95% CI 8.83 – 36.91) |
| Local recurrence | p<0.001 | HR=4.28 (95% CI 2.35 – 7.8) |
| Resection margin (R1 vs. R0) | p=0.04 | HR=2.03 (95% CI 1.01 – 4.11) |
ECOG: Eastern Cooperative Oncology Group performance status; CEA: carcinoembryonic antigen
Figure 3.
Overall survival rate by interval between surgery and first adjuvant treatment.
Figure 4.
Recurrence free survival curves according to interval between surgery and first adjuvant treatment.
Discussion
The purpose of this retrospective study was to evaluate long-term survival and prognostic factors, including the interval between surgery and radiation in rectal cancer patients treated with TME and postoperative chemoradiotherapy with or without adjuvant chemotherapy. The 5-year overall survival rate of 72.05% for all stages and 69.44% for stages II and III, was similar or better compared to those reported in other studies [10–12]. We were very satisfied with such a great survival rate of the cases studied, taking into account that for locally advanced rectal cancer stages NCCN guidelines recommend a different protocol from the one used in this study. These cases should have the benefit of neoadjuvant CRT, followed by surgery with or without adjuvant chemotherapy [3,9].
In our study lymphatic and perineural invasions were more frequent than venous invasion, most probably due to the fact that we included only locally advanced cases, and excluded those with distant metastasis. This might be an explanation also for the fact that we encountered only a small number of high CEA cases (12.5%). Even so, a statistically significant difference was highlighted regarding the survival of patients with normal CEA level versus those with high level and for those without vascular, lymphatic or perineural invasion (Table IV).
In our institution, we have a high number of patients who need radiotherapy, but a low number of radiotherapy machines, this being the reason why the time between surgery and radiotherapy might be longer than required. The most adequate interval between surgery and radiotherapy is not standardized and can vary in different institutions, but it is well known that a shorter interval has more survival advantages [10]. In this study only 13.04% of patients have started CRT in the first 6 weeks after surgery and in only 52.17% of cases CRT was the first adjuvant treatment. Because of the long time between surgery and CRT almost half of the patients started adjuvant 5-FU-based chemotherapy while pending for chemoradiotherapy, but only 21.12% started treatment in the first 4 weeks after surgery (Table II). It is well known that the patients with more advanced TNM stages, benefit more from radiotherapy [12]. But the data of this study outlines the incapacity of doctors to institute radiotherapy sooner, and the necessity to respect scheduled visits regardless of the TNM stage (Figure 1). Despite the limits related to infrastructure, good access to medical services of patients is still accomplishable as demonstrated by the fact that we did not identify a statistically significant difference between patients from the rural or urban area. Moreover the patients from rural areas had a shorter interval between surgery and adjuvant treatment than those from the urban area (Table III).
Univariate analysis showed that in this study the well known prognostic factors, such as TNM stage, performance status, CEA serum level, resection margins, perineural, vascular and lymphatic invasion, node capsular effraction and recurrences [13–17] have statistically significantly influenced overall survival (Table IV).
Most probably due to a small number of cases, this study has not been able to establish a significant correlation between survival rates and the anatomic location, histological grade, the type of first adjuvant treatment and a correct lymph node sampling. In retrospective studies published by Genovesi D et al. [18], which was conducted on 1,338 patients, as well as the one conducted by Bagatzounis et al [19] these factors have been described to have important impact on survival rates. However, even though univariate analysis has not identified an association between a correct lymph node sampling and survival, a percent of 55.9% of correct lymphadenectomies could be improved [4]. Because only on the basis of a correct lymph node sampling can we calculate the N stage and the lymph node ratio (LNR), which are well known prognostic factors for rectal cancer [20–23]. In order to improve the percentage of lymph nodes identified, a tight collaboration between the surgeon and the pathologist must exist. It is very important to have in the MDT experts in identifying perirectal lymph nodes. Almost three quarters of patients followed the radiotherapy protocol of 45 Gy/25 fractions (73.91%). Although there was no statistically significant difference (p=0.325), the univariate analysis outlined an advantage regarding survival in cases where the protocol of 50 Gy/25 fractions was used (Figure 2). For both protocols concurrent 5 FU-based chemotherapy was administered, and in some cases with distant recurrence even 5 lines of chemotherapy were necessary (Table II).
Moreover, univariate analysis has not identified significant differences regarding survival of patients depending neither on the interval between surgery and the onset of adjuvant treatment (Figure 3), nor on the interval between surgery and CRT. However, these factors have been described in several studies as having an important role in the survival of rectal cancer patients [10,11,24,25]. The same results were obtained regarding RFS according to the time between surgery and adjuvant treatment and the time between surgery and radiotherapy, without any statistical significance. Even so, a small advantage for OS was observed in cases in which the time to the onset of adjuvant therapy was smaller (Figure 3).
Even if in this study we haven’t identified statistically significant differences between OS and RFS related to the exact moment of initiation of the adjuvant treatment, international studies have demonstrated that it is very important to administer the adjuvant treatment as soon as possible, according to local resources [26–28]. That is why, in order to analyze all the resources that can be offered to each patient, close collaboration of a multidisciplinary team specialized in rectal cancer is of major importance. In locally advanced rectal cancer patients, the importance of the first therapeutic act is considerable. Radiotherapy has proven its advantage in neoadjuvant treatment, and this study emphasizes once again that using radiotherapy as an adjuvant treatment does not negatively influence OS, but it raises the risk of local relapses (16.77%) [5]. We did not identify any advantage in survival in the cases in which radiotherapy was started at 6 or at 12 weeks after surgery.
The major limitation of this study is the small number of patients. Other limitations of this study included its retrospective design and the variability of adjuvant chemotherapy regimens.
Conclusion
The present survival rates for patients with rectal cancer treated with surgery followed by chemoradiotherapy are better or similar to those reported in previous studies. Mostly, in rectal cancer, the importance of the first therapeutic act is crucial. Following international guidelines and including thorough therapeutic sequences provides a survival advantage and a better quality of life. The interval between neoadjuvant chemoradiotherapy and surgery or the interval between surgery and adjuvant treatments (chemoradiotherapy/chemotherapy) should be respected. In case of adjuvant treatment, it is recommended to start this treatment as soon as the local infrastructure allows it.
References
- 1.World Health Organization. Colorectal cancer. Available from: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/news/news/2012/2/early-detection-of-common-cancers/colorectal-cancer.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27(31):5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch SL, Nagtegaal ID. What is “good quality” in rectal cancer surgery? The pathologist’s perspective. Recent Results Cancer Res. 2014;203:41–46. doi: 10.1007/978-3-319-08060-4_5. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Fiorica F, Cartei F, Licata A, Enea M, Ursino S, Colosimo C, et al. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat Rev. 2010;36(7):539–549. doi: 10.1016/j.ctrv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Calvo FA, Morillo V, Santos M, Serrano J, Gomez-Espí M, Rodriguez M, et al. Interval between neoadjuvant treatment and definitive surgery in locally advanced rectal cancer: impact on response and oncologic outcomes. J Cancer Res Clin Oncol. 2014;140(10):1651–1660. doi: 10.1007/s00432-014-1718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck DE, Roberts PL, Saclarides TJ. ASCRS Textbook of Colon and Rectal Surgery. 2nd Edition. New York, NY: Springer; 2011. pp. 163–188. [Google Scholar]
- 9.NCCN Clincal practice guidelines in Onology, Rectal Cancer, Version 1.2016. Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 10.Kim JH, Byun SJ, Park SG, Oh YK, Baek SK. Interval between Surgery and Radiation Therapy Is an Important Prognostic Factor in Treatment of Rectal Cancer. Cancer Res Treat. 2012;44(3):187–194. doi: 10.4143/crt.2012.44.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JS, Kim NK, Min BS, Hur H, Ahn JB, Keum KC. Adjuvant radiotherapy following total mesorectal excision for stage IIA rectal cancer: is it beneficial? Int J Colorectal Dis. 2010;25(9):1103–1110. doi: 10.1007/s00384-010-0970-1. [DOI] [PubMed] [Google Scholar]
- 12.Moody JS, Sawrie SM, Kozak KR, Plastaras JP, Howard G, Bonner JA. Stage-specific survival differences associated with postoperative radiotherapy for gastrointestinal cancers. J Gastrointest Cancer. 2008;39(1–4):86–99. doi: 10.1007/s12029-009-9053-3. [DOI] [PubMed] [Google Scholar]
- 13.Huang CM, Huang CW, Huang MY, Lin CH, Chen CF, Yeh YS, et al. Coexistence of perineural invasion and lymph node metastases is a poor prognostic factor in patients with locally advanced rectal cancer after preoperative chemoradiotherapy followed by radical resection and adjuvant chemotherapy. Med Princ Pract. 2014;23(5):465–470. doi: 10.1159/000363604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cienfuegos JA, Rotellar F, Baixauli J, Beorlegui C, Sola JJ, Arbea L, et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol. 2015;22(3):916–923. doi: 10.1245/s10434-014-4051-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, Lee SY, Kim HR, Kim YJ. Prognostic Effect of Pretreatment Serum Carcinoembryonic Antigen Level: A Useful Tool for Prediction of Distant Metastasis in Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiotherapy and Total Mesorectal Excision. Medicine (Baltimore) 2015 Aug;94(31):e1291. doi: 10.1097/MD.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HH, Chang YY, Lin JK, Jiang JK, Lin CC, Lan YT, et al. The role of adjuvant chemotherapy in stage II colorectal cancer patients. Int J Colorectal Dis. 2014;29(10):1237–1243. doi: 10.1007/s00384-014-1943-6. [DOI] [PubMed] [Google Scholar]
- 17.Genovesi D, Cèfaro GA, Vinciguerra A, Augurio A, D’Alessandro M, Borzillo V, et al. Retrospective long-term results and prognostic factors of postoperative treatment for UICC stages II and III rectal cancer. Tumori. 2009;95(6):675–682. doi: 10.1177/030089160909500606. [DOI] [PubMed] [Google Scholar]
- 18.Genovesi D, Myerson RJ, Cèfaro GA, Vinciguerra A, Augurio A, Trignani M, et al. Postoperative 5-FU based radiochemotherapy in rectal cancer: retrospective long term results and prognostic factors of a pooled analysis on 1,338 patients. Anticancer Res. 2013;33(10):4557–4566. [PubMed] [Google Scholar]
- 19.Bagatzounis A, Willner J, Oppitz U, Flentje M. The postoperative adjuvant radiation therapy and radiochemotherapy for UICC stage II and III rectal cancer. A retrospective analysis. Strahlenther Onkol. 2000;176(3):112–117. doi: 10.1007/pl00002335. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Kulaylat M, Rockette H, Hassett J, Rajput A, Dunn KB, et al. Should total number of lymph nodes be used as a quality of care measure for stage III colon cancer? Ann Surg. 2009;249(4):559–563. doi: 10.1097/SLA.0b013e318197f2c8. [DOI] [PubMed] [Google Scholar]
- 21.Junginger T, Goenner U, Lollert A, Hollemann D, Berres M, Blettner M. The prognostic value of lymph node ratio and updated TNM classification in rectal cancer patients with adequate versus inadequate lymph node dissection. Tech Coloproctol. 2014;18(9):805–811. doi: 10.1007/s10151-014-1136-x. [DOI] [PubMed] [Google Scholar]
- 22.Gill A, Brunson A, Lara P, Jr, Khatri V, Semrad TJ. Implications of lymph node retrieval in locoregional rectal cancer treated with chemoradiotherapy: a California Cancer Registry Study. Eur J Surg Oncol. 2015;41(5):647–652. doi: 10.1016/j.ejso.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Ye M, Bai Y, Rong L, Hou Y. Prognostic value of lymph node ratio in survival of patients with locally advanced rectal cancer. Can J Surg. 2015;58(4):237–244. doi: 10.1503/cjs.001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Li Z, Gu X, Fang Y, Xiang J, Chen Z. Prognostic factors associated with locally recurrent rectal cancer following primary surgery (Review) Oncol Lett. 2014;7(1):10–16. doi: 10.3892/ol.2013.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song C, Song S, Kim JS, Oh HK, Kim DW, Lee KW, et al. Impact of Postoperative Chemoradiotherapy versus Chemotherapy Alone on Recurrence and Survival in Patients with Stage II and III Upper Rectal Cancer: A Propensity Score-Matched Analysis. PLoS One. 2015 Apr 22;10(4):e0123657. doi: 10.1371/journal.pone.0123657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JX, Wang Y, Chen N, Chen LC, Bai PG, Pan JJ. In the era of total mesorectal excision: adjuvant radiotherapy may be unnecessary for pT3N0 rectal cancer. Radiat Oncol. 2014 Jul 22;9:159. doi: 10.1186/1748-717X-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cihan S, Kucukoner M, Ozdemir N, Dane F, Sendur MA, Yazilitas D, et al. Recurrence risk and prognostic parameters in stage I rectal cancers. Asian Pac J Cancer Prev. 2014;15(13):5337–5341. doi: 10.7314/apjcp.2014.15.13.5337. [DOI] [PubMed] [Google Scholar]
- 28.Cerezo L, Ciria JP, Arbea L, Liñán O, Cafiero S, Valentini V, et al. Current treatment of rectal cancer adapted to the individual patient. Rep Pract Oncol Radiother. 2013;18(6):353–362. doi: 10.1016/j.rpor.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]