Abstract
Background and aims
Kidney cancer is among the cancers that have the highest growth rate in all age and racial groups in the world and is as the most deadly type of urinary tract cancer. Since awareness about this cancer incidence status and mortality is essential for better planning, this study aimed to investigate the incidence and mortality rate of kidney cancer and its relationship with the development index in the world in 2012.
Method
This study was an ecological study conducted based on GLOBOCAN project of the World Health Organization (WHO) for the countries in the world. The correlation between Standardized Incidence Rates (SIRs) and Standardized Mortality Rates (SMRs) of kidney cancer with HDI and its components was assessed using SPSS18.
Results
In total, 337,860 incidence cases (213,924 were men and 123,936 women) and 143,406 deaths (90,802 cases in men and 52,604 in women) of kidney cancer were recorded in 2012. A positive correlation of 0.731 was seen between SIR of kidney cancer and HDI (p≤0.001). Also, a negative correlation of 0.627 was seen between SMR of kidney cancer and HDI (p≤0.001).
Conclusion
The incidence and mortality rate of kidney cancer is higher in developed countries. A significant positive correlation has been seen between the standardized incidence and mortality rate of kidney cancer with the Human Development Index and its components. We need more studies to examine variation in incidence and mortality of kidney cancer and its related factors in the world.
Keywords: incidence, mortality, kidney cancer, Human Development Index
Introduction
With an annual incidence of 14 million new cases and about 8 million deaths, cancer is a global problem and is responsible for 13 percent of deaths worldwide [1]. Kidney cancer is one of the many types of this disease, with an annual incidence of 338 new cases and 144 thousand deaths equal to 1.7% of all deaths worldwide; it is known as the most deadly urinary tract caner [2,3].
More than 90 percent of all kidney tumors are associated with renal cell carcinoma (RCC) that occurs in adults of both sexes and is the twelfth most common cancer in men and the second most common cancer in women, generally [4]. This cancer is responsible for 2.4% of total cancer burden worldwide [2]. Its incidence is higher in men than in women and also increases with age [5]. The highest incidence of kidney cancer is for North America, Australia, New Zealand and East Europe countries. The lowest incidence rate is for South Africa, desert countries, the Pacific Island and India, its incidence rate in Africa is less than 1.5% per one hundred thousand population [2,6]. Generally, a huge difference exists in incidence of (RCC) in the world, being 15 times higher in the areas with the highest rates than in those with the lowest rate [6].
Various factors affect the incidence and mortality of cancer between different ethnic and geographical areas in the world including: the screening rate done in order to manage the risk of kidney cancer, accidental diagnosis, genetic and environmental risk factors and socio-economic status [7–9]. Although the pathogenesis of renal cell carcinoma is unclear [10], potential risk factors such as genetic factors, chronic kidney disease, a history of kidney cancer in first degree relatives, known birth defects, previous kidney stones, smoking, obesity, high blood pressure, occupational exposures including exposure to trichloroethylene (TCE), cadmium and asbestos may have an important role in kidney cancer detection [11–18]. On the other hand, it seems that moderate alcohol consumption is associated with decreased risk of RCC [19,20].
One of the causes of the difference in incidence and mortality in different regions is difference in access to knowledge (based on a combination of the adult literacy rate and enrollment rates of primary education), having a long and healthy life (based on life expectancy at birth) and good standard of living (based on GDP per capita due to purchasing power parity) that are mentioned as the Human Development Index (HDI). HDI is the arithmetic average of three mentioned indices that is a number between zero and one [21].This indicator is one of the indicators used for evaluation of diseases and deaths between countries and is considered as a useful classification to compare cancers at a global level [22–25]. It seems that this index and its components have a correlation with kidney cancer incidence and mortality, therefore the results of various studies indicate a relationship between the incidence and mortality of this cancer and the development index [26,27]. In view of the fact that awareness about kidney cancer incidence and mortality can be useful for health programs and research activities and considering the possible role of the Human Development Index, this study was performed with the aim of investigating the incidence and mortality of kidney cancer and its relationship with development index and its components in the world in 2012.
Materials and methods
This study was a global ecologic study for assessing the correlation between age-specific incidence and mortality rate (ASR) with HDI and its components such as life expectancy at birth, mean years of schooling, and Gross national income (GNI) per capita. Data about the age-specific incidence and mortality rate (ASR) for every country in 2012 were obtained from the global cancer project available at http://globocan.iarc.fr/Default.aspx [28] and HDI from Human Development Report 2013 [29], that includes information about HDI and its details for every country in the world in 2012.
Method for estimating the age-specific incidence and mortality rates in global cancer project by international agency for research on cancer
Age-specific incidence rate estimate
The methods of estimation are country specific, and the quality of the estimation depends upon the quality and on the amount of the information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system which independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with an aim of providing a broad indication of the robustness of the estimation. The methods to estimate the gender- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories, in priority order:
1. Rates projected to 2012 (38 countries); 2. Most recent rates applied to 2012 population (20 countries); 3. Estimated from national mortality by modeling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries); 4. Estimated from national mortality estimates by modeling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (9 European countries); 5. Estimated from national mortality estimates using modeled survival (32 countries); 6. Estimated as the weighted average of the local rates (16 countries); 7. One cancer registry covering a part of a country is used as representative of the country profile (11 countries); 8. Age/gender specific rates for “all cancers” were partitioned using data on relative frequency of different cancers (by age and gender) (12 countries); 9. The rates are those of neighboring countries or registries in the same area (33 countries) [28].
Age-specific mortality rate estimate
Depending on the degree of detail and accuracy of the national mortality data, six methods have been utilized in the following order of priority:
1. Rates projected to 2012 (69 countries); 2. Most recent rates applied to 2012 population (26 countries); 3. Estimated as the weighted average of regional rates (1 country); 4. Estimated from national incidence estimates by modeling, using country-specific survival (2 countries); 5. Estimated from national incidence estimates using modeled survival (83 countries); 6. The rates are those of neighboring countries or registries in the same area (3 countries) [2].
Human Development Index (HDI)
HDI is a composite measure of indicators along three components, including life expectancy, educational attainment, and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low and medium HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within countries of both the North and the South, and income inequality within and between many countries has been rising. According to HDI, countries in the world are divided into four categories as follows: countries with very high HDI (HDI≥=0.80), countries with a high HDI (0.80>HDI>0.710), medium HDI countries (0.710≥HD≥0.535), and countries with a low HDI (HDI<0.535) [29].
Statistical analysis
In this study, we used correlation bivariate method for assessment of the correlation between age-specific incidence and mortality rate (ASR) with HDI and its details, which include life expectancy at birth, mean years of schooling, and GNI per capita. Statistical significance was assumed if P<0.05. All reported P-values are two-sided. Statistical analyses were performed using SPSS (Version 15.0, SPSS Inc.).
Results
Worldwide, about 337,860 cases of kidney cancer occurred in 2012, among which 213,924 were men and 123,936 women. In very high HDI countries 183,867 cases, in countries with high HDI 54,493 cases, in medium HDI countries 89,465, and in countries with low HDI 9,896 cases occurred. The five countries with the largest number of kidney cancer are: China with 66,466 cases, the United States of America (USA) with 58,222 cases, The Russian Federation with 19,313 cases, Germany and Japan with 19,615 and 16,830 cases, respectively. Five countries that had the most cases of kidney cancer in men include: China with 44,372 cases, the USA with 36,345 cases, Germany with 11,353 cases, The Russian Federation and Italy with 10,921 and 7,681 cases, respectively. Also, five countries that had the largest number of cancer in women include: China with 22,094 cases, the USA with 21,877 cases, The Russian Federation with 8,392 cases, Germany and Japan with 7,262 and 5,689 cases, respectively.
The age-standardized incidence [ASIR] of kidney cancer in the world
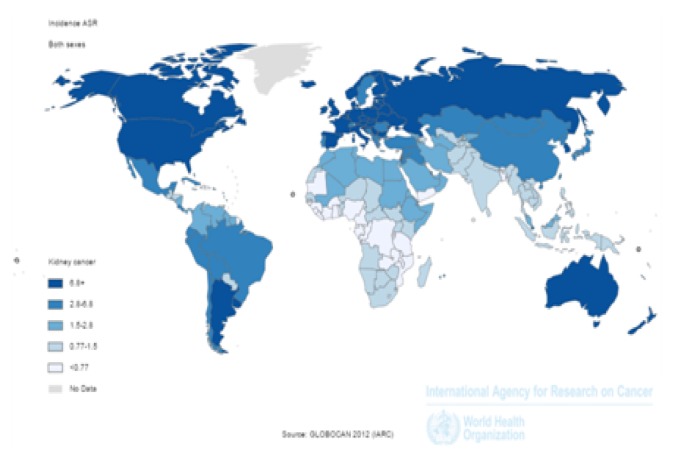
Standardized incidence rate of kidney cancer in the world was 4.4/100,000 population people, 6/100,000 population in men, and 3/100,000 population in women. The incidence of kidney cancer in very high HDI countries was 9.1 per 100,000 population, in countries with HDI 4.7/100,000 population, in countries with moderate HDI 2.5/100,000 population, and in countries with low HDI 1/100,000 population. Five countries with the highest ASIR included: Czech Republic with 16.7/100,100 population, Lithuania with 13.2/100,100 population, Slovakia with 12.5/100,100 population, USA with 12/100,100 population and Estonia with 11.7/100,100 population, respectively. Five countries with the highest ASIR in men include Czech Republic with 24.1/100,100 population, Lithuania with 20.5/100,100 population, Estonia with 17.6/100,100 population, Slovakia with 17.5/100,100 population, and Latvia with 16.4/100,100 population, respectively. Five countries with the highest ASIR in women included: the Czech Republic with 10.5/100,100 population, Slovakia with 8.7/100,100 population, USA with 8.5/100,100 population, Lithuania with 8.2/100,100 population and Belarus with 7.9/100,100 population, respectively (Figure 1).
Figure 1.
Distribution of standardized incidence of kidney cancer worldwide (extracted from GLOBOCAN 2012).
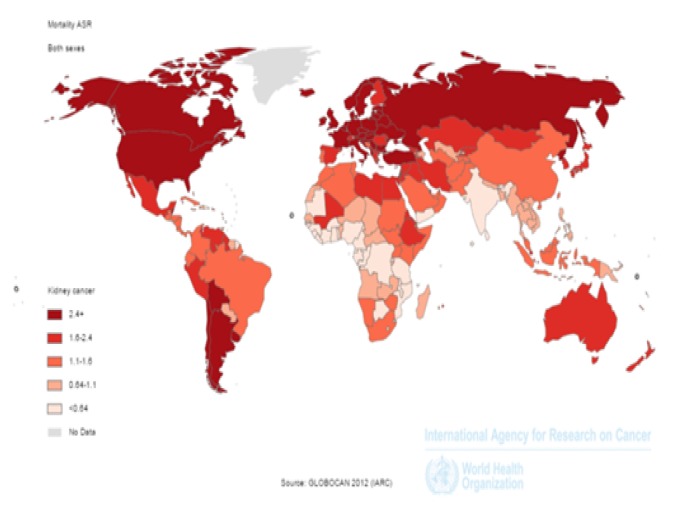
The number of deaths from kidney cancer in the world
In the world, 143,406 deaths from kidney cancer occurred in 2012, 90,802 cases in men and 52,604 in women. The number of deaths from kidney cancer in very high HDI countries was 65,853 cases, in high HDI countries, 27,917 cases, in medium HDI countries 41,103 cases, and in countries with low HDI 8,486 cases, respectively. Five countries with the most cases of the deaths included: China with 25,583 cases, the USA with 14,919 cases, the Russian Federation with 9,025 cases, Japan and Germany with 7,540 and 8,124 cases, respectively. Five countries with the highest death rates in men included: China with 16,712 cases, the USA with 9,567 cases, the Russian Federation with 5,601 cases, Japan with 5,177 cases, and Germany with 4,713 cases. Five countries with the highest death rates in women included: China with 8871 cases, the USA with 5,333 cases, the Russian Federation with 3,424 cases, Japan with 2,947 cases, and Germany with 2,827 cases.
Age-Standard death rates [ASDR] of kidney cancer in the world
In the world in 2012, age-standardized mortality rate for kidney cancers was 1.8/100,100 population, 2.5 in men and 1.2/100,100 population in women. ASDR in very high HDI countries was 2.6/100,100 population, in countries with high HDI 2.4/100,100 population, in medium HDI countries 1.1/100,100 population, and in countries with low HDI was 0.9/100,100 population. Five countries that had the highest ASDR, respectively, include: Lithuania with 4.9/100,100 population, Czech Republic with 4.8/100,100 population, Latvia with 4.7/100,100 population, Estonia with 4.6/100,100 population, and Uruguay with 4.4/100,100 population. Five countries with the highest ASDR in men include: Lithuania with 8.5/100,100 population, Estonia with 7.6/100,100 population, Latvia with 7.4/100,100 population, Uruguay with 7.2/100,100 population, and the Czech Republic with 7.1 per cent thousand people, respectively. Five countries with the highest ASDR in women include: Mauritius with 3.1/100,100 population, Czech Republic with 3/100,100 population, Latvia with 3/100,100 population, Turkey and Slovakia with 2.9 per and 2.8/100,100 population, respectively.
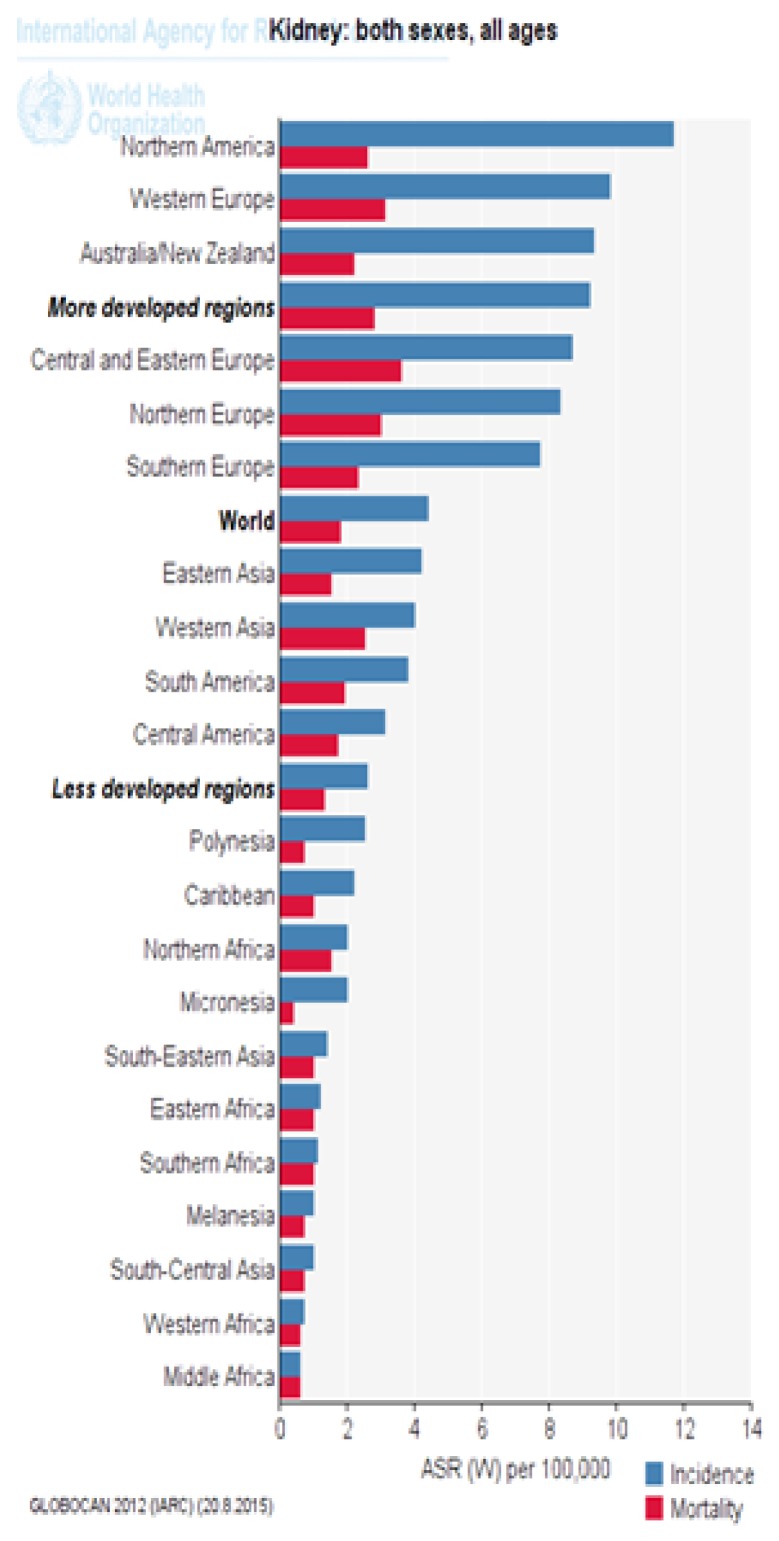
Figure 3 shows the standardized incidence and mortality of kidney cancer in different parts of the UN. As it is evident, most of developed countries have a higher standardized incidence and mortality of kidney cancer than underdeveloped countries (Figure 1).
Figure 3.
Standardized incidence and mortality of kidney cancer in different parts of the UN (extracted from GLOBOCAN 2012).
The relationship between the standardized incidence rate of kidney cancer and the human development index
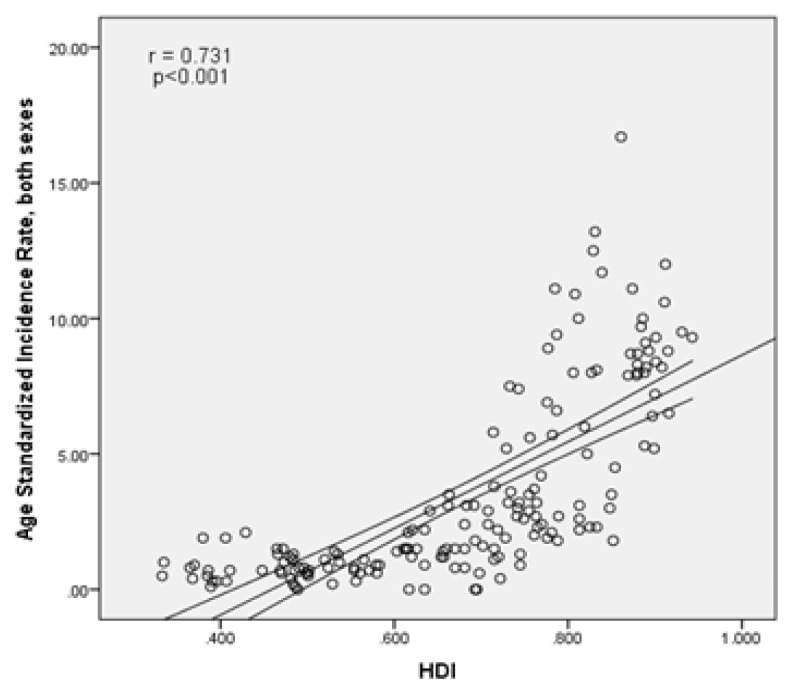
There is a statistically significant positive correlation of 0.731 between standardized incidence of cancer and the human development index (p<0.001). Components of human development index, as well, had a positive correlation with standardized cancer incidence, so that the standardized incidence rate was positively correlated with life expectancy at birth equal to 0.617 (p<0.001), with mean years of education equal to 0.725 (p<0.001) and the income level per person of population equal to 0.533 (p<0.001), respectively (Figure 4).
Figure 4.
The association between standardized incidence of kidney cancer and human development index.
The relationship between the standardized mortality of kidney cancer and the human development index
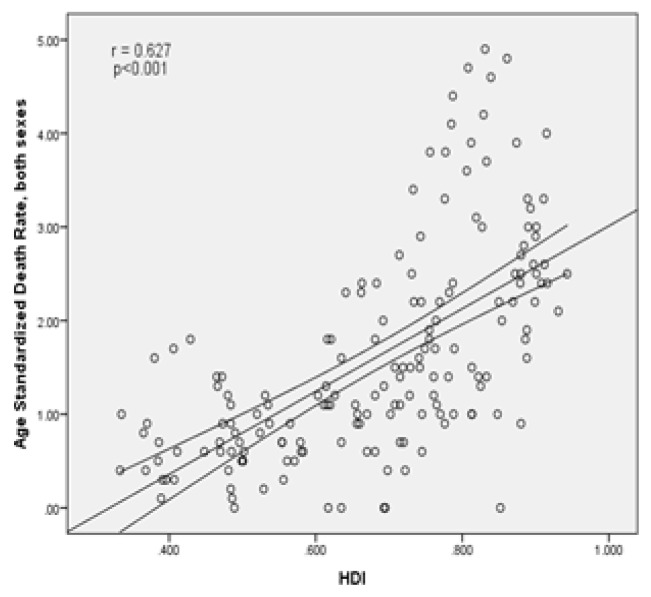
There was, as well, a statistically significant positive correlation (0.627) between standardized cancer mortality rate and the human development index (p<0.001). There was also a positive correlation between human development index components with standardized cancer mortality rates, so that the relationship between the standardized mortality with life expectancy at birth had a positive correlation equal to 0.532 (p<0.001), with mean years of schooling equal to 0.63 (p<0.001) and with income level per person of population had a positive correlation of 0.395 (p<0.001), respectively (Figure 5).
Figure 5.
The association between standardized mortality of kidney cancer and human development index.
Discussion
Kidney cancer is among the cancers that have shown highest growth rate for all age groups and races in the world and is known as the world’s sixteenth common cause of cancer death [2,30,31]. In developed countries, the age-standardized incidence and death rate of this cancer is higher, so that 52 percent of deaths due to this disease occur in developed countries of the world [2,32]. But it is predicted that the number of patients with kidney cancer increases year by year under the influence of aging and population growth in next coming decades as its incidence is equal to 62% in areas with low and middle income and reaches to 39% in high and very high income areas by 2030 [33]. According to the findings of this study, the age standardized incidence and mortality rate of kidney cancer is associated with the Human Development Index and its components in the world. The highest standardized incidence rate of kidney cancer is related to countries with very high and high Human Development Index, respectively. Results of other studies have also shown that kidney cancer age-standardized incidence rates have increased about 23 percent (3.82 to 4.7) for both sexes between 1990 and 2013. These rates are lower in developing countries with lower HDI rates than developed countries with higher HDI. But, their relative increase is the same. So that in these years, it has had an increase rate of 34 percent (1.69 to 2.27) in countries with lower HDI and 36 percent (7.15 to 9.71) in countries with higher HDI. One possible explanation for the similar incidence of kidney cancer in these countries is that, even if the risk factors only for kidney cancer is different with development status, the general pattern of risk factors may compensate this difference [32]. But for deaths of kidney cancer it is noteworthy that this rate reached its peak in Europe union in early 1900s, generally (4.8 per 100 thousand people in men and 2.1 per 100 thousand people in women) [34]. Then, a downward trend was seen in several Central and Eastern European countries with high HDI including France, Germany, Italy, Austria and the Netherlands; this decreasing trend may be due to improvements in diagnosis, treatment and reducing tobacco consumption [35].
Among the dimensions of the Human Development Index, we can point to life expectancy at birth that had a significant positive relationship with standardized incidence and mortality of kidney cancer in this study. The results of other studies show that the highest incidence rate of cancer is seen in elderly people and as the development of new tools for better diagnosis and treatment of cancer was targeted in 1990s, it is expected that the incidence rate of cancer increases and death rate decreases [36,37]. Another study showed that kidney cancer in elderly people is a real challenge for oncologic urologists. With increased life expectancy, incidence of renal cell carcinoma increases and its mortality also increases due to higher invasiveness of the disease and thus reduces survival chance [38]. Among other indicators of the Human Development Index is access to knowledge that had a significant positive correlation with standardized incidence and mortality rate of kidney cancer. The level of education is used as a social level indicator in such a way that a deep social difference between the highest and lowest educational level has been observed in women or men for some cancers [39]. The level of education can reflect health literacy or a person’s ability to manage his disease [40]. Many chronic diseases such as kidney cancer have more incidence and prevalence among groups with lower educational levels [41,42]. This difference in high and low level of education in age group 25–59 years was more than in age group 60–79 and is caused by risk factors related to this cancer such as smoking habits or other factors such as consumption of alcohol which seems to be a protective factor against kidney cancer in such a way that alcohol consumption is higher in people with higher education [42,43]. The results of one study conducted in the Netherlands also revealed that patients with higher levels of education have better 5-year survival and lower mortality rates compared to those with lower education for all cancers including kidney cancer [44]. Education level may be related to behaviors, health conditions, access to knowledge and resources, diagnosis, timeliness and type of cancer treatment and psychological support level that have direct or indirect effect on cancer survival and mortality caused by it [45–51]. From other indicators of the Human Development Index, we can point to appropriate income level which is determined by Gross Domestic Product. In our study, income level had a positive significant correlation with standardized incidence and mortality rate of kidney cancer. Other studies have shown that overall, social and economic disparities are seen in morbidity and mortality across Europe [52–54]. Regarding the incidence of kidney cancer, it is higher in countries with higher income and economic status than countries with lower economic status and among the reasons we can mention increased accidental diagnosis chance of tumors, higher prevalence of obesity and hypertension in high-income countries such as: the United States of America and the United Kingdom compared to lower income countries such as Brazil and China, as well as in urban areas than in rural ones and other risk factors associated with this cancer [55–60]. Despite the fact that the increased diagnosis at early stages of kidney cancer leads to increased incidence, due to lack of efficacy of treatment in early stages the mortality rates have not been decreased [61].
Conclusion
The incidence and mortality rate of kidney cancer is higher in developed countries. A significant positive correlation has been seen between the standardized incidence and mortality rate of kidney cancer with the Human Development Index and its components. We need more studies to examine variations in incidence and mortality of kidney cancer and its related factors in the world.
Figure 2.
Distribution of standardized mortality of kidney cancer worldwide (extracted from GLOBOCAN 2012).
References
- 1.Mohammadian M, Soroush A, Mohammadian-Hafshejani A, Towhidi F, Hadadian F, Salehiniya H. Incidence and Mortality of Liver Cancer and Their Relationship with Development in Asia. Asian Pac J Cancer Prev. 2016;17(4):2041–2047. doi: 10.7314/apjcp.2016.17.4.2041. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Lindblad P. Epidemiology of renal cell carcinoma. Scan J Surg. 2004;93:88–96. doi: 10.1177/145749690409300202. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Bray F, Pisani P, et al. IARC CancerBase No 5. Lyon: IARCPress; 2001. Globocan 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. [Google Scholar]
- 5.Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352(9141):1691–1696. doi: 10.1016/S0140-6736(98)01041-1. [DOI] [PubMed] [Google Scholar]
- 6.Adibi M, Karam JA, Wood CG. Reporting geographic and temporal trends in renal cell carcinoma: why is this important? Eur Urol. 2015;67(3):531–532. doi: 10.1016/j.eururo.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Olschwang S, Serova-Sinilnikova OM, Lenoir GM, Thomas G. PTEN germ-line mutations in juvenile polyposis coli. Nat Genet. 1998;18(1):12–14. doi: 10.1038/ng0198-12. [DOI] [PubMed] [Google Scholar]
- 8.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104( Suppl 3):S377–S387. doi: 10.2105/AJPH.2013.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weikert S, Ljungberg B. Contemporary epidemiology of renal cell carcinoma: perspectives of primary prevention. World J Urol. 2010;28(3):247–252. doi: 10.1007/s00345-010-0555-1. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, et al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol. 2003;14(1):197–207. doi: 10.1097/01.asn.0000039608.81046.81. [DOI] [PubMed] [Google Scholar]
- 11.Ferreccio C, Smith AH, Durán V, Barlaro T, Benítez H, Valdés R, et al. Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol. 2013;178(5):813–818. doi: 10.1093/aje/kwt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho E, Lindblad P, Adami H-O. Kidney cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of cancer epidemiology. 2nd edn. Oxford: Oxford University Press; 2008. 2008. pp. 597–612. [Google Scholar]
- 13.Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM. 2015;108(3):205–212. doi: 10.1093/qjmed/hcu195. [DOI] [PubMed] [Google Scholar]
- 14.Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2014;134(2):384–396. doi: 10.1002/ijc.28093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, et al. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med. 2011;171(16):1487–1493. doi: 10.1001/archinternmed.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow WH, Gridley G, Fraumeni JF, Jr, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343(18):1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JD, Van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114(1):101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 18.Mandel JS, McLaughlin JK, Schlehofer B, Mellemgaard A, Helmert U, Lindblad P, et al. International renal-cell cancer study. IV. Occupation. Int J Cancer. 1995;61(5):601–605. doi: 10.1002/ijc.2910610503. [DOI] [PubMed] [Google Scholar]
- 19.Song DY, Song S, Song Y, Lee JE. Alcohol intake and renal cell cancer risk: a meta-analysis. Br J Cancer. 2012;106(11):1881–1890. doi: 10.1038/bjc.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellocco R, Pasquali E, Rota M, Bagnardi V, Tramacere I, Scotti L, et al. Alcohol drinking and risk of renal cell carcinoma: results of a meta-analysis. Ann Oncol. 2012;23(9):2235–2244. doi: 10.1093/annonc/mds022. [DOI] [PubMed] [Google Scholar]
- 21.Sagar AD, Najam A. The human development index: a critical review. Ecological economics. 1998;25(3):249–264. [Google Scholar]
- 22.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 23.Razi S, Ghoncheh M, Mohammadian-Hafshejani A, Aziznejhad H, Mohammadian M, Salehiniya H. The incidence and mortality of ovarian cancer and their relationship with the Human Development Index in Asia. Ecancer medical science. 2016 Mar 24;10:628. doi: 10.3332/ecancer.2016.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015;3(4):135–140. doi: 10.1016/j.prnil.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res. 2015;4(6):763–774. doi: 10.3978/j.issn.2218-6751.2015.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17(1):381–386. doi: 10.7314/apjcp.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 27.Ghoncheh M, Mirzaei M, Salehiniya H. Incidence and Mortality of Breast Cancer and their Relationship with the Human Development Index (HDI) in the World in 2012. Asian Pac J Cancer Prev. 2015;16(18):8439–8443. doi: 10.7314/apjcp.2015.16.18.8439. [DOI] [PubMed] [Google Scholar]
- 28.Ferlay J, et al. IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. Available from: http://globocan.iarc.fr. [Google Scholar]
- 29.Malik K. UNDP-HDRO Human Development Reports. 2013. Human Development Report 2013. The rise of the South: Human progress in a diverse world. The Rise of the South: Human Progress in a Diverse World (March 15, 2013) [Google Scholar]
- 30.De P, Otterstatter MC, Semenciw R, Ellison LF, Marrett LD, Dryer D. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 2014;25(10):1271–1281. doi: 10.1007/s10552-014-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute. SEER Stat Fact Sheets: Kidney and Renal Pelvis. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/kidrp.html. [Google Scholar]
- 32.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Levi F, Lucchini F, Negri E, La Vecchia C. Declining mortality from kidney cancer in Europe. Ann Oncol. 2004;15(7):1130–1135. doi: 10.1093/annonc/mdh270. [DOI] [PubMed] [Google Scholar]
- 35.Levi F, Ferlay J, Galeone C, Lucchini F, Negri E, Boyle P, et al. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008;101(8):949–958. doi: 10.1111/j.1464-410X.2008.07451.x. [DOI] [PubMed] [Google Scholar]
- 36.Xing M. BRAF mutation in papillary thyroid microcarcinoma: the promise of better risk management. Ann Surg Oncol. 2009;16(4):801–803. doi: 10.1245/s10434-008-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 38.Kirkali Z. Kidney cancer in the elderly. Urol Oncol. 2009;27(6):673–676. doi: 10.1016/j.urolonc.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Faggiano F, Lemma P, Costa G, Gnavi R, Pagnanelli F. Cancer mortality by educational level in Italy. Cancer Causes Control. 1995;6(4):311–320. doi: 10.1007/BF00051406. [DOI] [PubMed] [Google Scholar]
- 40.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proc Natl Acad Sci U S A. 2002;99(16):10929–10934. doi: 10.1073/pnas.162086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain SK, Lenner P, Sundquist J, Hemminki K. Influence of education level on cancer survival in Sweden. Ann Oncol. 2008;19(1):156–162. doi: 10.1093/annonc/mdm413. [DOI] [PubMed] [Google Scholar]
- 42.Dalstra JA, Kunst AE, Borrell C, Breeze E, Cambois E, Costa G, et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Int J Epidemiol. 2005;34(2):316–326. doi: 10.1093/ije/dyh386. [DOI] [PubMed] [Google Scholar]
- 43.Braaten T, Weiderpass E, Kumle M, Lund E. Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2591–2597. doi: 10.1158/1055-9965.EPI-05-0345. [DOI] [PubMed] [Google Scholar]
- 44.Aarts MJ, Kamphuis CB, Louwman MJ, Coebergh JW, Mackenbach JP, van Lenthe FJ. Educational inequalities in cancer survival: a role for comorbidities and health behaviours? J Epidemiol Community Health. 2013;67(4):365–373. doi: 10.1136/jech-2012-201404. [DOI] [PubMed] [Google Scholar]
- 45.van Vliet EP, Eijkemans MJ, Steyerberg EW, Kuipers EJ, Tilanus HW, van der Gaast A, et al. The role of socio-economic status in the decision making on diagnosis and treatment of oesophageal cancer in The Netherlands. Br J Cancer. 2006;95(9):1180–1185. doi: 10.1038/sj.bjc.6603374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25(3):277–284. doi: 10.1200/JCO.2006.08.3063. [DOI] [PubMed] [Google Scholar]
- 47.Spiegel D. Effects of psychotherapy on cancer survival. Nat Rev Cancer. 2002;2(5):383–388. doi: 10.1038/nrc800. [DOI] [PubMed] [Google Scholar]
- 48.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54(3):269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 49.Spiegel D, Kraemer H, Bloom J, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2(8668):888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 50.Lehto US, Ojanen M, Dyba T, Aromaa A, Kellokumpu-Lehtinen P. Baseline psychosocial predictors of survival in localised breast cancer. Br J Cancer. 2006;94(9):1245–1252. doi: 10.1038/sj.bjc.6603091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kogevinas M, Porta M. Socioeconomic differences in cancer survival: a review of the evidence. IARC Sci Publ. 1997;138:177–206. [PubMed] [Google Scholar]
- 52.Cavelaars AE, Kunst AE, Geurts JJ, Crialesi R, Grötvedt L, Helmert U, et al. Differences in self reported morbidity by educational level: a comparison of 11 western European countries. J Epidemiol Community Health. 1998;52(4):219–227. doi: 10.1136/jech.52.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunst AE, Leon DA, Groenhof F, Mackenbach JP. Occupational class and cause specific mortality in middle aged men in 11 European countries: comparison of population based studies. Commentary: Unequal inequalities across Europe. BMJ. 1998;316(7145):1636–1642. doi: 10.1136/bmj.316.7145.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huisman M, Kunst AE, Andersen O, Bopp M, Borgan JK, Borrell C, et al. Socioeconomic inequalities in mortality among elderly people in 11 European populations. J Epidemiol Community Health. 2004;58(6):468–475. doi: 10.1136/jech.2003.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80(6):827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 56.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 57.Monteiro CA, D’A Benicio MH, Conde WL, Popkin BM. Shifting obesity trends in Brazil. Eur J Clin Nutr. 2000;54(4):342–346. doi: 10.1038/sj.ejcn.1600960. [DOI] [PubMed] [Google Scholar]
- 58.WHO. Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity; Geneva. 3–5 June 1997; Available from: whqlibdoc.who.int/trs/WHO_TRS_894.pdf. [Google Scholar]
- 59.Martorell R, Khan LK, Hughes ML, Grummer-Strawn LM. Obesity in women from developing countries. Eur J Clin Nutr. 2000;54(3):247–252. doi: 10.1038/sj.ejcn.1600931. [DOI] [PubMed] [Google Scholar]
- 60.Seidell JC. The epidemiology of obesity. In: Björntorp P, editor. International Textbook of Obesity. Chichester: John Wiley & Sons Ltd; 2001. pp. 23–29. [Google Scholar]
- 61.Sun M, Thuret R, Abdollah F, Lughezzani G, Schmitges J, Tian Z, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59(1):135–141. doi: 10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]