Abstract
Background and aim
Comparison of different irrigation and agitation methods for the removal of two types of calcium hydroxide medicaments from the root canal walls.
Methods
Fifty extracted single rooted teeth were selected for this study. After decoronation, the root canals of these teeth were prepared to the size F3 (30 no.) using rotary ProTaper file system. These samples were randomly divided into four groups. Group 1 (n=20) were filled completely with water based calcium hydroxide (CH), Group 2 (n=20) were filled with oil based CH using lentulo spiral, Group 3 (n=5) - the positive control group received the CH as intracanal medication, but no subsequent removal, Group 4 (n=5) - the negative control did not receive CH placement. Further on, Group 1 and Group 2 were divided into four sub-groups (n=5). In sub-group A we performed conventional syringe irrigation with side-vented needle sub-group B) manual dynamic agitation, sub-group C sonic agitation using endoactivator, sub-group D passive ultrasonic irrigation (PUI). Roots were split longitudinally into mesial and distal halves. Digital images of the root canal walls were acquired by a Dental Operating Microscope (DOM) and assessed by using a scoring criteria at different thirds (coronal, middle and apical) of the root canal as follows: score 1, score 2, score 3, and score 4. Data were analyzed applying one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests at a 95% confidence interval (P < 0.05).
Results
Statistically significant differences were not found between the experimental groups and the negative group in any one third of the root canal (P>0.05). However, a difference did exist between the experimental groups and the positive control group (P<0.05). None of the experimental groups totally removed CH substances from root canal walls.
Conclusion
Among all experimental groups, removal of CH was best achieved by sonic agitation using endoactivator followed by passive ultrasonic irrigation (PUI), manual dynamic agitation and conventional syringe irrigation with side-vented needle.
Keywords: calcium hydroxide, endoactivator system, intracanal medication, sonic irrigation
Introduction
The presence of microbes within the root canal plays a key role in causing endodontic infections [1]. The main aim of root canal therapy is getting a microbial diminution and subsequent elimination of their byproducts from the root canal system [2]. However, chemo-mechanical debridement cannot completely eliminate the microbes from the root canal [3]. Hence, the use of intracanal medicaments has been recommended [4].
CH is commonly used as an intracanal medicament with excellent antibacterial activity against most of the commonly seen bacterial strains identified in root canal infections [5,6]. CH along with a suitable vehicle, and placed in the root canal for several days or weeks, has been widely accepted in endodontic therapy [7]. However it should be completely removed before the final obturation of the root canals because its remnants may prevent the penetration of the sealers into the dentinal tubules, [8] hinders the sealer adhesion to dentin, and may increase the micro apical leakage of the canal obturation [9].
Mechanical instrumentation with a master apical file (MAF) and copious irrigation with Sodium hypochlorite (NaOCl) and Ethylenediaminetetraacetic acid (EDTA) is the most frequently described method for the removal of CH from the root canal [10,11]. However, several other methods have also been proposed over the years for e.g. Using rotary nickel-titanium (Ni-Ti) instruments, [12] using a patency file, [13] and using various devices for the agitation of an intracanal irrigating solution to increase its efficacy [14]. It has been reported that NaOCl solely and in combination with EDTA effectively removes all or most of CH.[15,16]. The mechanical agitation provided by ultrasonic (Passive Ultrasonic Irrigation) and sonic (Endoactivator) instrumentation or a rotary file together with irrigation enhance the removal of CH. However, there is no general consensus among the researchers regarding the best method for the removal of CH [17].
Material and methods
Fifty human single rooted teeth with completely formed apex were used in this in-vitro study (Figure 1). Teeth were stored in 3% NaOCl solution (Prime Dent, India) at room temperature to remove organic debris for 48 hrs. The external root surfaces of the teeth were cleaned with manual scaling and washed under running tap water. Teeth were kept stored in normal saline solution. They were decoronated at the cemento-enamel junction with a diamond disk, leaving 14mm of root length. Roots were radiographed from mesiodistal and buccolingual directions by placing # 10 K-file (Mani, Japan) to ensure the presence of single canal. Final working length was established at 1mm short of the apical foramen. Cleaning and shaping of the root canals was completed with ProTaper Ni-Ti rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland) to the size F3. Then 3% NaOCl and 17% EDTA (Anabond Stedman, India) were used as an irrigating solution. The samples were randomly divided into four groups. Group 1 samples (n=20) were filled completely with water based (Prime Dent, India) CH, Group 2 (n=20) were filled with oil based (Metapex, Aluro Healthcare, NZ) CH using Lentulo Spiral, Group 3 (n=5) - the positive control - received the CH as intracanal medication, but no subsequent removal was done, Group 4 (n=5) -the negative control - did not receive CH.
Figure 1.
QFifty single rooted samples with completely formed apex.
Group 1 and Group 2 were further divided into four sub-groups (n=5). In Sub-Group A, conventional syringe irrigation with side-vented needle (Prime Dent, India), sub-group B, manual dynamic agitation, sub-group C, sonic agitation using Endoactivator, sub-group D, PUI were used. Each sample had the same total post instrumentation irrigation/agitation time of 6 minutes. The access cavities were temporarily sealed with a Cavitemp Temporary Filling Material (Ammdent, India). Samples were stored at 37°C and 100% humidity (Tricks Incubator, India) for 7 days. After this storage period, the temporary filling materials were removed.
In Groups 1 and 2 (sub-group A), CH was removed from root canals using a 30 gauge side-vented endodontic needle (Prime Dent, India). The root canals were irrigated with 5 ml of 3% NaOCl, followed by using an 25# K-file (Mani, Japan) instrument in a circumferential filing action and were irrigated again with 5 ml of 3% NaOCl for 1 minute. Then the irrigant was left undisturbed in the canal space for 1 minute and canals were washed by using normal saline. This was followed by irrigation with 5 ml of 17% EDTA for 1 minute and left untouched in a canal for 1 minute and canals were washed by using normal saline. The last cycle was performed again with 5 ml of 3% NaOCl solution for 1 minute and followed by leaving the canal full of irrigant for 1 minute.
In Groups 1 and 2 (sub-group B), 5 ml of 3% NaOCl was delivered to the root canal with the side-vented needle and agitated with a ProTaper gutta-percha cone (size F3) (Dentsply Maillefer, Switzerland) well-fitted to the working length and moved for 1 minute in a corono-apical direction using back and forth strokes of approximately 1 mm with a frequency of 100 movements per minute, followed by leaving the irrigant in the root canal space for 1 minute and canals were washed by using normal saline. Finally the root canals were irrigated with 5 ml of 17% EDTA solution and agitated in the same manner as mentioned above.
The irrigant was left undisturbed in the canal space for 1 min and canals were washed by using normal saline. For the last agitation cycle, a final rinse was received with 5 ml of 3% NaOCl followed by the agitation via the gutta-percha cone with the same size and taper for 1 minute. The root canal was left undisturbed again for 1 minute with the irrigant inside and later on washed by using normal saline.
In Groups 1 and 2 (sub-group C) root canals were irrigated with agitation of 5 ml of 3% NaOCl using a piezoelectronic unit (Acteon Satelec, P-5 booster, Germany), which was achieved to the full WL for 1 min by using #25 U-files (Mani, Japan). Afterwards the irrigant was left undisturbed in the canal space for 1 min. This was followed by irrigation with 5 ml of 17% EDTA and ultrasonic activation with size-25 U-files (Mani, Japan) for 1 min, and then the solution left untouched in a full canal for 1 min. The final rinse was achieved with 5 ml of 3% NaOCl in conjunction with similar ultrasonic agitation, followed by leaving the canal full of irrigant for 1 min.
In Groups 1 and 2 (sub-group D) root canals were irrigated with agitation using Endoactivator System (Dentsply Tulsa Dental Specialties, Tulsa). This system was introduced as a new technique to improve the efficacy of irrigation procedure with usage of strong, flexible medical grade polymer tip. Endoactivator is a sonic device with disposable flexible non cutting polymer tips of various sizes. The design of the Endoactivator System allows activation of various intracanal irrigation and could produce vigorous intracanal fluid agitation [18]. The Endo Activator System in conjunction with demineralizing agents like EDTA was reported to remove the smear layer and disrupts the simulated biofilm within curved canals [19].
Root canals were irrigated with agitation of 5 ml of 3% NaOCl using Endoactivator System (Dentsply Tulsa Dental Specialties, Tulsa) which was achieved to the full WL for 1 min by using tip size 25/04 (Yellow). Afterwards the irrigant was left undisturbed in the canal space for 1 min. This was followed by irrigation with 5 ml of 17% EDTA and sonic activation for 1 min, and then the solution left untouched in a full canal for 1 min. The final rinse was achieved with 5 ml of 3% NaOCl in conjunction with sonic agitation, followed by leaving the canal full of irrigant for 1 min.
The remaining samples were prepared and used as a control groups. In the positive control group (Group 3) (n=5), no medicament was used to ensure the proper analysis of cleaning attempts. In the negative control group (Group 4) (n=5) canals were filled with CH but no subsequent removal was attempted. This ensured that CH was uniformly delivered throughout the entire canal length.
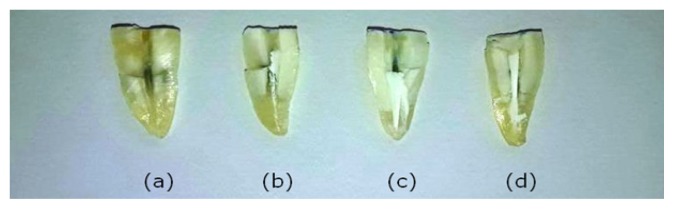
After each technique, root canals were irrigated with 5 ml of sterile saline and dried with multiple paper points (Sure Endo, Korea). Two longitudinal grooves on the buccal and lingual aspects were prepared along the external root surface at the maximum buccolingual diameter to facilitate subsequent splitting of the root to expose the instrumented canal. For this purpose, a cylindrical diamond bur and a diamond disk were used in a high-speed handpiece under copious water cooling with utmost caution to avoid iatrogenic perforation of the canal space. A new razor blade was placed in the buccal or lingual groove; and while the root was secured between two fingers, gentle tapping of the razor blade caused the splitting of the root into two longitudinal halves. The appropriate half of each root with visible semi canal lumen having more amounts of CH remnants was selected and photographs were taken. Digital images were taken by Dental Operating microscope at 19.2x magnification (Global Microscope, St. Louis, MO, U.S.A.). Two observers individually evaluated the amount of the residual medicament of each canal third using a 4-grade scoring system as suggested by Lambrianidis et al. [20] whenever disagreements existed between the assesses during the scoring of the images; they discussed in order to reach an agreement on the scores. The difference in scoring never exceeded score one and the higher score was recorded. The scoring criteria of degree of canal cleanliness and removal of medicament were as follows: Score 1 no visible remnants of medicaments, score 2 scattered remnants of medicament, score 3 distinct masses of medicament, and score 4 densely packed remnants of medicaments (Figure 2). The highest scores of cervical, middle and apical third of root canals were recorded.
Figure 2.
Representative images of removal scores from different groups: score 1 (negative control), score 2, score 3, score 4 (positive control).
Data thus obtained were statistically analyzed using one way analysis of variance (ANOVA) and Tukey’s multiple comparison tests at a confidence interval of 95% (P<0.05).
Results
The CH removal scores and mean and standard deviation values of each group according to the canal third levels are shown in Table I. No statistically significant differences were found between all experimental groups and negative control (P>0.05). CH remnants were seen on the canal walls irrespective of the technique used. The positive control group displayed complete coverage of the entire canal lumen with the densely packed remnants (P<0.05). Among all experimental groups, removal of CH was best achieved by sonic agitation using Endoactivator followed by PUI, manual dynamic agitation and conventional syringe irrigation with side-vented needle. Results show that water based CH medicament is easier to remove than oil based CH medicament.
Table I.
The calcium hydroxide removal scores and mean and standard deviation values of Group 1, 2, 3 and 4 according to the canal third levels are shown. SD=Standard Deviation.
| Groups | Scores | Mean±SD | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Group 1 | ||||||
| Cervical | 5 | 3 | 2 | 0 | 1.7 | 0.82 |
| Middle | 6 | 2 | 2 | 0 | 1.6 | 0.84 |
| Apical | 4 | 3 | 2 | 1 | 2.0 | 1.05 |
| Total | 4 | 3 | 2 | 1 | 2.0 | 1.05 |
| Group 2 | ||||||
| Cervical | 7 | 2 | 1 | 0 | 1.4 | 0.69 |
| Middle | 8 | 1 | 1 | 0 | 1.3 | 0.67 |
| Apical | 4 | 3 | 2 | 1 | 2.0 | 1.05 |
| Total | 5 | 3 | 1 | 1 | 1.8 | 1.03 |
| Group 3 | ||||||
| Cervical | 6 | 4 | 0 | 0 | 1.4 | 0.51 |
| Middle | 5 | 3 | 2 | 0 | 1.7 | 0.82 |
| Apical | 3 | 3 | 2 | 2 | 2.3 | 1.15 |
| Total | 5 | 2 | 1 | 2 | 2.0 | 1.24 |
| Group 4 | ||||||
| Cervical | 10 | 0 | 0 | 0 | 1.0 | 0.00 |
| Middle | 9 | 1 | 0 | 0 | 1.1 | 0.31 |
| Apical | 7 | 2 | 1 | 0 | 1.4 | 0.69 |
| Total | 7 | 2 | 1 | 0 | 1.4 | 0.69 |
| Positive | ||||||
| Cervical | 0 | 0 | 0 | 3 | 4.00 | 0.00 |
| Middle | 0 | 0 | 0 | 3 | 4.00 | 0.00 |
| Apical | 0 | 0 | 0 | 3 | 4.00 | 0.00 |
| Total | 0 | 0 | 0 | 3 | 4.00 | 0.00 |
| Negative | ||||||
| Cervical | 3 | 0 | 0 | 0 | 1.0 | 0.00 |
| Middle | 3 | 0 | 0 | 0 | 1.0 | 0.00 |
| Apical | 3 | 0 | 0 | 0 | 1.0 | 0.00 |
| Total | 3 | 0 | 0 | 0 | 1.0 | 0.00 |
Discussion
In the present study, the main goal was to compare the effectiveness of conventional side vented needle irrigation in removing the CH and comparing it with the manual dynamic agitation, Endoactivator and PUI systems in combination with 3% NaOCl and 17% EDTA. The results of this study showed that none of the techniques removed CH completely from the root canal walls.
EDTA has the ability to neutralize CH residues, which could prevent an interaction with the sealer, or chelate CH residues and these by facilitates easier removal. However, it has been reported that removal of CH from the apical root canal wall, when this method is used, is difficult. This can be explained as the instrumentation and irrigation alone cannot completely clean the entire root canal wall. When CH is removed from the main canal with a file, remnants will remain in canal extensions or irregularities. From these anatomical irregularities it is only possible to remove the CH by irrigation [17].
CH remnants left in the root canal can result in a thicker non-homogenous appearance of root canal sealers. The sealer thickness could have an effect on the sealing ability of root canal fillings. The CH remnants could also result in a chemical reaction with the sealer resulting in a reduction of flow or change in working time. CH remnants could also prevent the sealer from entering into the dentinal tubules resulting in a poor adaptation of the sealer. The dimensional instability of CH and its potential to dissolve in water and dissociate into hydroxide and calcium ions could cause the leakage of root canal fillings in the long run [17].
The Endoactivator uses sonic energy to irrigate root canal systems. This system has 2 components, a handpiece and activator. The battery-operated handpiece activates from 2,000–10,000 cycles/min. During use, the sonic action of the Endoactivator tip usually produces a mass of debris that can be observed within a fluid filled pulp chamber. The main function of the Endoactivator is to produce vigorous intracanal fluid agitation through its swirling movement and cavitation. This hydrodynamic mechanism of activation serves to improve the penetration, circulation, and flow of irrigant into the difficult-to-reach areas of the root canal system. Proper cleaning of root canal systems helps the clinician to achieve three dimensional obturation and these by ensuring long term success [21].
During PUI, acoustic micro streaming and cavitation occur, which cause a specific streaming pattern within the root canal from the apical to the coronal. Because of this micro streaming, more dentine debris can be removed from the root canal compared with syringe delivery of the irrigant, even from remote places in the root canal. Probably the same mechanisms are responsible for the more effective removal of CH during PUI in comparison with syringe delivery of the irrigant [17].
Conventional irrigation with syringes has been advocated as an effective method of irrigant delivery before the advent of passive ultrasonic activation. This technique is still widely accepted by both general practitioners and endodontist. The technique involves dispensing an irrigant into a canal through needles of variable gauges, either passively or with agitation. The latter is achieved by moving the needle up and down the canal space. The latter design has been proposed to improve the hydrodynamic activation of an irrigant and these by reducing the chance of apical extrusion. It is very crucial that the needle should remain loose inside the canal during irrigation. This allows the backflow of irrigant and causes more debris to be displaced coronally, while avoiding the inadvertent extrusion of the irrigant into periapical tissues. One of the advantages of syringe irrigation is easy control of the depth of needle penetration within the canal and the quantity of irrigating solution that is flushed through the canal [22].
There are methods used for measuring remnants of the medicaments on the root canals such as direct visualization, digital microscopy, scanning electron microscopy, and volumetric analysis by using cone beam computed tomography (CBCT). In several studies, the removal efficiency of different techniques was assessed by the percentage ratio of medicament coated surface area to the total canal surface area. On the other hand, a scoring CH evaluates only the superficial layer of CH remnants and does not allow for the three dimensional evaluation. This scoring system was considered to be a more reliable technique because of the difficulties in automatically selecting the areas covered with CH remnants by using appropriate software as previously reported. Moreover, digital image analysis for quantitative assessment of the CH remnants evaluates only the superficial layer. In the present study, the scoring of the images was made individually by two observers because of the attempts for the highest agreement. Following thorough calibration, they discussed to achieve an agreement on the scores [23].
Regardless of the irrigation and agitation methods used in the present study, CH remnants were found on the walls of the root canal, especially in the most apical part of the canal lumen. None of the techniques used have completely removed CH remnants from the canal walls as previously reported [15,22]. However, the presence of CH remnants as an apical barrier has been advocated for its prolonged antimicrobial activity. Nevertheless, it is preferable to remove CH because of the possible increase of apical leakage when contacted with tissue fluids [24].
When the overall canal areas were considered, the lowest results on the removal of CH remnants were found in conventional syringe irrigation and manual dynamic irrigation groups than the other two irrigation techniques. Depending on the anatomical complexities of the root canal in conjunction with depth of penetration and diameter of the needle, the conventional syringe irrigation has a relatively weak flushing action. The other techniques like PUI and be explained by the fact that the higher velocity and volume of irrigant flow achieved by PUI [12] and Endoactivator.
Different agitation techniques such as PUI and Endoactivator system showed significantly better results. Furthermore, better scores of canal cleanliness were found in the cervical and middle thirds in comparison with the apical third. However, there were no statistically significant differences between the experimental groups or the canal thirds evaluated.
Conclusion
In the present study it seems that neither technique removed the medicament from root canal walls completely. CH is widely used in endodontic treatment of infected root canals between multiple sessions because of its antibacterial activity. However, the passive ultrasonic irrigating system and Endoactivator System were more effective than the other techniques. The canal cleanliness efficiency of all experimental groups was similar.
Acknowledgement
The authors acknowledge Dr. Manjushree Bhandari, Chairman, Sri Aurobindo College of Dentistry, SAIMS, and Dr. Mahak Bhandari, Director, Mohak Superspecialty Hospital for their guidance and support for this manuscript.
References
- 1.Siqueira JF., Jr Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:281–293. doi: 10.1067/moe.2002.126163. [DOI] [PubMed] [Google Scholar]
- 2.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–328. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 3.Hülsmann M, Peters OA, Dummer PM. Mechanical preparation of root canals: shaping goals, techniques and means. Endod Topics. 2005;10:30–76. [Google Scholar]
- 4.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–175. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Winkler J, Hartwell G, Stewart J, Caine R. Current trends in endodontic practice: emergency treatments and technological armamentarium. J Endod. 2009;35:35–39. doi: 10.1016/j.joen.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Fava LR, Saunders WP. Calcium hydroxide pastes: classification and clinical indications. Int Endod J. 1999;32:257–282. doi: 10.1046/j.1365-2591.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 8.Calt S, Serper A. Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J Endod. 1999;25:431–433. doi: 10.1016/S0099-2399(99)80273-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Kim YO. Influence of calcium hydroxide intracanal medication on apical seal. Int Endod J. 2002;35:623–628. doi: 10.1046/j.1365-2591.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- 10.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Metzler RS, Montgomery S. Effectiveness of ultrasonics and calcium hydroxide for the debridement of human mandibular molars. J Endod. 1989;15:373–378. doi: 10.1016/s0099-2399(89)80076-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Wu MK, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004;37:672–678. doi: 10.1111/j.1365-2591.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- 13.Lambrianidis T, Margelos J, Beltes P. Removal efficiency of calcium hydroxide dressing from the root canal. J Endod. 1999;25:85–88. doi: 10.1016/S0099-2399(99)80002-8. [DOI] [PubMed] [Google Scholar]
- 14.Salgado RJ, Moura-Netto C, Yamazaki AK, Cardoso LN, de Moura AA, Prokopowitsch I. Comparison of different irrigants on calcium hydroxide medication removal: microscopic cleanliness evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:580–584. doi: 10.1016/j.tripleo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Kenee DM, Allemang JD, Johnson JD, Hellstein J, Nichol BK. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J Endod. 2006;32:563–565. doi: 10.1016/j.joen.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Alturaiki S, Lamphon H, Edrees H, Ahlquist M. Efficacy of 3 different irrigation systems on removal of calcium hydroxide from the root canal: a scanning electron microscopic study. J Endod. 2015;41:97–101. doi: 10.1016/j.joen.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 17.van der Sluis LW, Wu MK, Wesselink PR. The evaluation of removal of calcium hydroxide paste from an artificial standardized groove in the apical root canal using different irrigation methodologies. Int Endod J. 2007;40:52–57. doi: 10.1111/j.1365-2591.2006.01182.x. [DOI] [PubMed] [Google Scholar]
- 18.Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997;23:725–727. doi: 10.1016/S0099-2399(97)80342-1. [DOI] [PubMed] [Google Scholar]
- 19.Ruddle CJ. Endodontic disinfection: tsunami irrigation. Endod Pract. 2008;11:7–15. [Google Scholar]
- 20.Caron G, Nham K, Bronnec F, Machtou P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod. 2010;36:1361–1366. doi: 10.1016/j.joen.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 21.Lambrianidis T, Kosti E, Boutsioukis C, Mazinis M. Removal efficacy of various calcium hydroxide/chlorhexidine medicaments from the root canal. Int Endod J. 2006;39:55–61. doi: 10.1111/j.1365-2591.2005.01049.x. [DOI] [PubMed] [Google Scholar]
- 22.Malentacca A, Uccioli U, Zangari D, Lajolo C, Fabiani C. Efficacy and safety of various active irrigation devices when used with either positive or negative pressure: an in vitro study. J Endod. 2012;38:1622–1626. doi: 10.1016/j.joen.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Balvedi RP, Versiani MA, Manna FF, Biffi JC. A comparison of two techniques for the removal of calcium hydroxide from root canals. Int Endod J. 2010;43:763–768. doi: 10.1111/j.1365-2591.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- 24.Ballal NV, Kumar SR, Laxmikanth HK, Saraswathi MV. Comparative evaluation of different chelators in removal of calcium hydroxide preparations from root canals. Aust Dent J. 2012;57:344–348. doi: 10.1111/j.1834-7819.2012.01710.x. [DOI] [PubMed] [Google Scholar]