Abstract
For decades, many studies have linked maternal smoking to an increased risk of preterm birth. As a result, the scientific community has long hypothesized that exposure to environmental tobacco smoke (ETS), commonly referred to as second-hand smoke, is also associated with an increased risk of preterm birth. Multiple studies have examined this proposed association through different strategies and approaches. Recently, a small number of epidemiology studies have examined preterm birth trends before and after the implementation of anti-smoking legislation in various jurisdictions. We found that these studies have largely revealed a significant trend of decreasing population-level preterm birth rates after the implementation of smoking bans. However, most of the studies reviewed did not distinguish the impact of maternal smoking from ETS in their analyses, making it difficult to specifically evaluate the effects of smoking bans on ETS exposure. Other studies have taken the approach of directly measuring maternal ETS exposure and associations with preterm birth within particular study populations. In contrast to smoking ban studies, the latter group of studies had more inconclusive results. The use of a variety of exposure assessment methods ranging from different self-reporting techniques to biomarker measurements posed a challenge to compare studies. We evaluated current scientific literature for evidence of an association between maternal ETS exposure and risk of preterm birth. We also discuss the strengths and weaknesses of the different approaches to study this association, as well as methods used for ETS exposure assessment. We propose that more studies, specifically, evaluating rates of preterm birth among non-smoking women before and after smoking bans, are needed, as well as using better ETS exposure assessments methods in studies measuring maternal ETS exposure.
Graphical abstract
INTRODUCTION
Preterm birth is both a common and devastating adverse health outcome with global impacts. On average, approximately 11.1% of all babies born globally are classified as preterm. In 2010 alone, an estimated 15 million babies were born prematurely worldwide, including over half a million in the United States.1 The World Health Organization generally defines preterm birth as a woman giving birth before 37 weeks gestation.2 Babies who are born prematurely face a myriad of health complications upon arrival which greatly increase their likelihood of death. These include factors such as lung immaturity, gastrointestinal feeding intolerance, skin problems including the inability to regulate body temperature, immune system deficiencies, and cardiovascular, hearing and vision problems.3 Complications from preterm birth are the leading cause of death in children under the age of 5.2 These complications are not just limited to the days and weeks after birth. Premature babies often face a lifetime of chronic health problems and devastating disabilities, costing an estimated twenty-six billion dollars a year in the United States alone.3
ENVIRONMENTAL TOBACCO SMOKE
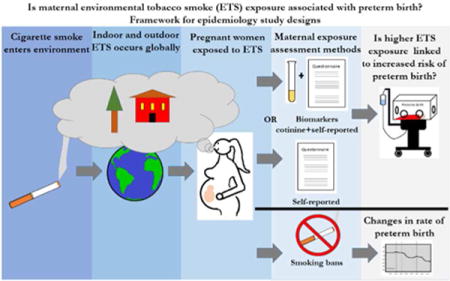
The scope of the preterm birth problem makes it a pressing global public health issue and as a result, extensive research has been conducted on potential causes for many decades. One potential cause of preterm birth that has been examined in the past few decades is environmental tobacco smoke (ETS). ETS, also known as secondhand smoke or passive smoke, is defined as any smoke from a tobacco-containing product that is emitted into the ambient air through the exhalation of an active smoker (mainstream smoke) or through the burning end of a lit cigarette (side stream smoke). Exposure to ETS primarily occurs in indoor environments such as at home, in cars or in restaurants.4 However, it can also occur outdoors such as near the entrances of smoke-free buildings or bus stops.4 Although a wealth of evidence dating back to the 1950s indisputably links active maternal smoking to preterm birth, considerably less research has examined the relationship between ETS and preterm birth.5 The comparatively small number of studies examining associations between ETS and preterm birth have examined the issue utilizing different strategies and approaches (Figure 1).
Figure 1.
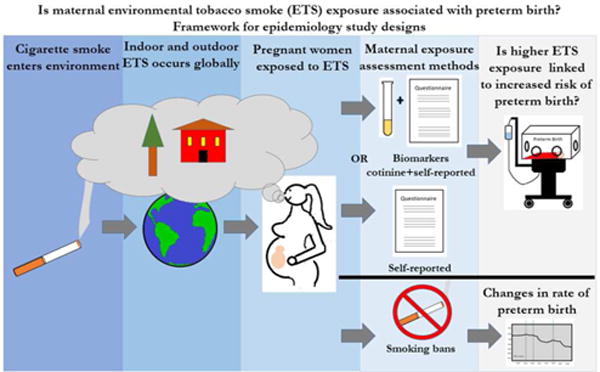
Framework for epidemiological study designs evaluating associations between maternal ETS exposure and preterm birth. Studies specifically evaluating maternal ETS exposure and risk of preterm birth use different methods to assess and/or quantify individual level maternal ETS exposure. These methods include maternal self-reporting via interviews or questionnaires or a combination of biomarker biochemical validation and self-reporting. Studies specifically evaluating ETS exposure and population level changes in preterm birth rate use data before and after the implementation of smoking bans.
ANTI-SMOKING LEGISLATION
As a result of the overwhelming evidence of adverse health outcomes and mortality attributed to smoking, in the past several decades a number of different types of smoking bans have been implemented across different parts of the world. These regulations vary widely in their details, scope, enforcement and geography. For example, some of the bans cover only bars and restaurants, while others also include parks, public spaces and workplaces. In addition, bans apply to whole countries, individual states, counties or cities.6 Timing and implementation of the anti-smoking regulations have varied. In Scotland, a single ban passed at one point in time and was implemented in phases over the course of years.7 In other examples, different bans were passed at different times or a single ban was passed and implemented at one time. As the number of smoking bans continues to increase, it is necessary to assess the effects of these bans in reducing smoking-related morbidities.
EVIDENCE FROM ANTI-SMOKING LEGISLATION AND PRETERM BIRTH STUDIES
Several epidemiology studies have examined the potential association between maternal ETS exposure and the risk of preterm birth by using the strategy of evaluating preterm birth trends before and after the implementation of anti-smoking legislation locally, regionally, and nationally. In order to characterize the results thus far of smoking bans and preterm birth studies, we searched PubMed to identify relevant studies. Different combinations of keywords were entered into the regular search bar. These keywords included “smoking bans,” “anti-smoking legislation,” “tobacco control,” “smoking regulation,” “smoke-free legislation,” “preterm birth,” “birth outcomes,” “neonatal outcomes,” “gestation,” and “premature.” In addition, we identified studies using citations from the Been et al. meta-analysis review.6 All studies that examined the associations of anti-smoking laws with preterm birth rates were included in the analysis. We identified five studies which are discussed here.7–11 In addition to preterm birth, most of the studies included evaluations of other outcomes such as low birth weight, small for gestational age, maternal smoking and childhood asthma. Only the sections of the studies relevant to the preterm birth outcome were evaluated.
Each of the five studies were conducted on populations in northern and western Europe and the United States. They encompassed geographical areas ranging from nations (Norway, Ireland) to regions (Flanders, Belgium and Scotland) to cities (Pueblo and El Paso, Colorado). The earliest smoking ban was implemented in 2003 and the last in 2010. Three of the five studies assessed laws with one implementation date that banned smoking in workplaces and public places.8–10 One study assessed laws that were passed at the same time and were implemented in three phases over the course of four years, also banning smoking in work-places and public places.7 One study assessed a law that built upon a previous ban on smoking in workplaces and public places by adding bans in restaurants and bars.11 The studies all used retrospectively available routine health data collected during and after pregnancies to perform their analyses. Interrupted time series and difference-in-difference study designs were used. All of the studies identified increased odds of preterm birth after the implementation of each ban. Several studies also reported gradual (slope) changes in preterm birth rates. These logistic regression models also included adjustment for several confounders. The most commonly included confounders in final regression models were: parity (4 studies), sex of baby (3 studies), age of mother (3 studies), alcohol consumption (2 studies) and marital status (2 studies).7–11
These studies have largely revealed a significant trend of decreasing population-level preterm birth rates after the implementation of smoking bans. The results of the five studies are summarized in Table 1. Four of the five studies showed overall immediate (step) statistically significant reductions in the rates of preterm birth7–10, while the fifth study showed a trend towards a reduction that was not significant.11 The largest reduction in the overall preterm birth rate category was −25% [95% Confidence Interval: −41.0%, −4.0%] reported by Kabiretal.8, while the smallest reduction was −0.59% [95% CI: −2.63%, 1.49%]7 reported by Cox et al. following the first ban of three implemented over the course of the study. Even though Cox et al. reported relatively small, insignificant reductions after the first and third bans were implemented, their final model for overall preterm birth rate, which included all three bans, was a larger significant reduction of −3.18% [95% CI: −5.38%, −0.94%]7. By contrast, Cox et al. reported significant reductions in the spontaneous preterm birth rate category following the implantation of each of the three smoking bans, as well as a reduction of −3.13% [95% CI: −4.37%, −1.87%] in their final model for the spontaneous category (Figure 2)7. In addition to Cox et al., Bhardawaj et al. also reported a reduction that was not statistically significant of −2.55% [90% CI: −5.52%, 0.42%].11 Page et al., who looked at a city-wide smoking ban in Colorado found a large −23.1% [95% CI: −40.1, −1.3]10 reduction in preterm birth rates, while Mackay et al. found a modest overall reduction of −11.72% [95% CI: −15.87%, −7.35%].9 Ameta-analysis conducted by Been et al. in 2014 is consistent with our finding that these studies have largely revealed a significant trend of decreasing preterm birth rates after the implementation of smoking bans. Been et al. included data from four of the five studies summarized here in their meta-analysis of over 1.3 million births.6 They found a significant reduction of −10.4% [95% CI: −18.8%, −0.2%] in the rate of preterm births after the implementation of smoking bans.6
Table 1.
Summary of Smoking Bans and Preterm Birth Studies
| First Author | Location of Study | Population | Year of Ban | Details of Smoking Ban | Preterm Birth Definition | Immediate Change in Preterm Birth Rate (overall adjusted)* | Findings |
|---|---|---|---|---|---|---|---|
| Page10 | USA: Pueblo & El Paso, CO | 470,000 | 2003 | Workplaces and Public places in Pueblo; none in El Paso | Preterm <37 weeks | −23.1% [95% CI: −40.1, −1.3] | Post-ban: Preterm birth rates reduced by 23.1 % in Pueblo compared to El Paso pre-ban |
| Bharadwaj11 | Norway | 4,950,000 | 2004 | Restaurants and Bars; Previous ban on Workplaces and Public places | Preterm <36 weeks | −2.55%** [95% CI: −5.52, 0.42] | Post-ban: Preterm birth rates reduced by 2.55% % in mothers working in bars and restaurants. |
| Kabir8 | Ireland | 4,580,000 | 2004 | Workplaces and Public places | Preterm <37 weeks | −25% [95% CI: −41.0, −4, 0] | Post-ban: Preterm birth rates reduced by 25% |
| MacKay9 | United Kingdom: Scotland | 5,300,000 | 2006 | Workplaces and Public places | Among smokers & non-smokers: Mild Preterm <37 weeks; Moderate Preterm <34 weeks; Extreme Preterm <32 weeks | −11.72% [95% CI: −15.87, −7.35] | Among both smokers & non-smokers: Post-ban: Preterm birth rates reduced by 11.72% |
| Stratified by: Current Smokers | −5.51% [95% CI: −13.84, 3.63] | Among currently smoking mothers: Post-ban: Preterm birth rates reduced by 5.51% | |||||
| Stratified by: Non-smokers | −15.44% [95% CI: 21.02, −9.47] | Among never smoking mothers: Post-ban: Preterm birth rates reduced by 15.44% | |||||
| Cox7 | Belgium: Flanders | 6,250,000 | 2006 | Phase I: Workplaces and Public places | Preterm <37 weeks; Very Preterm <32 weeks | Overall: −0.59%** [95% CI: −2.63, 1.49]; Spontaneous: −3.24% [95% CI: −4.40, −2.07] | Phase I Post-ban: Preterm birth rates reduced by 0.59% overall and 3.24% for spontaneous preterm birth. |
| 2007 | Phase II: Restaurants | Preterm <37 weeks; Very Preterm <32 weeks | Overall: −2.28% [95% CI: −4.37, −0.15]; ; Spontaneous: −3.69% [95% CI: −4.81, −2.55] | Phase II Post-ban: Preterm birth rates reduced by 2.28% overall and 3.69% for spontaneous preterm birth. | |||
| 2010 | Phase III: Bars Serving food | Preterm <37 weeks; Very Preterm <32 weeks | Overall: −1.24%** [95% CI: −3.05, 0.60]; Spontaneous: −3.36% [95% CI: −4.73, −1.9] | Phase III Post-ban: Preterm birth rates reduced by 1.24% overall and 3.36% for spontaneous preterm birth. | |||
| All Phases | Preterm <37 weeks; Very Preterm <32 weeks | Overall: −3.18% [95% CI: −5.38, −0.94]; Spontaneous: −3.13% [95% CI: −4.37, −1.87] | All phases post-ban: preterm birth rates reduced by 3.18% overall and 3.13% for preterm birth. |
p<0.05
not statistically significant; absolute change
Figure 2.
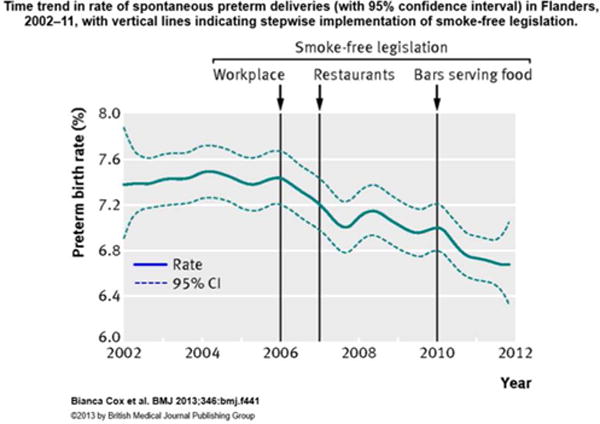
Change in rate of spontaneous preterm deliveries before and after these quential implementation of three smoking bans in Flanders, Belgium between 2006 and 2010. Reproduced from Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data, Cox, B., Martens, E., Nemery, B., Vangronsveld, J., and Nawrot, T. S, Vol. 346, Page f441, Copyright 2013 with permission from BMJ Publishing Group Ltd.7
A key limitation of this group of studies looking at the effects of smoking bans on preterm birth rates is the fact the majority of them were not able to differentiate the effects of the bans on active maternal smoking exposure versus second hand exposure because the researchers used previously collected health data and had no input into what questionswere asked of the mothers.7, 8, 10 Only two of the studies were able to report on preterm birth rates for women who never smoked versus current smokers.9, 11 Bhardawaj et al. reported no significant reduction for either group (but the stratified estimates were not shown), while MacKay et al. showed a significant reduction in preterm birth rates for both current smokers and never-smokers.9,11 Interestingly, they reported that never-smokers showed a 3-fold greater reduction in preterm births rates at −15.44% [95% CI: −21.02%, −9.47%] than current smokers at −5.51% [95% CI: −13.84%, 3.63%].9 This underscores the substantial need for more studies that are able to specifically examine the effects of smoking bans on non-smoking women. Such studies could use smoking bans as a proxy for reduced ETS exposure and eliminate similar exposures that active smokers receive from their direct smoking.
ETS EXPOSURE ASSESSMENT METHODS
In addition to the small group of studies using the smoking ban approach to study changes in preterm birth rates at a population level, a considerably larger group of studies have taken the approach of directly measuring maternal ETS exposure and associations with preterm birth within particular study populations. These studies faced substantial challenges in developing methods for accurately measuring ETS exposure among pregnant women.4 These challenges exist because assessing individual exposure accurately is difficult and many individual variables affect bodily absorption and disposition of ETS. A few of these challenges include assessing intensity and duration of exposure, repetitive or non-continuous exposure, cumulative effects, knowledge of exposure, indoor ventilation and outdoor weather patterns.4, 12 Despite these challenges, the number of studies assessing ETS and preterm birth has markedly increased since 2000, utilizing methods that have been evolving since they were first conceived in the 1960s.
SELF-REPORTING: INTERVIEWS AND QUESTIONAIRES
The most widely used method for assessing exposure to ETS has been through self-reporting, utilizing either interviews or self-reported questionnaires. The self-reporting methodology has many strengths, including its non-invasive nature, its cost-effectiveness and feasibility in larger studies and developing countries and its ease of obtaining large amounts of information in short periods of time. In particular, recent studies have done a better job than older studies of more accurately classifying and stratifying exposure levels over the course of the entire pregnancy and categorizing the severity of preterm birth. These efforts aim to establish a dose-response relationship, account for confounding, and gain detailed information on exposure sources.
Despite the wide spread use of self-reporting, many limitations for this method exist. These include early studies using too few simplistic questions about exposure, no classification of levels, location, intensity, or duration of ETS exposure, differences in how variables are defined and stratified and a wide array of methods for obtaining self-reported information.12–17 In addition, in accuracies in self-reporting have led to high misclassification rates between non ETS-exposed and ETS-exposed pregnant women as shown by studies using biochemical validation of self-reported ETS exposure information.18 The limitations that exist with using this method alone to classify ETS exposure have prompted the development and use of another assessment method, biomarkers.
ETS EXPOSURE MEASUREMENTS: BIOMARKERS
Biomarkers are measurable indicators of the presence of substances or their metabolites in the human body.19 In contrast to self-reported exposure information, which can offer imprecise information on potential exposure levels, biomarkers provide tangible proof of exposure and quantifiable measurements of the amount absorbed into the body from the exposure.4 In order for a specific biomarker to provide an accurate and precise quantification of an exposure, it should ideally meet several criteria. These criteria include specificity to the exposure, a relationship to the specific substance within the exposure that is thought to cause adverse health outcomes, a sufficiently long half-life, and a laboratory technique for quantification with a reasonable level of detection.20
Cotinine, the primary metabolite of nicotine, has emerged as both a highly accurate and practical biomarker to use when assessing ETS exposure because it has been shown to have a high sensitivity and specificity to tobacco smoke.20 Cotinine is a relatively stable molecule specific to tobacco smoke exposure. Its half-life is approximately 20 hours, making it detectable in serum, saliva, and urine for 3 to 4 days after exposure and in hair for 1–2 months.21,22 Cotinine, as a metabolite of nicotine, is also readily distributed in all issues, which implies that its presence indicates exposure in all compartments of the body.20, 23, 24 Even early studies confirmed the high correlation of cotinine levels in bodily fluids and known tobacco exposure. In a study conducted in 1983, Haley et al. found that daily cotinine levels in plasma and saliva samples taken from smokers correlated very highly with the number of cigarettes they reported smoking, when compared with another metabolite.25 Although biomarkers have been shown to be highly effective tools for measuring ETS exposure, they alone still cannot solely be used to assess ETS exposure because they do not provide any contextual information about the possible sources of ETS exposure or information about variables that could confound any associations discovered between ETS exposure and preterm birth.20
To address the deficits in both self-reporting and biomarker methodology, the best study design that has emerged in the past decade is one that utilizes a combination of both methods to capture the most accurate information about ETS exposure.26 Carefully considered and detailed questions about ETS exposure and possible confounders are combined with biomarker measurements of cotinine in bodily fluids or nicotine in hair. Although the combined study design is the gold standard, for very large epidemiology studies and studies where biochemical validation is not feasible, well-designed self-reported-based study designs are still used and viewed as acceptable. Finally, a few other methods for ETS exposure assessment have undergone limited testing but show great potential to detect ETS exposure even more precisely and accurately than the methods currently available. One method, the use of personal exposure monitoring devices, has been shown to accurately capture ETS exposure. However, the technology needs improvement and the costs are substantial.4, 20
EVIDENCE FROM DIRECT ETS EXPOSURE ASSESSMENT AND PRETERM BIRTH STUDIES
Studies that have taken the approach of directly measuring maternal ETS exposure and incidences of preterm birth within particular study population shave largely utilized the exposure assessment methods out lined above. In order to characterize the results thus far of maternal ETS exposure and preterm birth studies, we searched PubMed to identify relevant studies. Different combinations of keywords were entered into the regular search bar. These key words included “environmental tobacco smoke,” “ETS,” “passive smoke,” “second hand smoke,” “passive tobacco smoke,” “preterm birth,” “birth outcomes,” “neonatal outcomes,” “gestation,” and “premature.” Several studies were identified using the works cited section of the Meeker and Benedict review and several of the original research articles.4 All studies that examined an association between ETS exposure and preterm birth were included in the summary. In addition to the primary predictor and outcome of interest, some of the studies included other predictors such as active smoking and other out comes such as birth weight; however, only the relevant parts of those studies were reviewed. Ultimately, ten studies were included in the analysis.12–17, 26–29
Overall, the studies assessing the association between ETS exposure and preterm birth showed inconclusive results. The results are summarized in Table 2. All of the studies except Martin and Bracken and Mathai et al. used logistic regression for statistical analysis and included variables that could potentially confound any association found between ETS exposure and preterm birth. The odd ratios comparing the odds of giving birth prematurely among non-ETS exposed versus different levels of ETS exposed pregnant were obtained.13–17,26,27,29 Some of the studies such as Martin and Bracken, Crane et al., and Kharrazi et al., conducted initial univariate analyses between the individual maternal characteristics and ETS exposure status, which were displayed on the distribution tables, in order to identify possible confounders. They used t-tests or Wilcoxon sign-ranked tests for continuous variables and Chi square or Fisher’s exact tests for categorical variables12, 13, 27. The most commonly included confounders were parity, maternal age, BMI, employment status, and education level (each of 4 studies), history of pregnancy complications, sex of baby, alcohol consumption, and marital status (each of 3 studies).13–17, 26, 27, 29
Table 2.
Summary of Maternal ETS Exposure and Preterm Birth Studies
| First Author | Year | Cohort Size | Location of Study | Exposure Assessment Method | ETS Exposure Definition | Association? | Odds Ratio |
|---|---|---|---|---|---|---|---|
| Martin12 | 1986 | 3891 | USA: MA | In-person interview | Exposed more Than 2 hours per week | No | NO OR given: ETS exposed preterm birth rate: 4.64%; ETS non-exposed preterm birth rate: 4.66% |
| Mathai28 | 1992 | 994 | India | In-person questionnaire | 1 Household member smokes | No | NO OR; ETS exposure: 5–8% preterm birth rate; non-ETS exposed 3–8% preterm birth rate |
| Fortier14 | 1994 | 4644 | Canada | Phone interview-Post pregnancy (up to 6 weeks) | Exposed more Than 1 hour per week | No | ETS exposed at work: 0.92 [CI: 0.64–1.31] |
| No | ETS exposed at home: 0.93 [CI: 0.58–1.51] | ||||||
| No | ETS exposed at home and work: 0.98 [CI: 0.56–1.73] | ||||||
| Windham17 | 2000 | 4454 | USA: CA | Phone interview-Early pregnancy | Exposed more Than 1/2 hour per day | Yes (not significant) | Low ETS (05–6.5hrs/day) & Very Preterm (<35wks): 1.5 [CI: 0.90–2.5]; |
| Yes (not significant) | Low ETS (05–6.5hrs/day) & Preterm (<37wks): 1.1 [CI: 0.79–1.6]; | ||||||
| Yes (not significant) | High ETS (>7hrs/day) & Very Preterm (<35wks): 2.4 [CI: 1.0–5.3]; | ||||||
| Yes (not significant) | High ETS (>7hrs/day) & Preterm (<35wks): 1.6 [CI: 0.87–2.9] | ||||||
| Jaakkola26 | 2001 | 389 | Finland | Questionnaire at Clinic - Post pregnancy & Maternal Hair nicotine-post pregnancy | <0.75ug/g Hair Nicotine concentration | Yes (not significant) | Hair Nicotine: (0.75-<4.00 ug/g): 1.30 [CI: 0.30–5.58] |
| Yes | Hair Nicotine: (>4.00 ug/g): 6.12 [CI: 1.31–28.7] | ||||||
| No | ETS at home: 0.65 [CI: 0.06–6.81] | ||||||
| Yes (not significant) | ETS at work: 2.35 [CI: 0.50–11.1] | ||||||
| Yes | ETS exposure home/work: 8.89 [CI: 1.05–75.3] | ||||||
| Kharazzi27 | 2004 | 3669 | USA:CA | Questionnaire at Clinic - Post pregnancy & Maternal serum cotinine (15–19 Weeks pregnant) | <0.026 ng/ml Serum Cotinine concentration | No | Serum Cotinine: (0.026–0.053 ng/ml): 0.90 [CI: 0.48–1.66]; |
| No | (0.054–0.0096 ng/ml): 0.76 [CI: 0.40–1.42] | ||||||
| No | (0.097–0.235 ng/ml): 0.68[CI: 0.35–1.30] | ||||||
| Yes | (>0.235 ng/ml): 1.78 [CI: 1.01–3.13] | ||||||
| Ashford29 | 2010 | 210 | USA: KY | Questionnaire and Hair maternal cotinine | Answered Yes to any exposure question | Yes (not significant) | ETS exposure: 2.3 [CI: 0.96–5.96] |
| Crane13 | 2011 | 11,852 | Canada | Questions asked by physician at first prenatal visit | Any exposure-Cutoff not defined | Very Preterm (<34 Wks): Yes (not significant) ; Preterm (<37 Wks): Not reported | Very Preterm (<34 Wks): 1.87[CI: 1.00–3.53] |
| Qiu16 | 2013 | 10,094 | China | In-person interview-Post pregnancy (1–3 days post-delivery) | Exposed more Than 1/2 hours per month | Yes | ETS During Whole Pregnancy: Very Preterm (<32Wks): 1.98 [CI: 1.41–2.27]; |
| No | Moderate Preterm (32–36wks): 0.98 [CI: 0.81–1.19] | ||||||
| Yes (not significant) | Preterm (32–36wks): 1.12 [CI: 0.95–1.32] | ||||||
| Miyake15 | 2013 | 1565 | Japan | 2 questionnaires (1 during pregnancy, 1 post pregnancy) | At least 1 person in home or at work smoked during 1 st questionnaire | No | ETS at home: 0.91 [CI: 0.48–1.65] |
| No | ETS at work: 0.97 [CI: 0.36–2.23] |
The earlier studies that used simple self-reporting methodology all showed no significant association between ETS exposure and preterm birth.12, 15, 28 The more recent studies that use more sophisticated self-reporting methodology showed mixed results. The Windham et al. study calculated odds ratios for 4 groups stratified based on high and low ETS exposure and preterm and very preterm birth. The odds ratio for very preterm and preterm births, respectively, among mothers with high versus low ETS exposure were2.4 [95% CI: 1.0–5.3] and 1.5 [95% CI: 0.90–2.5].17 Two other studies, Qiu et al. and Crane et al., both evaluated large cohorts of over 10,000 people each and saw similar results. Qiu et al. reported an odds ratio of 1.98 [95% CI: 1.41–2.76]), and Crane reported 1.87 [95%CI: 1.00–3.53], specifically for their respective very preterm birth categories.13,16 Neither study found strong or significant associations for preterm or moderate preterm birth categories.13, 16 Miyake et al. also found no association.15
Studies that combined self-reporting and biomarker measurements in their study designs collectively showed results that indicate a relationship between preterm birth and ETS exposure. One study, Ashford et al., which used maternal hair cotinine and a questionnaire to assess ETS exposure, reported a trending association with an odds ratio of 2.3 [95% CI: 0.96–5.96].29 Kharrazi et al. used an extremely sensitive detection assay for serum cotinine measurements and observed an odds ratio for preterm birth of 1.78 [95% CI: 1.01–3.13], but only within the group with the highest ETS exposure confirmed by serum cotinine levels above 0.235 ng/ml.27 The results of Kharrazi et al. suggested that very low ETS exposure is not associated with preterm birth, but higher levels may be associated.27 Jaakkola et al. used hair nicotine to quantify ETS exposure and reported the highest odds ratio of these studies, 6.12 [95% CI: 1.31–28.7], suggesting a strong association between ETS exposure above 4.00 ug/g in hair and preterm birth. However, below that threshold, the results were not significant.26 Jaakkola et al. also simultaneously calculated odds ratios based on self-reported ETS exposure from the same study participants. Only those who reported ETS exposure both at home and at work had a statistically significant odds ratio of 8.89 [95% CI: 1.05–75.3], while the other odds ratios were not significant.
Our assessment of studies examining the association between ETS exposure and preterm birth differs somewhat from a meta-analysis conducted by Leonardi-Bean et al. which concluded that there is no association between ETS exposure and preterm birth.30 The meta-analysis included studies published prior to 2008 and did not include results based on the stratification of different prematurity categories such as very pre-term.30 In our assessment, we included several large studies published after 2008 that reported either a significant or trending association between ETS exposure and very preterm birth catagories.13,16 This evidence, in addition to the evidence of a positive association from several combined self-reporting and biomarker studies, led us to interpret the evidence available thus far as being inconclusive.26, 29
DISCUSSION AND CONCLUSION
Preterm birth is a severe adverse health outcome that may have lifelong health implications for babies. Assessing possible causes of increased risk of preterm birth is essential for identifying public health interventions that can reduce incidence of preterm birth.6 In the past several decades, numerous studies have examined a potential association between ETS and risk of preterm birth through different strategies and approaches. The small number of studies that have used the approach of examining preterm birth trends before and after the implementation of anti-smoking legislation have largely demonstrated a significant trend of decreasing population-level preterm birth rates. Unfortunately, most of these studies do not differentiate the impact of maternal smoking from ETS in their analyses, making it difficult to specifically evaluate the effects of smoking bans on ETS exposure. The larger group of studies that have taken the approach of directly measuring maternal ETS exposure and calculating associations of ETS and preterm birth within particular study populations have had much more in conclusive results. They have utilized both self-report exposure assessment methods and biomarker measurements.4,20,26 Although the information revealed about the association between ETS exposure and preterm birth in the studies that have been conducted thus far serves as a starting point, more research must be done in order to clarify the nature of a potential association and to develop appropriate public health interventions. Based on this review, we propose that more studies specifically evaluating rates of preterm birth among non-smoking women before and after smoking bans are needed, as well as use of better ETS exposure assessments methods in studies measuring maternal ETS exposure.
Acknowledgments
Funding Sources: This work was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants P30ES017885, R01ES016932, R01ES017022, T32ES00706, P42ES0171982), National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention (Grant T42OH008455) and Rackham Graduate Student Research Grant, University of Michigan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
ABBREVIATIONS
- ETS
Environmental Tobacco Smoke
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Preterm Birth Factsheet. World Health Organization; 2014. [Google Scholar]
- 3.Behrman RE, Butler AS. Preterm Birth. National Academies Press; 2007. Committee on Understanding Premature, B., Assuring Healthy, O., Board on Health Sciences, P., and Medicine, I. o. [Google Scholar]
- 4.Meeker JD, Benedict MD. Infertility, Pregnancy Loss and Adverse Birth Outcomes in Relation to Maternal Secondhand Tobacco Smoke Exposure. Current women’s health reviews. 2013;9:41–49. doi: 10.2174/1573404811309010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel EL. Smoking during pregnancy: a review of effects on growth and development of offspring. Human biology. 1980;52:593–625. [PubMed] [Google Scholar]
- 6.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet (London, England) 2014;383:1549–1560. doi: 10.1016/S0140-6736(14)60082-9. [DOI] [PubMed] [Google Scholar]
- 7.Cox B, Martens E, Nemery B, Vangronsveld J, Nawrot TS. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMJ (Clinical research ed) 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabir Z, Clarke V, Conroy R, McNamee E, Daly S, Clancy L. Low birthweight and preterm birth rates 1 year before and after the Irish workplace smoking ban. BJOG : an international journal of obstetrics and gynaecology. 2009;116:1782–1787. doi: 10.1111/j.1471-0528.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackay DF, Nelson SM, Haw SJ, Pell JP. Impact of Scotland’s smoke-free legislation on pregnancy complications: retrospective cohort study. PLoS medicine. 2012;9:e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page RL, Slejko JF, Libby AM. A citywide smoking ban reduced maternal smoking and risk for preterm births: a Colorado natural experiment. Journal of women’s health (2002) 2012;21:621–627. doi: 10.1089/jwh.2011.3305. [DOI] [PubMed] [Google Scholar]
- 11.Bharadwaj P, Johnsen J, Løken K. Smoking Bans, Maternal Smoking and Birth Outcomes. IZA Discussion Paper No. 7006. 2012 Available at SSRN: http://ssrn.com/abstract=2177204.
- 12.Martin TR, Bracken MB. Association of low birth weight with passive smoke exposure in pregnancy. American journal of epidemiology. 1986;124:633–642. doi: 10.1093/oxfordjournals.aje.a114436. [DOI] [PubMed] [Google Scholar]
- 13.Crane JMG, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG : an international journal of obstetrics and gynaecology. 2011;118:865–871. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 14.Fortier I, Marcoux S, Brisson J. Passive smoking during pregnancy and the risk of delivering a small-for-gestational-age infant. American journal of epidemiology. 1994;139:294–301. doi: 10.1093/oxfordjournals.aje.a116997. [DOI] [PubMed] [Google Scholar]
- 15.Miyake Y, Tanaka K, Arakawa M. Active and passive maternal smoking during pregnancy and birth outcomes: the Kyushu Okinawa maternal and child health study. BMC pregnancy and childbirth. 2013;13:157. doi: 10.1186/1471-2393-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu J, He X, Cui H, Zhang C, Zhang H, Dang Y, Han X, Chen Y, Tang Z, Zhang H, Bai H, Xu R, Zhu D, Lin X, Lv L, Xu X, Lin R, Yao T, Su J, Liu X, Wang W, Wang Y, Ma B, Liu S, Huang H, Lerro C, Zhao N, Liang J, Ma S, Ehrenkranz RA, Liu Q, Zhang Y. Passive smoking and preterm birth in urban China. American journal of epidemiology. 2014;180:94–102. doi: 10.1093/aje/kwu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11:427–433. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Riboli E, Haley NJ, Tredaniel J, Saracci R, Preston-Martin S, Trichopoulos D. Misclassification of smoking status among women in relation to exposure to environmental tobacco smoke. Eur Respir J. 1995;8:285–290. doi: 10.1183/09031936.95.08020285. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt CW. Signs of the times: biomarkers in perspective. Environ Health Perspect. 2006;114:A700–705. doi: 10.1289/ehp.114-a700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaakkola MS, Jaakkola JJ. Assessment of exposure to environmental tobacco smoke. The European respiratory journal. 1997;10:2384–2397. doi: 10.1183/09031936.97.10102384. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nafstad P, Jaakkola JJ, Hagen JA, Zahlsen K, Magnus P. Hair nicotine concentrations in mothers and children in relation to parental smoking. J Expo Anal Environ Epidemiol. 1997;7:235–239. [PubMed] [Google Scholar]
- 23.Binnie V, McHugh S, Macpherson L, Borland B, Moir K, Malik K. The validation of self-reported smoking status by analysing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral diseases. 2004;10:287–293. doi: 10.1111/j.1601-0825.2004.01018.x. [DOI] [PubMed] [Google Scholar]
- 24.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Haley NJ, Axelrad CM, Tilton KA. Validation of self-reported smoking behavior: biochemical analyses of cotinine and thiocyanate. American journal of public health. 1983;73:1204–1207. doi: 10.2105/ajph.73.10.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaakkola JJ, Jaakkola N, Zahlsen K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environmental health perspectives. 2001;109:557–561. doi: 10.1289/ehp.01109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharrazi M, DeLorenze GN, Kaufman FL, Eskenazi B, Bernert JT, Graham S, Pearl M, Pirkle J. Environmental tobacco smoke and pregnancy outcome. Epidemiology (Cambridge, Mass) 2004;15:660–670. doi: 10.1097/01.ede.0000142137.39619.60. [DOI] [PubMed] [Google Scholar]
- 28.Mathai M, Vijayasri R, Babu S, Jeyaseelan L. Passive maternal smoking and birthweight in a south Indian population. Br J Obstet Gynaecol. 1992;99:342–343. doi: 10.1111/j.1471-0528.1992.tb13736.x. [DOI] [PubMed] [Google Scholar]
- 29.Ashford KB, Hahn E, Hall L, Rayens MK, Noland M, Ferguson JE. The effects of prenatal secondhand smoke exposure on preterm birth and neonatal outcomes. J Obstet Gynecol Neonatal Nurs. 2010;39:525–535. doi: 10.1111/j.1552-6909.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:F351–361. doi: 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]


