Abstract
Background/Objectives
Obesity is a risk factor in the development of type 2 diabetes mellitus (DM2), which is associated with increased morbidity and mortality, predominantly as a result of cardiovascular complications. Increased adiposity is a systemic condition characterized by increased oxidative stress (ROS), increased inflammation, inhibition of anti-oxidant genes such as HO-1 and increased degradation of epoxyeicosatrienoic acids (EETs). We previously demonstrated that EETs attenuate mitochondrial ROS. We postulate that EETs increase peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which controls mitochondrial function, oxidative metabolism and induction of HO-1.
Subjects/Methods
Cultured murine adipocytes and mice fed a high fat (HF) diet were used to assess functional relationship between EETs, HO-1 and (PGC-1α) using an EET analogue (EET-A) and lentivirus to knock down the PPARGC1A gene.
Results
EET-A increased PGC-1α and HO-1 in cultured adipocytes and increased the expression of genes involved in thermogenesis and adipocyte browning (UCP1 and PRDM16, respectively). PGC-1α knockdown prevented EET-A-induced HO-1expression, suggesting that PGC-1α is upstream of HO-1. MRI data obtained from fat tissues showed that EET-A administration to mice on a HF diet significantly reduced total body fat content, subcutaneous and visceral fat deposits and reduced the VAT: SAT ratio. Moreover EET-A normalized the VO2 and RQ (VCO2/VO2) in mice fed a HF diet, an effect that was completely prevented in PGC-1α deficient mice. In addition, EET-A increased mitochondrial biogenesis and function as measured by OPA1, MnSOD, Mfn1, Mfn2, and SIRT3, an effect that was inhibited by knockdown of PGC-1α.
Conclusion
Taken together, our findings show that EET-A increased PGC-1α thereby increasing mitochondrial viability, increased fusion potential thereby providing metabolic protection and increased VO2 consumption in HF-induced obesity in mice, thus demonstrating that the EET-mediated increase in HO-1 levels require PGC-1α expression.
Keywords: HO-1, metabolic dysfunction, epoxyeicosatrienoic acid
Introduction
Obesity is a global epidemic and a major risk factor in the development of metabolic syndrome and diabetes and associated complications that include cardiovascular disease, increased oxidized HDL levels, kidney disease, hypertension, and neuropathies (1–4). Epoxyeicosatrienoic acids (EETs) are arachidonic acid derived metabolites generated by a family of cytochrome P450 (CYP) monooxygenases and epoxygenases (5;6). Although rapidly subjected to hydrolysis by soluble epoxide hydrolase (sEH) to their respective dihydroxyepoxytrienoic acids as well as by esterification primarily to glycerophospholipids, their vasodilatory, anti-inflammatory, anti-adipogenic and antiapoptotic actions are well established and sEH-inhibition increases cellular and circulating EET levels (7–9). EET agonists prevent both adiposity and vascular complications in vitro and in vivo and obesity-induced adipose tissue expansion impairs the CYP epoxygenase pathway and the generation of EET in vivo (10–13).
Heme oxygenase (HO) consists of an inducible and a constitutive form (HO-1 and HO-2, respectively) that catalyzes the degradation of heme into equimolar amounts of biliverdin/bilirubin, carbon monoxide (CO) and iron (Fe2+ ion) (14;15). HO-2 maintains normal metabolic cellular functions, such as vascular tone, renal channel function and activity (15), as well as control of body weight, insulin sensitivity, and blood pressure (16). HO-2 deficiency is associated with oxidative stress, chronic inflammation, and reduced protection against diabetes-induced renal injury (17;18). HO-1, a stress responsive enzyme, offers increased protection against high fat diet (HF)-induced obesity (19–21) and regulates adipocyte stem cell differentiation (22;23), attributable to the potent antioxidant and anti-inflammatory properties of biliverdin/bilirubin.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a major regulator of mitochondrial function, oxygen consumption and oxidative phosphorylation (24;25). PGC-1α along with the transcriptional regulator PR Domain Containing 16 (PRDM16), are the major regulators of adipocyte browning and thermogenic activation of brown fat (26–28). Transgenic mice with mildly elevated muscle PGC-1α levels are resistant to age-related obesity and diabetes and have a prolonged lifespan suggesting that PGC-1α stimulates the secretion of factors from skeletal muscle that affect the function of other tissues (29). In fact, PGC-1α and several of its responsive genes involved in oxidative phosphorylation display reduced protein and mRNA expression levels in muscle and adipose tissue of patients with chronic heart failure and diabetes mellitus type 2 (DM2) (30–34). Furthermore mice lacking PGC-1α in adipose tissue fed a HF diet develop insulin resistance and increased circulating lipid levels (35). Additionally, the uncoupling proteins are intramembranous of the mitochondrial proteins that play a key role in thermogenesis. UCP1 is present exclusively in brown adipose tissue (36) and myokines drive UCP1 in a PGC-1-dependent manner to increase energy expenditure (36) and possibly oxygen consumption. These studies suggest that PGC-1α is a key moderator of energy metabolism and in preventing the development of metabolic syndrome and DM2. As a transcriptional co-activator, PGC-1α enhances the expression of many transcription factors and can bind to members of the nuclear receptor family, including nuclear respiratory factor (NRF)-1 and -2 (35;37).
Adipocyte differentiation is associated with an increase in ROS and inflammatory cytokine levels and a concomitant decrease in mitochondrial function. Although ROS are being generated in several cellular compartments, the bulk of ROS (about 90%) contribute to mitochondrial energy metabolism (38). Sirtuin 3 (SIRT3), one of the seven mammalian sirtuins, is a major mitochondrial deacetylase and has recently been discovered to be the target of PGC-1α and is important in mitochondrial processes, such as suppression of ROS, mitochondrial biogenesis, and energy metabolism (38), including mitochondrial fatty-acid oxidation (39). The mitochondrial network morphology is tightly linked to energy and metabolic demands as well as viability and depends greatly on quality control, involving mitochondrial fusion (the merge of dysfunctional to functional) and fission (budding and isolation of dysfunctional mitochondria, orchestrated by the dynamin-related protein 1 (DRP1) and the fission, mitochondrial 1 (Fis1) proteins (40;41)). The autosomal dominant optic atrophy 1 (OPA1) protein is situated on the mitochondrial inner membrane that, along with the mitochondrial fusion proteins, Mitofusin 1 and 2 (Mfn 1 and 2), located on the mitochondrial outer membrane, facilitate the mitochondrial fusion process (42;43). That the dynamics of the mitochondrial fusion/fission processes need to be tightly regulated is exemplified by the link between the reduction of mitochondrial fusion and the development of obesity and insulin resistance (41;44;45). Conversely, reduced hepatic steatosis, insulin resistance, and increased mitochondrial function and fusion potential is present in hepatocyte mitochondria of rats fed high-fish-oil diet as compared to rats fed a high-lard diet (41).
We recently reported that EETs increase HO-1 expression and mitochondrial viability, and exert their function on adipocyte differentiation via activation of PGC-1α in vitro (46). The present report examines whether the EET-mediated regulation of adiposity is due to activation of PGC-1α and an increase in HO-1 levels in cultured adipocytes in vitro and in mice fed a high fat diet in vivo and investigates the mechanistic EET-mediated actions on mitochondrial function in reference to mitochondrial viability and fusion mediators mitochondrial superoxide dismutase (MnSOD/SOD2), SIRT3, Mfn1, Mfn2, and OPA1. We further examined the impact of HF, EETs, and lack of PGC-1α on metabolic alterations and on VO2 and respiratory quotient (RQ). We demonstrate that EET is upstream of the PGC-1α signaling pathway responsible for increased levels of HO-1 and insulin phosphorylation and further that the EET-mediated induction of HO-1 is dependent on PGC-1 α. Our results indicate that the EET-PGC1α-mediated reduction in adiposity in mice fed a HF diet involves an increase in VO2. This study highlights the existence of an EET-PGC-1α axis that is associated with increased levels of HO-1 that, concomitantly, upregulates mitochondrial function, MnSOD and SIRT3, and fusion mediators, Mfn1 and Mfn2, and OPA1, which, together, serve to regulate adipocyte cell differentiation and obesity-induced hypertension and improve metabolic homeostasis.
Materials and Methods
Cell culture
3T3-L1 murine pre-adipocytes were purchased from ATCC (ATCC, Manassas, VA). After thawing, 3T3-L1 cells were cultured in α-minimal essential medium (α-MEM, Invitrogen, Carlsbad CA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) and 1% antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). The cultures were maintained at 37°C in a 5% CO2 incubator and the medium was changed after 48h and every 3~4 days thereafter as described previously (10). For adipogenesis studies the medium was replaced with adipogenic medium (Dulbecco’s modified Eagle Medium (DMEM) with high glucose (Invitrogen), supplemented with 10% (v/v) FBS, 10 μg/ml insulin (Sigma-Aldrich, St. Louis, MO), 0.5 mM dexamethasone (Sigma-Aldrich), and 0.1mM indomethacin (Sigma-Aldrich) and the cells were cultured for an additional 7 days. Cells were cultured in the absence and presence of the EET analog EET-A ((S)-2-(11-(nonyloxy) undec-8(Z)- enamido) succinic acid) in a dose of 10 μM. To examine the effect of inhibition of HO activity, SnMP (5 μM, Tin mesoporphyrin, Frontier Scientific) was added for the duration of EET-A treatment. At the experimental endpoints, cells were collected by trypsinization, washed once with PBS and then lysed for protein measurements and for RNA extraction.
Development of PGC-1α deficient adipocytes
SMART vector lentiviral shRNA-PPARGC1A or scrambled RNA (Dharmacon, Lafayette, CO) was applied to 3T3-L1 cells to establish a stably transduced cell line. Briefly, 1×106 cells were seeded in 6-well plates 1 day prior to transduction. On the day of transduction, the transduction medium was made by 1×106 transducing units (TU) of lentiviral particles with 0.5ml α-MEM growth medium, applied to each well and incubated for 3h to maximize the contact between each cell and lentiviral particles. Cells were also treated with the transduction medium without lentiviral particles which served as un-transduced control. Growth Medium (1.5 ml) was then added to each well in the presence of 8 μg/ml polybrene (final concentration). After 48 h incubation, antibiotic selection medium (α-MEM growth medium with 10 μg/ml puromycin) was used to kill all the un-transduced cells. Cells were then cultured and maintained as outlined above.
Animal experimentation
All animal experiments followed an NYMC IACUC institutionally approved protocol in accordance with the NIH Guidelines. C57bl6 mice were used in the studies. Mice were divided into 4 groups: (1) lean, (2) HFD, (3) HFD + EET-A, (4) HFD+EET−A+PGC-1α lentivirus. Lean mice (group 1) were fed ad libitum a normal chow diet containing 11 % fat, 62 % carbohydrate, and 27.0 % protein with a total calories of 12.6 KJ/g. The remaining animals (groups 2, 3, and 4) were fed a high-fat diet containing 58 % fat (from lard), 25.6 % carbohydrate, and 16.4 % protein with total calories of 23.4 KJ/g (Harlan, Teklad Lab Animal Diets, US) for 8 Weeks. Mice were treated as following, group (1) normal chow diet, group (2) HF diet, group (3) EET-A was injected, intraperitoneally, for 2 weeks daily at a dose of 1.5 mg/100 gm of body weight as previously described (1), group (4) EET-A was injected, intraperitoneally, for 2 weeks daily at a dose of 1.5 mg/100 gm of body weight and each animal received a 2 bolus injection of PGC-1α Lentivirus was injected into the retro orbital vein at a concentration of 40–70 *106 TU/mouse in 80–100 μl (Dharmacon, Lafayette, CO, US), as previously described (1). At the end of the 8-week period, mice were anesthetized with sodium pentobarbital (65 mg/Kg, i.p.) and, at the time of sacrifice, body weight and fat content were measured.
Real-time qPCR and Western Blot Analysis
Total RNA was extracted from 3T3 cells using TRIzol® (Ambion, Austin, TX) and from frozen adipose tissue by RNeasy® Lipid Tissue (Qiagen), as indicated by the manufacturer. RNA was determined by measuring the absorbance at 260 nm (A260) with a Biotek™ plate reader and the Take3™ plate (Biotek, Winooski, VT), and assessed by the A260/A280 ratio. cDNA was synthesized from total RNA (Applied Bio systems) using a High Capacity cDNA Reverse Transcription Kit (Applied Bio systems). Real-time PCR was performed using TaqMan® Fast Universal Master Mix (2x), on a 7500 HT Fast Real-Time PCR System (Applied Bio systems). Specific TaqMan® Gene Expression Assays probes for mouse PGC1α, PRDM16, UCP1, adiponectin, SIRT3, Mfn1, Mfn2 and GAPDH were used as described previously (4). For in vitro western blot analysis pelleted cells were lysed with lysis buffer supplemented with protease and phosphatase inhibitors (cOmplete™ Mini and PhosSTOP™, Roche Diagnostics, Indianapolis, IA). Frozen adipose tissues were ground under liquid nitrogen and suspended in homogenization buffer (mmol/l: 10phosphate buffer, 250 sucrose, 1.0 EDTA, 0.1 PMSF, and 0.1% v/v tergitol, pH 7.5). Immunoblotting for HO-1, UCP1, PEG1/MEST, MnSOD, Mfn-2, OPA-1, PGC-1α, COX-IV (Cytochrome c oxidase subunit-IV), adiponectin, β-actin and GAPDH was performed on cell and mouse adipose tissue lysates as previously described (2; 5).
Magnetic Resonance Imaging
Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) volumes were determined by MRI. Total fat, SAT, and VAT volumes, VAT/SAT ratio, and fat/body volume ratio were calculated as described earlier (7).
Oxygen consumption
Animals were acclimatized in the oxygen consumption chambers for 2 hours three times a week for 3 weeks. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured using the Oxylet gas analyzer and air flow unit (Oxylet; Panlab-Bioseb, Vitrolles, France). Individual mice were placed in the chambers and had the flow rate set to maintain the dCO2 between 0.4 and 0.8 as specified by the manufacturer’s instructions. Hourly respiratory quotients were measured using the VCO2 and VO2 obtained by the gas analyzer. This process was performed twice on each mouse. The data for VO2 are expressed as the consumed volume of oxygen per kilogram body weight per minute (ml/kg/min). The respiratory quota is expressed as CO2 eliminated/O2 consumed.
Statistical analysis
Data are expressed as means ± S.E.M. Significance of difference in mean values was determined using one-way analysis of variance followed by Bonferroni’s post-test for comparison between groups. P< 0.05 was considered to be significant.
Results
EET mediated induction of PGC1-α, UCP1, PRDM16 and adiponectin
Western blot analysis and real-time PCR demonstrated a significant (p<0.05) increase in PGC-1α protein and mRNA expression after treatment of adipocyte cells with EET-A (Figure 1 A–C). Concomitant treatment with EET-A and SnMP decreased PGC-1α protein and mRNA expression when compared to EET-A treated cells (Figure 1 A–C). Uncoupling protein 1 (UCP1) was significantly upregulated in cells treated with EET-A (p<0.05), an increase that was abrogated in cells treated with SnMP along with EET-A (Figure 1 A and D). We examined the effect of EET-A on the brown fat specific gene PRMD16 and found that PRMD16 mRNA levels were increased (p<0.05) in adipocyte cells treated with EET-A (Figure 1 E). SnMP attenuated the EET-A mediated increase in PRMD16 mRNA expression. The adiponectin mRNA expression level in the adipocyte cells treated with EET-A was significantly higher (p<0.05) compared to untreated adipocytes and was attenuated by SnMP administration (P<0.05) (Figure 1 F).
Figure 1.
Western blot and densitometry analysis for PGC-1α protein (A and B) and mRNA (C) levels in differentiated adipocyte cells in the presence of EET-A, or EET-A+SnMP. Western blot and densitometry analysis for the expression of the UCP1 protein levels in differentiated adipocyte cells in the presence of EET-A, or EET-A+SnMP (D). PRDM16 (E), and adiponectin (F) levels in differentiated adipocyte cells in the presence of EET-A, or EET-A+SnMP. *p<0.05 vs. control, #p < 0.05 vs. EET-A alone, n=3.
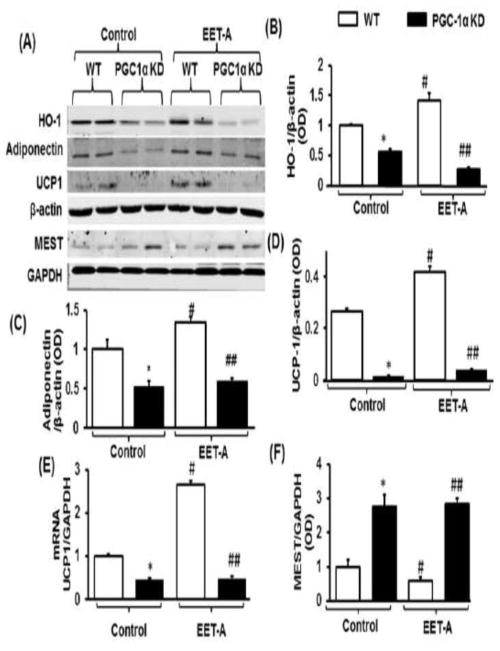
PGC-1α knockdown prevents EET-mediated HO-1 induction, decreases UCP1, adiponectin, and COX-IV, while increasing PEG1/MEST expression
To examine whether EET induction of HO-1 is mediated through PGC-1α we knocked down PGC-1α in adipocyte cells using PGC-1α shRNA. As seen in Figure 2 A and B, the EET-mediated increase in HO-1 protein was completely abolished in PGC-1α deficient cells. Adiponectin protein levels were significantly lower in PGC-1α deficient cells as compared to WT cells (Figure 2 A and C). While treatment with EET-A increased adiponectin protein levels in WT cells (p<0.05), it did not affect the adiponectin levels in PGC-1α deficient cells (Figure 2 A and C). UCP1 mRNA expression and protein levels were lower (p<0.05) in PGC-1α deficient cells as compared to WT cells (Figure 2 D and E, respectively). Treatment of adipocyte cells with EET-A significantly (p<0.05) reduced PEG1/MEST protein expression as compared to vehicle control cells (Figure 2 A and F). Moreover, the expression of the COX-IV protein was increased in control cells treated with EET-A (p<0.05). Importantly, the protein level of COX-IV in PGC-1α deficient cells was lower (p<0.05) as compared to WT control cells (Figure 2 G and H).
Figure 2.

Protein and mRNA expression analysis in WT and PGC-1α deficient differentiated adipocyte cells adipocytes in the presence and absence of EET-A (10 μM) treatment. Representative blots of heme oxygenase-1 (HO-1), adiponectin, uncoupling protein 1 (UCP1), paternally-Expressed Gene 1/mesoderm specific transcript (PEG1/MEST), glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), and β-Actin (A), Densitometric analysis of HO-1 (B), adiponectin (C), and UCP1 protein levels (D), normalized to β-Actin. Analysis of UCP1 mRNA expression levels (E). Densitometric analysis PEG1/MEST protein normalized to GAPDH (F). Protein expression analysis of COX-IV in WT and PGC-1α deficient differentiated adipocyte cells in the presence and absence of EET-A (10 μM) treatment. Representative blots of COX-IV and β-Actin (G), and densitometric analysis (H). n=3, *p<0.05 vs control. *p<0.05 vs. WT, #p< 0.05 vs. WT, ##p < 0.05 vs. WT-EET-A. White bars WT, black bars PGC-1 α deficient cells.
Magnetic Resonance Imaging of adipose tissues
Total body fat volumes of subcutaneous adipose and visceral adipose tissues (SAT and VAT, respectively) were determined by MRI in mice fed a HF diet with and without treatment with EET-A (Figure 3 A–D). As seen in Figure 3 C and D, the total, SAT, and VAT volume of obese mice was significantly reduced in mice treated with EET-A. More importantly, the SAT-to-VAT ratio was markedly reduced in the obese mice treated with EET-A.
Figure 3.
Sagittal magnetic resonance imaging (MRI) and representative axial cross-sections of a mouse fed high fat diet alone (A), and a mouse fed a HF diet and treated with EET-A (B). Subcutaneous and visceral adipose tissues (SAT and VAT, respectively) are highlighted with red arrows. Analysis of total body, and fat volumes in HF diet fed mouse (C), and a HF diet fed mouse treated with EET-A (D). VO2 oxygen consumption (ml/kg/min) (D) and RQ (CO2 eliminated/O2 consumed) (F) in mice fed regular chow (lean), mice fed a HF diet, mice fed a HF diet treated with EET-A (HF+EET), and PGC-1α-deficient mice fed a HF diet treated with EET-A (HF+EET+PGC1α KD). n=4, *p<0.05 vs lean, #p<0.05 vs HF, ##p<0.05 vs HF+EET.
Effect of EET on oxygen consumption
We examined the effect of EET-A on mice fed a HF diet on both O2 consumption and the ratio of CO2/O2. As expected, mice on a HF diet displayed a significant (p<0.05) decrease in VO2 consumption. However, HF-diet fed mice treated with EET-A, exhibited a significant (p<0.05) increase in oxygen consumption with a concomitant lowering of VCO2/VO2. PGC-1α deficient animals on a HF-diet treated with EET-A also displayed a significant (p<0.05) decrease in VO2 consumption and an elevated VCO2/VO2. (Figure 3 E and F)
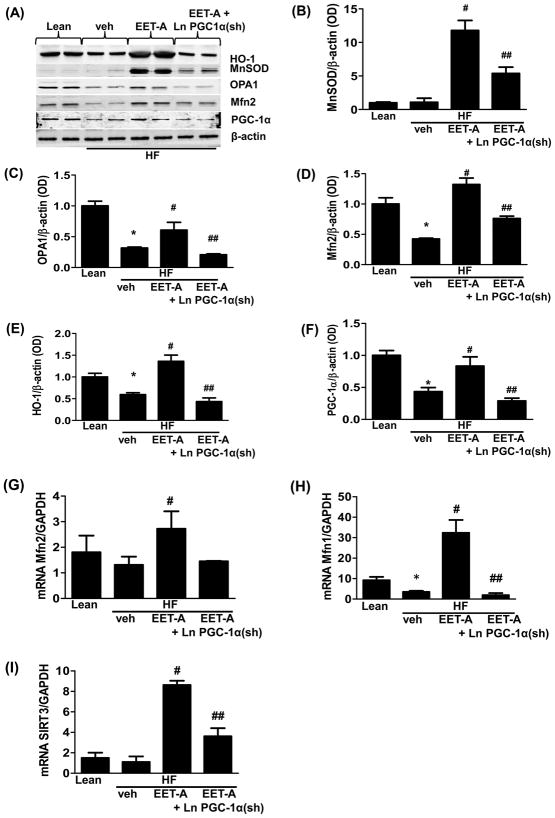
Effect of EET-A administration on HO-1, PGC-1α, Mfn1, Mfn2, MnSOD, OPA-1, and SIRT3 levels in adipose tissues of high fat diet fed control and PGC-1α deficient mice
The levels of MnSOD and HO-1 in adipose tissue were not affected by a HF diet (Figure 4 A, B, and E). Interestingly, EET-A treatment of mice on a HF diet significantly (p<0.05) increased the adipose tissue levels of both MnSOD and HO-1 as compared to both untreated lean and obese mice (Figure 4 A, B, and E). The observed increase was PGC-1α-dependent as the levels of both MnSOD and HO-1 in adipose tissue from PGC1α-deficient mice were significantly (p<0.05) reduced as compared to HFD mice treated with EET-A alone (Figure 4 A, B, and E). The levels of PGC-1α, and the mitochondrial fusion-associated proteins Mfn2 and OPA1, in visceral adipose tissue of mice fed a HF diet were significantly (p<0.05) reduced as compared to control mice fed regular chow (Figure 4 A, C, D and F). Importantly, the levels of these proteins were normalized in HF diet mice treated with EET-A, an effect that was dependent on PGC-1α (Figure 4 A, C, D and F).
Figure 4.
Effect EET-A treatment of HF diet fed control and PGC-1α-deficient on protein and mRNA expression of genes involved in mitochondrial biogenesis and dynamics. Representative blots (A) and densitometric analysis of manganese superoxide dismutase (MnSOD) (B), optic atrophy 1 (OPA1) (C), mitofusin 2 (Mfn2) (D), heme oxygenase 1 (HO-1) (E), and Peroxisome proliferator-activated receptor gamma, coactivator 1 (PGC-1α) (F). mRNA expression of Mfn2 (G), Mfn1 (H), and sirtuin 3 (SIRT3) (I) in adipose tissues of lean, a HF diet fed (HF), a HFdiet EET-A-treated (HF+EET), and PGC-1α-deficient fed a HF diet mice (HF+EET+PGC1α KD). Results are means ±SE, n = 4, *p < 0.05 vs. lean, #p < 0.05 vs HF, ##p<0.05 vs HF+EET.
Analysis of mRNA levels confirmed the EET-A mediated increase of Mfn2 in adipose tissue (Figure 4 G). Furthermore, the Mfn1 mRNA levels were also significantly (p<0.05) increased in HF diet fed mice treated with EET-A as compared to mice fed a HF diet alone (Figure 4 H). Importantly, knockdown of PGC-1α completely prevented the EET-A mediated effect on Mfn1 mRNA levels (Figure 4 H).
Finally, the mRNA levels of the mitochondria associated histone deacetylase, SIRT3, were significantly (p<0.05) elevated in adipose tissues of mice fed a HF diet and treated with EET-A (Figure 4 I), meanwhile the EET-A mediated increase of SIRT3 mRNA levels were significantly (p<0.05) impaired in PGC1α-deficient mice fed a HF diet (Figure 4 I).
Discussion
This study demonstrates that EET is located upstream of PGC-1α in its signaling cascade and, in turn, that PGC-1α is crucial for the induction of HO-1. Together these factors play an important role in the regulation of the adipocyte stage of differentiation. We show that EET is a powerful inducer of PGC-1α-mediated downstream signaling and mitochondrial viability and function, including the processes of oxidative phosphorylation, fusion, and mitochondrial quality control, resulting in improved metabolic parameters in mice fed a HF diet, highlighting that the EET and PGC-1α interplay has a significant role in adiposity, diabetes and obesity. Four key findings substantiate this conclusion.
The first key finding is that EET-A-treatment increases PGC-1α, HO-1 and adiponectin levels in cultured murine adipocyte cells (in vitro) and, more importantly, that these EET-mediated actions do not take place in PGC-1α deficient cells, a strong indication that they are located downstream of PGC-1α in the signaling pathway. The transcriptional coactivator, PGC-1α, is a nuclear protein that was first identified as a regulator of peroxisome proliferator-activated receptor gamma (PPARγ) in brown adipose tissue rich with mitochondria and which has a special role in thermogenesis (24) and is a potent transcriptional coactivator of nuclear receptors and other transcription factors (47;48). PGC-1α is highly expressed in tissues with high energy demands, such as heart, skeletal muscle, and brown adipose tissue wherein it plays a critical role in the maintenance of mitochondrial function as well as in the cellular energy metabolism (49;50) and its induction regulates mitochondrial oxidative metabolism as well as mitochondrial biogenesis and the recruitment of chromatin modifying enzymes (51–54). The importance of PGC-1α in adipose tissue has been established in mice lacking PGC-1α, specifically in adipocytes, which develop insulin resistance and have increased circulating lipid levels when fed a HF diet (35). Thus, the role of PGC-1α in energy homeostasis suggests it to be a target for both anti-obesity and diabetes drugs (48).
This is supported by our observation that in in vitro cultures of murine adipocytes EET-A treatment increases the expression of UCP1 and PRDM16 that play an essential role in thermogenesis and in the browning of adipocytes, which is associated with an increased number of mitochondria (26–28). This EET-mediated increase was also shown, at least in part, to be dependent on HO-1 as both UCP1 and PRDM16 levels were decreased in cells treated with the HO-activity inhibitor SnMP. Furthermore, the EET-A mediated increase of PGC-1 α expression was prevented by SnMP, indicating that EET is working through the HO system that controls cellular levels of heme and the potential of heme-mediated terminal adipocyte stem cell differentiation and enlargement. Our results are corroborated by other studies that have shown that EET, together with SIRT1, regulates PGC-1α expression (55–57), suggesting that they are upstream regulators of PGC-1α. Although EET downregulates the BTB And CNC Homology 1, Basic Leucine Zipper Transcription Factor 1 (BACH1), an inhibitory transcriptional regulator of HO-1, in favor of increased HO-1 levels (58) we now show that the EET-mediated action on HO-1 is also dependent on PGC-1α expression. These results identify PGC-1α as a crucial component of the EET-augmented regulatory pathway involved in the control of HO-1 and adiponectin production as well as mitochondrial viability and function.
We have previously shown that heme levels are increased with, while also promoting, adipocyte differentiation, while the levels of HO-1 are reduced (1;46). Additionally, increased heme levels, either by exogenous addition of hemin or by the inhibition of HO-activity induce or enhance adipogenesis of pre-adipocytes (59), and mesenchymal stem cells alongside the development of oxidative stress and attenuation of SIRT1 expression (22;60). Although heme is critical for increased adipocyte differentiation and hypertrophy it is an essential substrate for numerous enzymes and its levels must be tightly regulated to minimize toxicity while HO-1/heme is essential to the maintenance of homeostatic regulation of mitochondrial respiration. Recently, the nuclear heme receptor, Rev-erbα, was identified as a negative regulator of heme levels via its ligand activated repression of PGC-1α expression that, in turn, leads to inhibition of 5′-Aminolevulinate Synthase 1 (ALAS1), the rate limiting enzyme in heme biosynthesis (61;62). Not only does HO-activity inhibition increase heme levels, but also results in lower levels of its metabolic products biliverdin and CO. Whether these products themselves play a role in PGC-1α regulation remains to be clarified. Simultaneously, we show that PGC-1α regulates HO-1 expression, thus putting PGC-1α in a central position with a capacity to both increase heme levels in favor of terminal adipogenic differentiation, and to reduce heme levels by increasing HO-1 expression, as a result of EET-treatment, that favors prevention of terminal adipocyte differentiation. Therefore, there is a balance of heme levels and stage of adipocyte differentiation and is regulated by the rate of heme degradation (HO-1 induction) and heme synthesis (PGC-1α-ALAS1 induction) necessary for increase of heme and terminal differentiation and hypertrophy (46).
HO-2 and HO-1 play a regulatory role on mitochondrial function and apoptosis(63–65). HO-2 deficiency has a negative impact on superoxide anion levels, EC-SOD and the mitochondrial signaling pathway (17;64), but its definite role in preservation of mitochondrial function is not fully understood. We have shown that HO-1 is present in the mitochondrial inner membrane and cortex (64) and we and others have in several reports shown that HO-1 plays a role in mitochondrial biogenesis, cytochrome oxidase levels as well as in mitochondrial quality control (66–68). Additionally, HO-1 induction increased mitochondrial function in ischemic cardiac damage (69) and experimental diabetes (66). In contrast, drug-mediated disruption of mitochondrial membranes and mitochondrial structure also had adverse effects on heme metabolism (70).
The second key finding is that EET-A treatment of obese mice decreases both VAT and SAT. More importantly, the VAT/SAT ratio was reduced in mice treated with EET-A. EET decreases adiposity and insulin resistance in an animal model of obesity and diabetes involving an increase in HO-1 gene expression and in the AMPK/AKT signaling cascade (16). In addition, EET inhibits BACH-1 expression, a negative transcriptional regulator of HO-1 expression (58). While EET has been shown to increase HO-1 expression, increased HO-1 levels lead to a recovery in EET levels in HO-2 null mice (71) as well as in EC-SOD null mice (72), thus completing a positive feed-back loop.
We have previously reported HO-1-EET interactions and their beneficial role in regulating adipogenesis. Specifically, EETs decrease mesenchymal stem cell-derived adipocyte differentiation in vitro via upregulation of HO-1-adiponectin-AKT signaling and a decrease in PPARγ expression (11). Moreover, endothelial overexpression of CYP2J2 in mice fed a HF diet normalized adipocyte function, insulin levels, blood glucose, body weight and inflammatory markers (9). Our results have been confirmed by others attesting to the regulation of adipocyte differentiation by EETs both in vivo and in vitro (13). These results clearly point to a regulation of PGC-1α that is directly dependent on EET and indirectly, via increased EET levels, on HO-1 (Figure 5).
Figure 5.
Schematic description of the EET-PGC-1α-HO-1 interplay, increased insulin sensitivity, mitochondrial function, and reduced inflammation. Epoxygenase-derived EETs increase of HO-1 expression in a process that is dependent on PGC-1α expression. The increase in PGC-1α – HO-1 interplay subsequently increases insulin sensitivity, mitochondrial biogenesis and function and expression of energy dependent enzymes, including Mfn-1, Mfn-2, SIRT-3, MnSOD, and OPA-1, thus attenuating the development of obesity, metabolic syndrome and ultimately diabetes mellitus type 2.
The third novel finding is that EET-A is associated with a normalization of both VO2 and the RQ in mice fed a HF diet as compared animals on regular chow (lean mice). This increase may be related to an alternate metabolic strategy of HF-fed mice to EET i.e., to increase the metabolic rate in response to increased EET-A-mediated PGC-1α expression and mitochondrial thermogenesis and function as indicated by the increased levels of UCP1 in vitro and SIRT3 in vivo leading to a reprogramming of adipose stem cells to brown-like cells thus allowing the maintaining of normal mitochondria and energy consumption and a subsequent decrease in body fat. Meanwhile, the EET-A-mediated normalization of VO2 and RQ was completely prevented in PGC-1α deficient mice on a HF diet.
Cytochrome c Oxidase Subunit IV (COX-IV) is a crucial component of the cytochrome c oxidase complex, which catalyzes the final step in the electron transfer chain and regulates oxidative phosphorylation and total respiration. Impaired activity of the cytochrome c oxidase complex has been linked to a wide range of disorders including stroke, hepatic failure, fatal lactic acidosis, and exercise intolerance, and is a major cause of subsequent mitochondrial abnormalities (73–75). A reduced total oxygen consumption in mice deficient in lung specific COX-IV subunit 2 has been reported (76). We measured the protein levels of COX-IV in vitro in WT and PGC-1α deficient adipocyte cells treated with EET-A and found that the COX-IV levels were elevated in WT cells treated with EET-A. However, the COX-IV levels of PGC-1α-deficient cells were significantly reduced.
The fourth key finding is that visceral fat from mice fed a HF diet showed impaired mitochondrial viability-, quality control- and fusion-associated parameters as compared to lean mice. While treated with EET-A normalized or enhanced these mitochondrial parameters, PGC-1α-deficiency prevented the EET-A-mediated effects, thus indicating that the EET-A-mediated normalization of diet-induced mitochondrial dysfunction is PGC-1α-dependent. Our results linking EETs to mitochondrial viability are supported by a report showing that EETs promote mitochondrial biogenesis and protect neurons from oxidative damage (77). However, to our knowledge our study is the first to show that EETs stimulate mitochondrial fusion associated processes as indicated by the increased expression of Mfn1 and 2, and OPA1. Furthermore, the EET-PGC1α axis increases MnSOD, which is a critical component of mitochondrial resistance to superoxide induced damage. Even though a HF diet itself did not reduce MnSOD levels in visceral fat, the EET-A mediated increase was effectively reduced in PGC-1α-deficient mice corroborating our recent report showing that MnSOD levels are impaired in PGC-1α deficient 3T3-L1-derived adipocytes, contributing to increased mitochondrial derived superoxide formation (46). Similarly, the levels of SIRT3 were reduced in PGC-1α-deficient mice on high fat diet treated with EET-A as compared to mice on a HF diet treated with EET-A alone. About 90% of ROS contribute to the mitochondrial energy metabolism (38). Furthermore, SIRT3 is the target of PGC-1α and important in mitochondrial suppression of ROS, mitochondrial biogenesis and metabolic homeostasis (38;78), including mitochondrial fatty-acid oxidation (39). The visceral adipose tissue levels of the mitochondrial fusion mediating proteins, Mfn1, Mfn2 and OPA1, were significantly downregulated with a HF diet. While, EET-A-treatment normalized, or enhanced the levels of these proteins in animals on high fat diet, PGC-1α-deficiency completely prevented the EET-A-mediated effects. Several reports have shown the importance of mitochondrial function and dynamics, health, and aging, for review see (43). Knockout mice Mfn1, Mfn2 and OPA1 are all embryonic lethal (78). In humans, mutations in OPA1 are linked to hereditary blindness, and Mfn2 mutations are known to cause Charcot-Marie-Tooth disease (42;78;79). In mice, a liver-specific knockout of Mfn2 was linked to impaired glucose metabolism and insulin signaling (80). Our results linking PGC-1α-dependent increase of HO-1, thus potentiating the beneficial effect of PGC-1α itself are supported by a recent report in which doxorubicin-induced oxidative stress increased mitochondrial fragmentation and Fis1 expression, an effect that was prevented by HO-1 induction favoring expression of Mfn1 and Mfn2 (68).
These results support our hypotheses linking EET-A to PGC-1α mediated regulation of HO-1 expression and levels of heme and potentially biliverdin and CO, to mitochondrial health as well as to metabolic adipocyte reprogramming that may influence adipocyte hemostasis and delay terminal differentiation. The reported findings suggest that induction of HO-1, possibly via its metabolic products biliverdin and CO, leads to an increase in PGC-1α through an increase in EET levels thereby providing metabolic protection as a mechanism to regulate HF diet induced obesity in mice. The present study is of considerable interest from both a basic and a clinical perspective clearly defining the existence of an EET-PGC-1α regulatory module that can be manipulated to ameliorate the deleterious effect of HF diet-induced insulin resistance, diabetes and metabolic syndrome via induction of HO-1 leading to control of mitochondrial dynamics and biogenesis. HO-1 expression is instrumental for mitochondrial integrity (68). A deeper understanding of the mechanism of EET- PGC-1α necessary to increase insulin sensitivity, HO-1 expression and HO activity will provide new approaches to the treatment of obesity-diabetes and improve the effectiveness of EET- PGC-1α downstream signaling in the treatment of vascular disease.
Acknowledgments
This work was supported by: National Institutes of Health grant HL34300 and The Brickstreet Foundation and The Huntington Foundation (NGA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors wish to thank Ms. Jennifer Brown, New York Medical College for her outstanding editorial assistance in the preparation of the manuscript.
Author contributions are as follows: S.P.S performed experiments; L.B. performed experiments, collected literature citations, and edited manuscript; J.S. conducted ELISA assay and edited manuscript; N.G.A. planned and designed the experimental protocol, and wrote the first draft of the manuscript; J.C. designed the PGC-1α sequence.
ABBREVIATIONS
- BACH1
BTB and CNC homology 1, basic leucine zipper transcription factor 1
- CO
Carbon monoxide
- COX-IV
Cytochrome C oxidase subunit IV
- DM2
Diabetes mellitus type 2
- EET
Epoxyeiocosatrienoic acid
- EET-A
EET agonist
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HO
Heme oxygenase
- Mfn
Mitofusin
- MnSOD/SOD2
Manganese-containing superoxide dismutase/superoxide dismutase 2, mitochondrial
- OPA1
Optic atrophy 1 (autosomal dominant)
- PEG1/MEST
Paternally-expressed gene 1 protein/Mesoderm specific transcript
- PGC-1α
Peroxisome proliferator-activated receptor gamma, coactivator 1
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PRDM16
PR domain containing 16
- ROS
Reactive oxygen species
- SAT
subcutaneous adipose tissue
- sEH
Soluble epoxide hydrolase
- SIRT
Sirtuin
- SnMP
Tin mesoporphyrin
- UCP1
Uncoupling protein 1
- VAT
visceral adipose tissue
Footnotes
Conflict of Interest
There are no conflicts of interest among the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peterson SJ, Vanella L, Gotlinger K, Jiang H, Bialczak A, Singh SP, et al. Oxidized HDL is a Potent Inducer of Adipogenesis and Causes Activation of the Ang-II and 20-HETE Systems in Human Obese Females. Prostaglandins Other Lipid Mediat. 2016 doi: 10.1016/j.prostaglandins.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3 Pt 2):625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88(11):1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 4.Vigili de KS, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J. 2003;24(13):1210–1215. doi: 10.1016/s0195-668x(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 5.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 7.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433(2):413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, et al. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension. 2014;64(6):1352–1361. doi: 10.1161/HYPERTENSIONAHA.114.03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol. 2008;4(2):165–174. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, et al. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19(12):1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prost Other Lipid Mediat. 2012;98(3–4):133–142. doi: 10.1016/j.prostaglandins.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res. 2014;55(10):2124–2136. doi: 10.1194/jlr.M053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39(1):1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Abraham NG, Junge JM, Drummond GS. Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharmacol Sci. 2015 Oct 26; doi: 10.1016/j.tips.2015.09.003. pii/00202–3: S0165–S6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331(3):906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, et al. Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction. J Am Soc Nephrol. 2006;17(4):1073–1081. doi: 10.1681/ASN.2004121082. [DOI] [PubMed] [Google Scholar]
- 18.Dennery PA, Spitz DR, Yang G, Tatarov A, Lee CS, Shegog ML, et al. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J Clin Invest. 1998;101(5):1001–1011. doi: 10.1172/JCI448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57(6):1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, Abraham NG. High fat diet enhances cardiac abnormalities in SHR rats: Protective role of heme oxygenase-adiponectin axis. Diabetol Metab Syndr. 2011;3(1):37. doi: 10.1186/1758-5996-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J, Inoue K, Sodhi K, Puri N, Peterson SJ, Rezzani R, et al. High-fat diet exacerbates renal dysfunction in SHR: reversal by induction of HO-1-adiponectin axis. Obesity (Silver Spring) 2012;20(5):945–953. doi: 10.1038/oby.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr, et al. Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4(2):28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46(1):236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–8890. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hondares E, Rosell M, Diaz-Delfin J, Olmos Y, Monsalve M, Iglesias R, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286(50):43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen P, Spiegelman BM. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes. 2015;64(7):2346–2351. doi: 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA. 2009;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Garnier A, Fortin D, Zoll J, N’Guessan B, Mettauer B, Lampert E, et al. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005;19(1):43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 31.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 32.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple RK, Crowley VC, Sewter CP, Laudes M, Christodoulides C, Considine RV, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord. 2004;28(1):176–179. doi: 10.1038/sj.ijo.0802482. [DOI] [PubMed] [Google Scholar]
- 34.Hammarstedt A, Jansson PA, Wesslau C, Yang X, Smith U. Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun. 2003;301(2):578–582. doi: 10.1016/s0006-291x(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 35.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci USA. 2012;109(24):9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arany Z, Wagner BK, Ma Y, Chinsomboon J, Laznik D, Spiegelman BM. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. Proc Natl Acad Sci USA. 2008;105(12):4721–4726. doi: 10.1073/pnas.0800979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lionetti L, Mollica MP, Donizzetti I, Gifuni G, Sica R, Pignalosa A, et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One. 2014;9(3):e92753. doi: 10.1371/journal.pone.0092753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alavi MV, Fuhrmann N. Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener. 2013;8:32. doi: 10.1186/1750-1326-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism, A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278(19):17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 45.Civitarese AE, Ravussin E. Mitochondrial energetics and insulin resistance. Endocrinology. 2008;149(3):950–954. doi: 10.1210/en.2007-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, et al. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1alpha Activation, Which is Required for HO-1 Expression and Increased Mitochondrial Function. Stem Cells Dev. 2016 doi: 10.1089/scd.2016.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko GT, Chan JC, Woo J, Lau E, Yeung VT, Chow CC, et al. Serum bilirubin and cardiovascular risk factors in a Chinese population. J Cardiovasc Risk. 1996;3(5):459–463. doi: 10.1177/174182679600300508. [DOI] [PubMed] [Google Scholar]
- 48.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 50.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, et al. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278(29):26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 51.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, et al. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286(5443):1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 53.Fandrey J, Bunn HF. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993;81(3):617–623. [PubMed] [Google Scholar]
- 54.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Chen M, Yuan L, Xiang Y, Zheng R, Zhu S. 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem Biophys Res Commun. 2014;450(1):604–609. doi: 10.1016/j.bbrc.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 58.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, et al. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96(1–4):54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen JJ, London IM. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981;26(1 Pt 1):117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- 60.Puri N, Sodhi K, Haarstad M, Kim DH, Bohinc S, Foglio E, et al. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J Cell Biochem. 2012;113(6):1926–1935. doi: 10.1002/jcb.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu N, Yin L, Hanniman EA, Joshi S, Lazar MA. Negative feedback maintenance of heme homeostasis by its receptor, Rev-erbalpha. Genes Dev. 2009;23(18):2201–2209. doi: 10.1101/gad.1825809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, et al. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122(4):505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 63.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 64.Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, et al. Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem. 2007;100(4):815–823. doi: 10.1002/jcb.21138. [DOI] [PubMed] [Google Scholar]
- 65.Olszanecki R, Rezzani R, Omura S, Stec DE, Rodella L, Botros FT, et al. Genetic suppression of HO-1 exacerbates renal damage: reversed by an increase in the antiapoptotic signaling pathway. Am J Physiol Renal Physiol. 2007;292(1):F148–F157. doi: 10.1152/ajprenal.00261.2006. [DOI] [PubMed] [Google Scholar]
- 66.Di Noia MA, Van DS, Palmieri F, Yang LM, Quan S, Goodman AI, et al. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem. 2006;281(23):15687–15693. doi: 10.1074/jbc.M510595200. [DOI] [PubMed] [Google Scholar]
- 67.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103(11):1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hull TD, Boddu R, Guo L, Tisher CC, Traylor AM, Patel B, et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight. 2016;1(2):e85817. doi: 10.1172/jci.insight.85817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, et al. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One. 2014;9(3):e92246. doi: 10.1371/journal.pone.0092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham NG, Lutton JD, Freedman ML, Levere RD. Benzene modulation of liver cell structure and heme-cytochrome P-450 metabolism. Am J Med Sci. 1986;292(2):81–86. doi: 10.1097/00000441-198608000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, et al. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cell Physiol Biochem. 2012;29(1–2):99–110. doi: 10.1159/000337591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawakami T, Puri N, Sodhi K, Bellner L, Takahashi T, Morita K, et al. Reciprocal Effects of Oxidative Stress on Heme Oxygenase Expression and Activity Contributes to Reno-Vascular Abnormalities in EC-SOD Knockout Mice. Int J Hypertens. 2012;2012:740203. doi: 10.1155/2012/740203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Park JS, Deng JH, Bai Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr. 2006;38(5–6):283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, Tzagoloff A. Cytochrome oxidase in health and disease. Gene. 2002;286(1):53–63. doi: 10.1016/s0378-1119(01)00803-4. [DOI] [PubMed] [Google Scholar]
- 75.Diaz F. Cytochrome c oxidase deficiency: patients and animal models. Biochim Biophys Acta. 2010;1802(1):100–110. doi: 10.1016/j.bbadis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 76.Huttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, et al. Cytochrome c oxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology. FASEB J. 2012;26(9):3916–3930. doi: 10.1096/fj.11-203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, Chen M, Yuan L, Xiang Y, Zheng R, Zhu S. 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem Biophys Res Commun. 2014;450(1):604–609. doi: 10.1016/j.bbrc.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, Kitsis RN, et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res. 2016 doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zuchner S, Vance JM. Charcot-Marie-Tooth Neuropathy Type 4A. 1993. [Google Scholar]
- 80.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA. 2012;109(14):5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]