Abstract
Objective
This 6-month pilot trial compared two strategies for weight loss in older adults with BMI’s ≥ 35 kg/m2 to assess weight loss response, safety, and impact on physical function.
Methods
We randomized 28 volunteers to a balanced deficit diet (BDD, 500 kcal/d below estimated energy needs) or an intensive low calorie meal replacement diet (ILCD, 960 kcal/day). Behavioral interventions and physical activity prescriptions were similar for both groups. Primary outcomes were change in body weight and adverse event frequency; secondary outcomes included measures of physical function and body composition.
Results
ILCD average weight change was −19.1±2.2 kg or 15.9±4.6% of initial body weight compared to −9.1±2.7 kg or 7.2±1.9% for BDD. ILCD lost more fat mass (−7.7 kg, 95%CI [−11.9, −3.5]) but had similar loss of lean mass (−1.7 kg, 95%CI [−4.1, 0.6]) compared to BDD. There were no significant differences in change in physical function or adverse event frequency.
Conclusions
Compared to a traditional balanced deficit diet intervention, older adults with severe obesity treated with intensive medical weight loss had greater weight loss and decreases in fat mass without a higher frequency of adverse events. In the short-term however, this did not translate into greater improvements in physical function.
Keywords: older adult, obesity, meal replacement, low calorie diet, body composition, physical function
Introduction
Adults aged ≥65 years represent one of the fastest growing segments of the US population, and among this sub-population the prevalence of obesity is increasing. In 2007–2010, the prevalence of obesity in older adults was 35%, and was increasing especially at the highest levels of body mass index (BMI) classification (BMI≥35 kg/m2).1 Obesity can worsen the decline in physical function associated with aging, leading to frailty.2–6 As a greater proportion of the population is surviving to old age, the public health burden of obesity-related disability and need for long-term care is of critical concern.1
Despite the high potential for functional decline, there is no consensus for the most effective and safe methods for lowering this risk in older adults with obesity. Bariatric surgery is one option, yet less than 3% of all bariatric surgeries are performed in older patients, often because of the attendant surgical risk and the unclear risk-benefit ratio in this age group.7 Trials of pharmacotherapy for weight reduction often do not include adults older than 70 years of age. More intensive lifestyle modification strategies that include use of medical monitoring and low calorie diets using meal replacements have not been studied in older adults either. 8,9 Even still, there is a traditional reluctance among health care providers to recommend weight loss due to the uncertainty of whether the benefits outweigh the risks of loss of lean mass, fracture risk, and worse prognoses with many age-related co-morbidities associated with weight loss in this population.10.11
As the proportion of older adults with severe obesity increases, there is limited comparative evidence on how to treat obesity and improve functional status in this group. Higher volume weight loss, such as that achieved through intensive medical weight loss treatment, may have the benefits of providing expedient improvement in physical function due to a higher rate of weight loss. This may be especially important in older adults that have limited time to realize benefits of weight reduction (e.g., in need of more urgent intervention to reverse significant morbidity). However, more rapid weight loss may be associated with greater risk of lean mass loss and adverse events. Therefore, the primary aim of this 24-week pilot study was to compare weight loss and adverse event outcomes for an intensive medical weight loss diet (ILCD) designed to produce weight loss of 0.9–1.4 kg/week to a moderate balanced deficit diet (BDD) intervention which would produce 0.45–0.9 kg weight loss per week in a group of older adults with BMI ≥ 35 kg/m2. Our secondary aim was to explore if ILCD would result in greater improvements in physical function compared to BDD, primarily as a function of the larger amount of weight loss. We hypothesized that, compared to BDD, the ILCD would lead to greater weight loss, primarily as fat, and greater improvements in physical function with no differences in the frequency of adverse events.
Methods
Study population
Volunteers who were ≥65 years old, weight stable (<10 lb weight change in past year), with a BMI ≥35 kg/m2 were recruited from the Winston-Salem, NC metropolitan area in April-May, 2014. Key exclusion criteria included cognitive impairment (Montreal Cognitive Assessment <20), uncontrolled/symptomatic depression (Centers for Epidemiologic Studies Depression Scale score >16), non-skin cancer in the last 2 years, major organ dysfunction, and poorly controlled type 2 diabetes (hemoglobin A1c >9%) or blood pressure (>159/>99 mm Hg). Participants could not be dependent on others for daily meals and food supplies. Following 2 screening and baseline study visits, 28 eligible volunteers were randomized via a secure web-based system that stratified participants based on sex and diagnosis of type 2 diabetes. All participants provided informed consent; this study was approved by the Wake Forest University School of Medicine Institutional Review Board.
Study interventions
Intensive low calorie diet
Participants randomized to ILCD were placed on complete meal replacement using the OPTIFAST medical weight loss protocol.12 Participants were prescribed 5–6 servings of meal replacement for a total of 960 kcal/day (45% carbohydrate; 20% fat; 35% protein). The meal replacements provided 100% of daily recommended needs for micronutrients and were provided to the participants. Participants began to incorporate food into their routine at week 13 with guidance from a dietitian. From weeks 13–26, caloric prescriptions were designed to be between 1100 to 1600 kcal/day, using a combination of meal replacements and food, for continued weight loss. The macronutrient goals were 35% of calories from protein, 40% from carbohydrate, and 25% from fat.
Balanced deficit diet
Participants randomized to BDD were prescribed a calorie restricted diet based on estimates of total energy expenditure (TEE) obtained from the measured resting metabolic rate (RMR) at baseline. TEE was estimated by multiplying RMR by a factor of 1.1–1.3 to cover estimates of activity energy expenditure. We created an individualized dietary prescription for each participant by subtracting up to 500 calories from the estimated TEE, with a minimum intake of 1200 kcal/day. Calorie reductions were preferentially taken from refined carbohydrates and saturated fats in the usual intake. Preference was given for lean proteins, whole grains, fruits, vegetables, and unsaturated fats. Macronutrient goals included a minimum of 25% of calories from protein and maximum of 30% of calories from fat. The study dietitian provided each participant with a detailed program manual that described the prescribed diet.
Adherence to both interventions was monitored by review of weekly food diaries in individual and group counseling sessions. Participants from both groups met individually with the study dietitian (6 visits), to discuss strategies to meet dietary goals, and medical physician (9 visits), to monitor for adverse events and adjust medications, with the same frequency. No appetite suppressants or other medications intended to induce weight loss were prescribed in either group.
Exercise
Both groups received the same standard exercise program designed to promote activity energy expenditure of approximately 1200 kcal/week. We prescribed resistance training for 2 days per week with a loading intensity of 60% of 1 repetition maximum and volume of 3 sets at 8 repetitions per exercise. Aerobic training was prescribed for 3 days per week. Participants had a total of 10 supervised training sessions of 30-minutes each to learn how to use resistance bands for resistance training at home, as well as a battery of aerobic activities that could be alternatives for walking. Other than the training sessions, participants completed the exercise program as home-based activities and recorded all of their exercise in a study-provided log.
Behavioral techniques to promote lifestyle change
Both intervention arms had group and individual behavioral counseling for the 6 months of the intervention. The behavioral interventions were facilitated by trained interventionists with skills in key counseling techniques such as motivational interviewing and cognitive behavioral therapy. During the 6-month behavioral intervention participants met individually with the interventionist for four sessions and in their assigned groups on a weekly basis. Intervention materials are based on those used in the OPTIFAST Lifestyle Education Series (ILCD) and the By Design Essentials Program from the Wake Forest Baptist Health Weight Management Center (BDD).
Measurements
Body weight and composition
Body weight was measured on standardized electronic scales with light clothing and without shoes. Height was measured on a wall-mounted stadiometer without shoes. BMI was calculated as weight (kg)/height (m2). Body composition, including total body and truncal fat mass, lean mass, and bone mineral density, was measured at baseline and 6 months by dual-energy x-ray absorptiometry (DXA, Hologic Delphi QDR, located in our Geriatric Research Center).
Physical function
The Short Physical Performance Battery (SPPB) was the primary measure of overall physical function. It is a measure of lower-extremity function consisting of walking speed, balance, and repeated chair stands. These 3 performance measures are scored from 0 to 4, with 4 indicating the highest level of performance and 0 the inability to complete the task. 13,14 The summary score ranges from 0 (worst) to 12 (best). Secondary physical function measures included the timed stair climb and the 400-meter walk. The timed stair climb task involves assessment of the time it takes an individual to ascend a standard flight of stairs (12 steps) with or without use of a handrail.15,16 In the 400-meter walk, participants are instructed to complete the 400m distance (on a flat indoor surface) as quickly as possible at a maintainable pace and the time to complete the walk is recorded in minutes and seconds. 17,18 Activities of daily living were assessed using the Mobility Assessment Tool – Short Form (MAT-sf) at baseline and the 6 month follow-up.19 Knee muscle strength was measured using an isokinetic dynamometer (Biodex) at one speed (60°/sec) with the participant sitting and the hips and knee flexed at 90°.
Adverse events
Adverse events were defined as any negative outcome or undesirable problem that occurred during the conduct of the study, whether or not it was associated with the study. Serious adverse events were defined as an adverse event that results in: 1) death; 2) a life-threatening situation; 3) hospitalization; 4) disability or permanent damage; 5) the immediate need for medical or surgical intervention to prevent one of these outcomes. All participants were screened at each individual clinic visit by questionnaire on symptoms and other medical events. Questionnaires were reviewed weekly by the study physician to catalog and classify events. We collected blood samples from all participants for medical monitoring of serum electrolytes, renal function, and liver function in the morning in a non-fasted state every other week through 12 weeks and again at week 16. The study physician could also order additional labs based on reported symptoms. Abnormal lab values are reported separately from the adverse events data.
Statistical analysis
Demographic variables and descriptive characteristics of the sample are presented as means and standard deviations. Our primary analysis was focused on testing the significance of the difference between intervention groups for outcomes related to weight change and body composition. Our secondary analyses estimated differences between intervention groups for outcomes related to physical function including the SPPB, 400m walk time, MAT-sf and stair climb task. We used mixed models analysis of covariance to estimate the effect of the intervention on follow-up measures of physical function. The model included the following covariates: the baseline value of the outcome, gender (a stratifying factor used in randomization), age (to control for possible imbalance due to the small number of participants randomized), visit, and a (visit x treatment) interaction. Differences between treatment groups are presented as Estimated Treatment Differences (ETD) and the 95% confidence intervals. Analyses were completed using SAS 9.4.
Results
Study participants
The demographics for study participants by group and overall are shown in Table 1. On average, the participants were 70.3 years old and had a BMI that is categorized as stage III obesity (BMI ≥ 40 kg/m2). A total of 9 participants had type 2 diabetes, including 4 in ILCD and 5 in BDD. Approximately 86% of participants had hypertension, and 54% were being treated for hyperlipidemia. One participant was lost to follow up (BDD group), dropping from the study after week 6 due to work related obligations. Attendance at weekly group sessions averaged 81% for BDD and 96% for ILCD.
Table 1.
Demographics at baseline
| Intensive Low Calorie Diet N=14 |
Balanced Deficit Diet N=14 |
|
|---|---|---|
| Mean ± SE | ||
| Age (years) | 70 ± 1.17 | 69.5 ± 0.95 |
| Sex | ||
| Male | 6 | 6 |
| Female | 8 | 8 |
| Race/Ethnicity | ||
| Non-Hispanic White | 9 | 9 |
| Non-Hispanic Black | 5 | 5 |
| Initial BMI (kg/m2) | 41.48±1.13 | 41.62±1.16 |
| Initial Body Weight (kg) | 122.36 ± 6.49 | 117.66 ± 4.39 |
Weight change
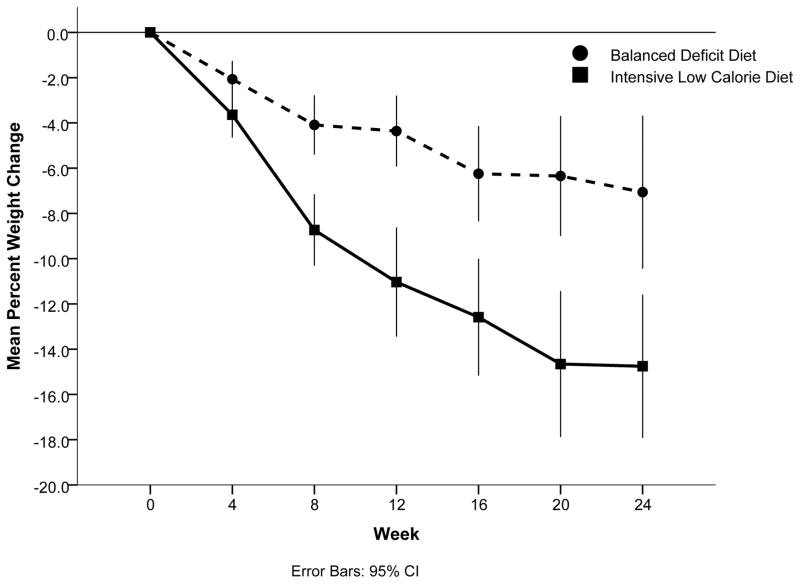
Weight change by treatment groups through 6 months is shown in Figure 1. All participants in ILCD lost some weight (range= −6.3 to −38.8 kg) while 13 of 14 participants in BDD lost some weight (range= 0.1 to −23.9 kg). The average weight change in ILCD was −19.1±2.2 kg or 15.9±4.6% of initial body weight, resulting in an average rate of weight loss of 0.8 kg per week. In the BDD group, the average weight change was −9.1±2.7 kg or 7.2±1.9 % of initial body weight, with an average rate of weight loss of 0.4 kg per week. The ILCD group had a 10±3.6 kg greater weight loss compared to the BDD group (p = 0.0120).
Figure 1.
Percent change in body weight based on weekly clinic measures
Lines represent mean change in percent of initial body weight over the course of the intervention
Body composition and risk factor changes
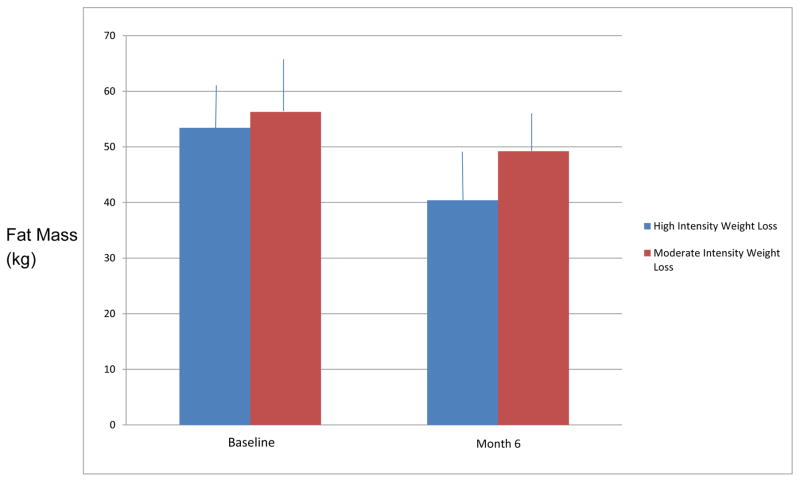
Based on DXA measurements at 6 months, the ILCD group lost more total fat mass (ETD= −7.7 kg, 95%CI [−11.9, −3.5]) (Figure 2) and percent fat (ETD= −3.7%, 95%CI [−5.7, −1.7]). Regional changes in fat mass were similarly favorable for the ILCD group. The ILCD group had a 4.6 kg greater decrease in trunk fat mass compared to the BDD group (95% CI [1.6,7.5]). Total lean mass loss was not significantly different between groups (ETD= −1.7 kg, 95%CI [−4.1, 0.6]). Regional changes in lean mass paralleled total lean mass changes, with the ILCD group having slightly greater, but non-significant, loss of lean mass in the arms and legs compared to BDD (ETD= 0.78 kg, 95% CI [−0.5, 2.1]).
Figure 2.
Changes in total fat mass (kg) by DXA
Bars represent mean fat mass in kg with standard deviation at baseline and 6 month follow up
Physical function
Table 2 shows changes in physical function from baseline to 6 months. SPPB scores did not change significantly in either group from baseline to 6 months (BDD: baseline =9.9; 6 months =10.0; ILCD= 10.4 to 10.2, ETD at 6 months=0.21, 95% CI=[−0.80,1.02]). There was no difference at 6 months in 400 m walk time by treatment group (BDD= 395.1 to 382.9 sec; ILCD= 389.8 to 389.8 sec, ETD at 6 months=−6.9 sec, 95% CI= [−51.0, 37.2]). Stair climb time decreased in ILCD (9.06 to 7.94 sec) but was not significantly different from BDD (9.56 to 9.34 sec, ETD at 6 months=−1.40 sec, 95% CI=[−3.50,0.66])
Table 2.
Physical function outcomes
| Time point | Balanced Deficit Diet | Intensive Low Calorie Diet | Estimated treatment difference [95% CI] | |
|---|---|---|---|---|
| Mean±SD | ||||
| Short Physical Performance Battery | Baseline | 9.93±0.41 (N=14) | 10.43±0.39 (N=14) | |
| 3 Month | 10.73±0.35 (N=13) | 10.03±0.34 (N=14) | 0.70 [−0.32,1.72] | |
| 6 Month | 10.03±0.39 (N=13) | 10.24±0.38 (N=14) | −0.21 [−1.29, 0.86] | |
| Stair climb (secs) | Baseline | 9.56±1.59 (N=14) | 9.06±1.14 (N=14) | |
| 3 Month | 8.92±0.46 (N=12) | 8.00±0.45 (N=12) | 0.92 [−0.34, 2.18] | |
| 6 Month | 9.34±1.04 (N=9) | 7.94±0.88 (N=13) | 1.40 [−1.42, 4.22] | |
| 400-m walk (secs) | Baseline | 395.1±21.78 (N=11) | 389.8±21.50 (N=13) | |
| 3 Month | 389.6±17.13 (N=10) | 400.3±15.02 (N=13) | −10.8 [−58.3, 36.8] | |
| 6 Month | 382.9±15.17 (N=9) | 389.8±12.85 (N=12) | −6.8 [−48.3, 34.6] | |
Footnote for Table 2: Mixed models analysis of covariance containing the baseline value of the outcome, gender, age, visit, and visit x treatment
Safety and adverse events
A total of 1551 separate clinical laboratory measures were completed for the ILCD group while 1371 measures were completed for the BDD group. Abnormal, but non-critical, values were seen on 97 measures in the ILCD group (6.3%), compared to 48 such values in the BDD group (3.5%) (Table 3). Abnormal values for blood urea nitrogen and creatinine represented the majority of abnormal values in both groups (44–62%). One participant in the ILCD group had several abnormal calcium measures and was eventually diagnosed with hyperparathyroidism. Reanalyzing the data without the abnormal serum calcium values due to the hyperparathyroid diagnosis, the number of abnormal values for ILCD group decreases to 82 or 5.3%.
Table 3.
Summary of abnormal labs
| Lab measure | ILCD N=14 |
BDD N=14 |
|---|---|---|
| (% abnormal) | ||
| Sodium | 3.9 | 0.9 |
| Potassium | 3.9 | 2.6 |
| Chloride | 5.4 | 0.9 |
| Bicarbonate | 3.1 | 0.9 |
| Blood urea nitrogen | 24.8 | 20.2 |
| Creatinine | 8.5 | 6.1 |
| Glucose | 2.3 | 0.9 |
| Calcium | 11.6 | 0 |
| Protein | 1.2 | 4 |
| Albumin | 3.6 | 1.3 |
| Bilirubin | 1.2 | 1.3 |
| Alkaline Phosphatase | 1.2 | 1.3 |
| Aspartate aminotransferase | 3.6 | 2.7 |
| Alanine aminotransferase | 4.8 | 0 |
There was no difference in the frequency of adverse events between the treatment groups (data not shown). A total of two serious adverse events were reported, including a transient ischemic attack in the BDD group and a retinal ischemic event in the ILCD group. Both participants had brief adjustments of their participation in the trial (exercise programming was modified as they recovered), but neither had to discontinue participation completely. The majority of adverse events reported were musculoskeletal in nature and generally exacerbations of previous, chronic conditions.
Discussion
This pilot trial comparing two weight loss interventions in older adults with severe obesity demonstrated the efficacy and safety of an intensive medical weight reduction strategy designed to promote 0.9–1.4 kg of weight loss per week. Those in the ILCD group lost approximately double the weight of BDD with no difference in frequency of adverse events. Changes in body composition also suggested a favorable result for those in the ILCD group, with non-significant group differences in loss of lean mass but highly significant differences in loss of total and trunk fat mass. However, despite the achievement of higher volume weight loss for the ILCD group, estimated changes in physical function were similar to those in the BDD group. For both groups, physical function as measured by a variety of measures did not change significantly in the 6-month intervention period.
This study provides a unique assessment of a treatment strategy that is generally targeted for high risk individuals, including those with severe obesity, but has never been studied in older adults. Some of the largest amounts of weight loss previously reported in studies focused on older adults have been in the range of 9.7 to 10.6 kg over 12–18 months.20,21 In the present study, the ILCD intervention led to 19.1 kg weight loss in 6 months. The potential benefits of higher volume weight loss in older adults include risk factor and quality of life improvements along with greater long-term weight loss maintenance. Risk factors and symptoms such as hyperlipidemia, hyperglycemia, hypertension and knee osteoarthritis do appear to have a dose-response relationship with weight loss, such that larger amounts of weight loss lead to greater improvements.16,20,22 In addition, short-term weight loss (during the first 6 months of treatment) is a predictive factor for long-term weight loss maintenance. 23 This behavioral strategy has not been studied in older individuals previously, perhaps because of the perception that higher volume, more intensive weight loss strategies are too high risk in older adults. However, our data suggest this approach could be well tolerated and may not be associated with a higher risk of safety concerns in appropriate clinical settings.
We hypothesized that higher volume weight loss in older adults would lead to greater and more rapid improvements in physical function. However, this was not the case in this study; in fact, there was limited improvement in physical function for both groups. This may have been due to the study sample, the functional measures used, and/or the interventions. Several prior studies report improvements in various aspects of physical function with loss of fat mass in older adults.24–26 For example, Beavers et al reported that loss of fat mass was independently associated with improvements in self-reported mobility disability and walking speed.24 However, in this combined analyses of 3 studies in 271 participants with a baseline BMI of 32.9 kg/m2, the average weight loss of 7.8 kg did not result in changes in SPPB scores. In contrast, Anton et al found that a 5.95 kg weight loss in a group of older women with a baseline BMI of 37.8 kg/m2 was associated with a significant improvement in SPPB scores of 1.82 compared to a control educational intervention.26 Aside from differences in measures and interventions, our study sample may have been fundamentally different at baseline. For example, this study sample had a much higher BMI than either of the aforementioned study populations (our mean BMI at baseline was 40 kg/m2); however, the baseline physical function scores were similar. If fat mass were the primary driver of declines in physical function, we would expect lower baseline function scores. Our study sample was older on average as well, suggesting that there are other drivers potentially related to the aging process, duration of obesity, and/or accumulated co-morbid conditions that may be influencing physical function at baseline, and as a result, the response to treatment.
While the initial improvement in physical function was limited, a potential health benefit could be that longer term changes in physical function will be impacted by the weight loss interventions. If larger volume of weight loss does not lead to dramatic improvements in physical function, could it delay ongoing declines in function associated with aging? This is especially interesting in the context of considering different volumes of weight loss. Over time, some weight regain can be expected in this setting, and data from the Health ABC study, as well as small intervention studies by our group, suggest that weight regain is preferentially fat compared to lean mass in this age group.10,27 It is still unclear if this type of weight loss strategy could lead to a net loss of lean mass and worse long-term physical function or maintenance of function because of a lower body weight.
All research findings have to be considered in the context of the limitations of the experimental conditions. This study is limited by the fact that it was a short-term intervention, designed primarily as a pilot trial to assess the safety and efficacy of the interventions. Additional long-term trials with larger samples will be required to provide more definitive assessment of physical function. As noted previously, our set of measures chosen for assessment of physical function are standard and have been shown to be sensitive to weight loss in previous studies. However, other measures may be more sensitive in this population with higher BMIs at baseline. Lastly, we used BMI as a criterion to define our study sample (BMI≥ 35 kg/m2) to find higher risk older adults. This may not have been as sensitive in identifying people whose physical function scores may be most responsive to weight reduction. Others have used frailty scores for example; however, BMI is readily available to clinicians and this population of older adults has not been studied frequently.
In summary, a short-term medically-supervised intensive low calorie diet intervention led to larger reductions in fat mass without evidence of increased risk in older adults with severe obesity compared to a moderate weight loss intervention based on a balanced deficit diet. This finding is notable as we continue to see an increase in the proportion of older adults who reach higher BMIs and are considering treatment options for obesity. Additional research is needed to identify if there are other domains of physical function that may be affected by higher volume weight loss and the timeline for when those effects may be realized. These pilot data serve as a basis for building longer-term trials of differential weight loss in severely obese older adults to definitively assess the impact on physical function and longer-term adverse events.
What is already known about this subject?
Obesity has significant effects on health risks and physical function in older adults. Prior research has shown some benefits of decreasing fat mass on some aspects of physical function in older adults at lower levels of obesity.
What does this study add?
Older adults with severe obesity treated with an intensive medical weight loss intervention were able to lose over 15% of their body weight safely in 6 months with similar losses of lean mass as those who lost about 7% of their body weight using a standard weight loss diet. Despite the significant differences in weight loss, physical function outcomes did not differ by group.
Acknowledgments
Funding: Wake Forest Claude D. Pepper Older Americans Independence Center (P30AG21332); Grant for meal replacements provided by Nestle Healthcare Nutrition
Footnotes
Disclosures: JDA is a medical consultant for Nestle Healthcare Nutrition
Contributor Information
Jamy Ard, Department of Epidemiology and Prevention, Wake Forest School of Medicine, Winston Salem, NC.
Miranda Cook, Department of Epidemiology and Prevention, Wake Forest School of Medicine, Winston Salem, NC. Weight Management Center, Department of Surgery, Wake Forest School of Medicine, Winston Salem, NC.
Julia Rushing, Department of Biostatistics, Wake Forest School of Medicine, Winston Salem, NC.
Annette Frain, Weight Management Center, Department of Surgery, Wake Forest School of Medicine, Winston Salem, NC.
Kristen Beavers, Department of Geriatrics, Wake Forest School of Medicine, Winston Salem, NC.
Gary Miller, Department of Health and Exercise Science, Wake Forest University, Winston Salem, NC.
Michael E. Miller, Department of Biostatistics, Wake Forest School of Medicine, Winston Salem, NC
Barb Nicklas, Department of Geriatrics, Wake Forest School of Medicine, Winston Salem, NC.
References
- 1.Fakhouri TH, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS data brief. 2012 Sep;(106):1–8. [PubMed] [Google Scholar]
- 2.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Experimental gerontology. 2013 Sep;48(9):888–897. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2010 Sep;11(9):671–685. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. Journal of the American Geriatrics Society. 2002 Nov;50(11):1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 5.Apovian CM, Frey CM, Rogers JZ, McDermott EA, Jensen GL. Body mass index and physical function in obese older women. Journal of the American Geriatrics Society. 1996 Dec;44(12):1487–1488. doi: 10.1111/j.1532-5415.1996.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. Jama. 1994 Apr 13;271(14):1093–1098. [PubMed] [Google Scholar]
- 7.O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obesity surgery. 2010 Sep;20(9):1199–1205. doi: 10.1007/s11695-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12; [Google Scholar]
- 9.Ard JD, Schroeder MC, Kivilaid K, Soliday NM. Practical Application of a Comprehensive Weight Management Program in Patients with and without Metabolic Syndrome. J Obes Wt Loss …. 2014 [Google Scholar]
- 10.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. The American journal of clinical nutrition. 2005 Oct;82(4):872–878. doi: 10.1093/ajcn/82.4.872. quiz 915–876. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. International journal of obesity. 2005 Sep;29(9):1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 12.Ard J, Schroeder M, Kivilaid K, Soliday N, Swanson J. Practical Application of a Comprehensive Weight Management Program in Patients with and without Metabolic Syndrome. J Obes Wt Loss Ther S. 2014;4:2. [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. New England Journal of Medicine. 1995;332(9):556. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 15.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity. 2006 Jul;14(7):1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 16.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and rheumatism. 2004 May;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 17.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. Journal of the American Geriatrics Society. 2001 Nov;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 18.Rejeski WJ, Brubaker PH, Goff DC, Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Archives of internal medicine. 2011 May 23;171(10):880–886. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Ip EH, Marsh AP, Barnard RT. Development and validation of a video-animated tool for assessing mobility. The journals of gerontology. Series A, Biological sciences and medical sciences. 2010 Jun;65(6):664–671. doi: 10.1093/gerona/glq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. Jama. 2013 Sep 25;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. The New England journal of medicine. 2011 Mar 31;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neiberg RH, Wing RR, Bray GA, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity. 2012 Oct;20(10):2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svetkey LP, Ard JD, Stevens VJ, et al. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity. 2012 Sep;20(9):1820–1828. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Kritchevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013 Jan;68(1):80–86. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. Journal of obesity. 2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anton SD, Manini TM, Milsom VA, et al. Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clinical interventions in aging. 2011;6:141–149. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? The American journal of clinical nutrition. 2011 Sep;94(3):767–774. doi: 10.3945/ajcn.110.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]