Abstract
Sphingoid bases are cytotoxic for many cancer cell lines, and are thought to contribute to suppression of intestinal tumorigenesis in vivo by ingested sphingolipids. This study explored the behavior of a sphingoid base analog, (2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane (“Enigmol”), that cannot be phosphorylated by sphingosine kinases and is slowly N-acylated, therefore, is more persistent than natural sphingoid bases. Enigmol had potential anti-cancer activity in a National Cancer Institute (NCI-60) cell line screen, and was confirmed to be more cytotoxic and persistent than naturally occurring sphingoid bases using HT29 cells, a colon cancer cell line. Although the molecular targets of sphingoid bases are not well delineated, Enigmol shared one of the mechanisms that has been found for naturally occurring sphingoid bases: to “normalize” the aberrant accumulation of β-catenin in the nucleus and cytoplasm of colon cancer cells due to defect(s) in the adenomatous polyposis coli (APC)/β-catenin regulatory system. Enigmol also had anti-tumor efficacy when administered orally to Min mice, a mouse model with a truncated APC gene product (C57Bl/6JMin/+ mice), decreasing the number of intestinal tumors by half at 0.025 % of the diet (w/w), with no evidence of host toxicity until higher dosages. Enigmol was also tested against the prostate cancer cell lines DU145 and PC-3 in nude mouse xenografts, and suppressed tumor growth in both. Thus, Enigmol represents a novel category of sphingoid base analog that is orally bioavailable and has the potential to be effective against multiple types of cancer.
Keywords: Sphingolipids, analogs, colon cancer, prostate cancer, metabolism, Min mouse
Introduction
Sphingolipids are highly bioactive compounds that modulate many cell signaling pathways that are relevant to tumor biology and cancer control (1, 2). Much attention has been given to ceramide (Cer) and Cer analogs (3, 4) as inhibitors of cell growth and inducers of apoptosis (5), and to sphingosine 1-phosphate (S1P), which can inhibit apoptosis, induce cell migration and other “pro-“carcinogenic behaviors (1, 2). However, sphingosine (So), sphinganine (Sa) and other sphingoid bases (such as safingol) also have the potential to be useful for cancer control because they inhibit transformation of normal cells by irradiation (6), and chemical carcinogens (7), induce differentiation of transformed cells (8), and are growth inhibitory and cytotoxic for many cancer cell types (9–13). The mechanism(s) for these effects are unclear, however, sphingoid bases and analogs affect multiple signaling pathways (14) and important processes such as autophagy (15).
Because dietary sphingolipids are hydrolyzed to the sphingoid base backbones that are taken up by intestinal cells (16, 17), many of the studies of cancer suppression in vivo have focused on colon cancer. Orally administered sphingolipids decrease pre-cancerous lesions and tumors in models for chemically induced and inherited colon cancer (18, 19), and sphingoid bases “normalize” one of the regulatory defects in colon cancer, the accumulation of β-catenin in the nucleus and cytosol of intestinal cells (20, 21).
The effectiveness of sphingoid bases appears to be limited by their phosphorylation by sphingosine kinase--an enzyme that has been called an oncogene (22); indeed, studies with sphingosine kinase 1 knockout mice have found that this enzyme is required for small intestinal tumor cell proliferation in Min mice (23). Based on this rationale, one would predict that compounds that cannot be phosphorylated would be more effective in cancer suppression than the naturally occurring sphingoid base sphingosine.
This study describes the findings with a sphingoid base analog, (2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane (named “Enigmol”), that cannot be phosphorylated by sphingosine kinase, and is also poorly N-acylated (24). Enigmol was compared with natural sphingoid bases for cytotoxicity for cancer cell lines, cellular uptake and metabolism, and reduction of nuclear β-catenin, which has previously been associated with suppression of colon cancer (20, 21); then, its efficacy was evaluated using mouse models for colon and prostate cancer. Enigmol displayed anti-cancer activity in all these systems, hence, represents a novel category of sphingoid base analog that is worthy of additional consideration in cancer control.
Materials and Methods
Addition information about the materials and methods has been provided in supplemental files that are available online.
Sphingolipids and analogs
Enigmol [(2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane] (Fig. 1B) was prepared using a highly diastereoselective chemical methodology developed in the Liotta laboratory (25) and references cited in supplementary materials and methods), except for the studies using mouse xenografts with PC-3 cells, where it was obtained from the NCI under the RAND program. Other compounds and reagents were obtained commercially.
Figure 1.
Sphingoid base structures, metabolic products, and effects on cells in culture. Panel A illustrates the metabolic fates of two sphingoid bases (sphinganine without dashed double bond; sphingosine with 4,5-trans-double bond): acylation by ceramide synthase and phosphorylation by sphingosine kinase. Panel B shows the structure of Enigmol and N-acyl-Enigmol. Panel C shows the effect of Enigmol, sphinganine and sphingosine on HT29 (left) and DU145 (right) cells measured by WST-1 assay after 24 h after addition of these compounds (as 1:1 complexes with fatty acid-free bovine serum albumin) in the concentrations shown. The data are shown as the difference from the control (fatty acid-free bovine serum albumin). For each panel, the data are means ± SEM (n=4) and points tagged with “*” are significantly different (P < 0.05) from the control.
Cell culture and treatments
The cell lines referred to in this manuscript and supplemental data were obtained from the American Type Culture Collection (Rockville, MD) and grown in media standard for each cell type as described in the supplemental files. The identity of the HT29, DU145 and PC-3 had been established by the ATCC by its criteria for authentication, which are described on their web site (http://www.atcc.org/Science/CollectionsResearchandDevelopment/CellBiology/tabid/205/Default.aspx) and the lines had not been passaged in culture for greater than 6 months from their receipt or resuscitation.
For treatment of the cells in culture, the sphingoid bases were prepared as the 1:1 molar complex with fatty acid-free bovine serum albumin (BSA) (Calbiochem, San Diego, CA) (26). Unless otherwise noted, viability was measured using the WST-1 reagent (Roche, Indianapolis, IN). The “NCI-60 Human Tumor Cell Line Screen” was conducted by the National Cancer Institute’s Developmental Therapeutics Program (NCI DTP) (http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html) using the BSA complex of Enigmol provided by our laboratory.
Analysis of sphingolipids by liquid chromatography, electrospray ionization tandem mass spectrometry (LC ESI-MS/MS)
Lipid analysis was conducted by extracting the sphingolipids from the cells followed by LC ESI-MS/MS in positive ion mode as described previously (27), with minor modifications described in Supplemental Materials and Methods.
Other assays
The localization of β-catenin in HT29 cells was determined using a primary anti-β-catenin antibody (Transduction Labs; 1:500 dilution) and Alexa 488-goat anti-mouse IgG (Molecular Probes, Eugene, OR) as the second antibody (20, 21).
Animals and treatments
Protocols involving animals were approved by the Institutional Animal Care and Use Committee and conducted according to National Research Council Guidelines. Male Min mice (C57Bl/6JMin/+ mice; 32 d of age; Jackson Laboratory, Bar Harbor, ME) were fed a semipurified AIN 76A diet (Dyets, Bethlehem, PA) that is essentially sphingolipid free (28) supplemented with 0, 0.025% or 0.1 % (w/w) Enigmol. At 39 d of age, the mice were changed to these diets for 45 d then killed for analysis of the tissues.
In the first mouse xenograft study, athymic nu/nu BALB/c mice (Charles River Laboratories, Portage, MI) of both sexes at 32 d of age were given subcutaneous injections of DU145 cells (5 × 106/100 µl PBS) in the right and left flanks, then the groups received Enigmol i.p. (0 or 8 mg/kg mouse body weight in 200 µl olive oil) daily for 5 days. Tumors were measured with calipers (width and length) two times a week and the tumor volume was calculated as 0.5 × (length × width2). In the second, male, athymic (nu/nu) mice (from the National Cancer Institute, Bethesda, MD) received 5 × 106 PC-3 cells, and when tumors became palpable, mice were assigned to groups (n=15) that received 0 or 10 mg/kg Enigmol orally by daily gavage until termination of the experiment. For these studies, Enigmol was dissolved in a small volume of 100% ethanol and then diluted into olive oil; the daily dosage was delivered in 200 µl/mouse.
Results
The hypotheses of this study were that Enigmol will display potential anticancer activity with human cancer cell lines; have greater potency than So and Sa because it will undergo slower metabolism; modulate at least some of the regulatory processes that have been seen previously for sphingoid bases; and, suppress tumorigenesis in vivo in animals models.
Results from an NCI-60 Human Tumor Cell Line Screen
When Enigmol was tested by the NCI (http://dtp.nci.nih.gov/branches/btb/ivclsp.html (29)), it displayed 50% growth inhibition for all 57 cell lines at concentrations between 0.4 and 14 µM, and had LD50 in the 5 to 10 µM range for 5 of the cell lines, and possibly as many as 40 (the latter estimate includes cell lines that displayed ≥50% toxicity at 10 µM but less toxicity at 100 µM, which might reflect a solubility problem at such a high level, or interference from the equimolar fatty-acid-free bovine serum albumin that is also added to assist with solubilization). For more results from this screen, see the Supplemental Data available online.
Additional cellular studies using cancer cell lines
Starting with HT29 cells because sphingolipids are known to inhibit colon carcinogenesis (20, 21), the IC50 for Enigmol was ~8 µM vs ~25 µM for So and Sa after 24 h (Fig. 1C) (it is worth mentioning that this IC50 refers to this specific time after treatment—i.e., whereas ~8 µM Enigmol caused a 50% reduction in 24 h, there was almost complete elimination of viable cells between 48 and 72 h). At the higher concentrations, cells that appeared to be dead had detached from the dishes and were seen floating in the medium; therefore, to confirm that these were not viable, floating cells from dishes treated with ≥20 µM Enigmol were pelleted and replated in new medium without Enigmol, and the dishes were examined for viable cells after 24 h, but none were seen. Likewise, new medium was added to the original dishes to determine if the few cells that remained were viable, but no colonies of resistant clones or any evidence of viable cells were seen in the dishes after several days in culture.
As has been noted before (26), the presence of albumin and other serum proteins has a major impact on the IC50 for sphingoid bases because they are bound by serum proteins. For example, in the case of Enigmol, when the ratio of Enigmol to BSA was increased from 1:1 to 2:1, the IC50 decreased by about half; likewise, removal of FBS from the medium decreased the IC50 by approximately 3 fold (data not shown). Therefore, all of the comparisons in these studies were made at the same Enigmol:BSA ratio (1:1) and with 10% FBS.
The mechanism(s) for cell death were not elucidated, but caspase activity was elevated by Enigmol (Supplemental Fig. 1), and Enigmol was also more potent than So in caspase activation.
Fig. 1C also shows the greater toxicity of Enigmol versus Sa or So for the prostate cell line DU145. The effect of Enigmol on a number of other cell lines (PC3, LnCAP, HL60 and MCF-7 cells) was also examined using the WST-1 assay (as in Fig. 1C) and all had IC50’s in the range of 8 to 12 µM (data not shown); therefore, this sphingoid base analog affects a wide variety of cancer cell lines, as was indicated by the NCI-60 Human Tumor Cell Line Screen.
Enigmol uptake and metabolism
The greater toxicity of Enigmol is not due to more being taken up by cells (Fig. 2A); however, Enigmol persisted longer, as seen in the pulse-chase portion of the experiment (right graph of Fig. 2A)—i.e., where the cells were treated with So or Enigmol for 1 h, then the medium was removed and replaced with new medium minus these compounds and incubated for varying times then analyzed by LC ESI-MS/MS. Whereas very little free So was found in the cells (only 10% at 3 h and <2% at 6 h), >50% of the Enigmol was present after 3 h and ~25% after 12 h. Under these conditions, Enigmol killed 98 ± 2 % of the cells, versus ~50% for So (Supplemental Fig. 2).
Figure 2.
Cellular amounts of Enigmol versus sphingosine in HT29 cells, and their effects on other lipids. In Panel A, left graph: HT29 cells were administered 30 µM sphingosine (So) or Enigmol and incubated for the shown times before analysis of the amounts of these compounds in the cells by LC ESI-MS/MS; right graph: amounts of So and Enigmol associated with the cells in a pulse-chase treatment, i.e., the medium from some of the dishes used for the left graph was removed after1 h, new medium without sphingoid base was added, and the lipids were analyzed at the times shown. The data are expressed in nmol/mg protein as the mean +/− SD, n=3. Panel B shows the amounts and subspecies (i.e., N-acyl-chain length variants) of Enigmol associated with the HT29 cells after the first hour and at each time point during the incubation in new medium. Panel C and D show the effects of the added Enigmol or So on other sphingolipids of interest: sphinganine (Sa), sphinganine 1-phosphate (Sa1P) and sphingosine 1-phosphate (S1P). In panel D, the lower limit of detection (LOD) for Enigmol 1-phosphate by LC ESI-MS/MS is shown by hashed lines.
A substantial portion (~5 nmol/106 cells) of the Enigmol that disappeared during the chase period (~6 nmol/106 cells) could be accounted for as N-acyl-Enigmols (Fig. 2B), which is consistent with this compound being a modest substrate for Cer synthase(s) (24) (the finding of multiple fatty acyl-derivatives suggests that it is acylated by several Cer synthases because each is relatively selective with respect to the fatty acyl-CoA’s that it utilizes) (30). HT29 cells also produced small amounts (~0.1 ± 0.02 nmol/106 cells at the 12 h time point) of N,N,N-trimethyl-Enigmol—a type of sphingoid base metabolite that has recently been found with safingol (31).
As a weak Cer synthase inhibitor (12, 24, 32), Enigmol elevated sphinganine (Sa) and sphinganine 1-phosphate (Sa1P) by several fold (Fig. 2C and D), but had little or no effect on endogenous cellular So (Fig. 2C) and S1P (Fig. 2D). The latter is somewhat surprising because Enigmol inhibited sphingosine kinase in vitro (see Supplemental Fig. 3). Enigmol had little or no effect on the amounts of Cer, SM and monohexosylCer (Supplemental Fig. 4 and 5).
The So that was added to the cells resulted in extremely large increases in S1P (Fig. 2D), from 0.07 ± 0.01 to 7 ± 1 nmol S1P/106 cells. There was also a large increase in Cer (from ~1 to 15 nmol/106 cells at 12 h) (Supplemental Fig. 2) and, interestingly, the fatty acid compositions of the Cer in So treated cells differed considerably from the N-acyl-Enigmols in Enigmol treated cells (Supplemental Fig. 6), therefore, it is possible that different CerS are involved. Sphingomyelins and monohexosylceramides also increased when the cells were treated with So, but by much lower amounts (~1 nmol/106 cells each) than was seen with Cer (Supplemental Fig. 5). These results demonstrate that addition of So alters many sphingolipid subspecies, with probably the most noteworthy being the 100-fold increase in S1P and 15-fold increase in Cer.
Other Effects of Enigmol on HT29 cells
Since a large number of signaling pathways are affected by sphingoid bases, evaluation of all of them was beyond the scope of this study. We selected one in particular—i.e., how Enigmol compares to So with respect to reduction of nuclear β-catenin—because this has been associated with colon cancer suppression by sphingoid bases (20). HT29 cells have large amounts of nuclear and cytosolic β-catenin (Fig 3A) due to truncation of adenomatous polyposis coli protein (APC) (33), and at the concentration where So shows a noticeable effect on nuclear β-catenin (i.e., at ~30 µM, middle panels of Fig. 3A), Enigmol eliminated most of the nuclear β-catenin. Close examination of the images also indicates that fluorescence at the cell-cell boundaries is enhanced by Enigmol and So (for example, see arrows with @ symbol in panels d and g of Fig. 3A), which resembles the “normal” localization of β-catenin with E-cadherin in differentiated intestinal cells (20, 21).
Figure 3.
Disappearance of nuclear β-catenin in HT29 cells treated with Enigmol or sphingosine. For panel A, HT29 cells were grown in chamber slides and treated for 6 h with vehicle (control) (a–c), 30 µM Enigmol (d–f), or 30 µM sphingosine (g–i), then double stained to label β–catenin (Alexa 488 labeled, green) (a, d, g), nucleus (Hoescht, blue) (b, e, h) or a merge of both (c, f, i). Arrows indicate representative cells containing nuclear β-catenin, # indicates cells with partial loss of nuclear β-catenin, * indicates cells with loss of nuclear β-catenin, and the @ symbol highlights fluorescent regions at the cell-cell juncture. The scale for all the images is shown by the bar in panel b (10 µm). Panel B shows pie charts representing the percentage of cells with or without nuclear β-catenin when treated with 30 µM of indicated sphingolipid for 0, 2.5, 6, or 12 h. Data represents 10 fields of view (90 ± 20 cells).
The relative degree of nuclear staining for β-catenin is also depicted in Fig. 3B using 3 categories: positive, partially positive, or negative scored for 5 randomly selected fields of view with 10 to 25 cells/field of view. Essentially all of the control cells were positive for nuclear β-catenin; Enigmol-treated cells displayed fewer nuclear β-catenin positive cells at all time points, with only 2% positive and 3% partially positive at 12 h. So treatment also decreased the percentage that were nuclear β-catenin positive, but not as extensively as Enigmol. Note in particular that the loss of nuclear β-catenin is 10 × greater with Enigmol than So at 2.5 h, which is a time point when HT29 cells contain essentially the same amounts of Enigmol and So (c.f., Fig. 2A).
Regulation of β-catenin is a complex process, but a major factor in the aberrant accumulation of this protein in many colon cancer cells, including HT29 cells, is mutation of the APC gene, which participates in β-catenin turnover by facilitating its phosphorylation, ubiquitination and proteasomal proteolysis (34). Western blot analysis (Fig. 4A, right) revealed that So reduces cytosolic β-catenin somewhat at 30 µM whereas Enigmol (Fig. 4A, left) caused a substantial decrease in soluble (cytosolic) β-catenin within 2 h, and its almost complete disappearance by 6 h.
Figure 4.
Effects of sphingoid bases on the amount of soluble β-catenin and regulators of β-catenin turnover in HT29 cells. Panel A displays the induction of β-catenin turnover by Enigmol. For the left gel, HT29 cells were treated with 30 µM Enigmol for varying time points; for the right gel, the cells were treated with vehicle control or 30 µM Enigmol or sphingosine for 6 h; in each case, soluble proteins were separated by SDS-PAGE and immunoblotted using a monoclonal anti-β-catenin antibody. Panel B displays the effect of a proteasomal inhibitor (MG132) on β-catenin degradation and attenuated of Enigmol-induced turnover of β-catenin by this proteasomal inhibitor. HT29 cells were pretreated with 50 µM MG132 for 1 h, followed by addition of 30 µM Enigmol for 6 h. In panel C, HT29 cells were treated with or without 30 µM Enigmol for 6 h and analyzed using phospho-specific antibodies for GSK-3β and β-catenin and cytosolic proteins (i.e., after centrifugation to remove nuclei and membranes) were analyzed by Western blotting.
Some of the upstream regulators of β-catenin turnover are summarized in the scheme in Fig. 4—i.e., β-catenin phosphorylation by casein kinase I-alpha (CKIα) and glycogen synthase kinase 3-beta (GSK3β) as part of the “Destruction complex” with APC and AXIN (34). To explore if these are involved, HT29 cells were treated with the inhibitor of proteasomal proteolysis MG-132 and the amounts of soluble β-catenin were compared by Western blot analysis. MG-132 completely blocked the disappearance of soluble β-catenin upon Enigmol treatment (Fig. 4B), which suggests that Enigmol is acting by increasing proteasome-dependent turnover. Likewise, the phosphorylation state of β-catenin was affected by Enigmol as analyzed using phospho-specific antibodies for S33/S37/T41- and T41/S45-β-catenin (Fig. 4C, right), which suggests that Enigmol enhances the phosphorylation of β-catenin by CKI-α and/or GSK-3β, which occurs at these sites (34). GSK-3β is also phosphorylated on S9 by protein kinase C (35), which might also be affected because PKC is inhibited by sphingoid bases (36), and indeed there was a decrease in phospho-S9-GSK-3β in cells treated with Enigmol (Fig. 4C, left).
These might not be the only mechanisms whereby Enigmol induces turnover of β-catenin in these cells because other proteases are also activated by sphingoid bases (as shown in Supplemental figure 1 for caspase activation by So and Enigmol). All in all, these results establish that Enigmol has effects similar to, but more potent than, So with respect to decreasing the amount of β-catenin in the nucleus and cytosol.
Inhibition of adenoma formation in Min mice
To determine if Enigmol has anti-tumor activity in vivo, Min mice (19, 20) were fed an essentially sphingolipid-free diet with no supplement (control) (n = 9); 0.025% (w/w) Enigmol (n = 8); or 0.1% (w/w) Enigmol (n = 8). Mice fed the control diet had 75.0 ± 13.6 tumors per mouse whereas those fed Enigmol had 52% and 37% fewer for the 0.025% and 0.1% fed groups, respectively (P < 0.05 versus control) (Fig. 5A). Suppression by Enigmol was seen throughout the proximal, mid, and distal portions of the intestine (Fig. 5A). Since only a few tumors develop in the colon of Min mice, Enigmol did not have a statistically significant effect although the averages appear lower (i.e., 0.63 ± 0.43 for 0.025% and 0.88 ± 0.44 for 0.1% versus 1.0 ± 0.4 for the control, with P=0.2 and 0.4, respectively).
Figure 5.
Effect of Enigmol on tumorigenesis and host toxicity in Min mice. Panel A shows the effect of Enigmol on tumor number in APCMin/+ mice after 6 weeks feeding starting at 32 d of age. Tumor number was assessed by light microscopy and adenomas throughout the entire intestinal tract were counted. Data bars represent mean +/− SEM (n=9, control; n=8, Enigmol-treated). *, P < 0.05, significant difference from untreated control (the groups given 0.025 and 0.1% Enigmol were not significantly different from each other, P < 0.1). Panel B shows the effect of Enigmol administered in the diet on the total weight gained at the end of 45 days feeding. Data represents mean ± SD. Panel C displays the blood levels of common hepato- and renal toxicity markers. Shaded area represents normal diagnostic range for values. Data represented as mean (―) and individual values (◆). *, P < 0.05, significant difference from untreated control.
Enigmol had no adverse effect on body weight (20.2 ± 0.9 g for the control mice and 20.2 ± 1.3 g) nor on any of the biomarkers for liver and kidney function (Fig. 5C) at 0.025% of the diet. However, 0.1% Enigmol reduced weight gain to 4.3 ± 0.7 g during the course of the 5.5 wk study (versus 6.0 ± 0.5 g for the control mice and 6.2 ± 0.4 g for the 0.025% Enigmol group, both of which were significantly different from the 0.1% group with P < 0.05) for a final weight of 18.0 ± 0.8 g (P < 0.05). We do not know if this is due to an effect of 0.1% Enigmol on food consumption, which was not recorded, however, we did not notice differences in the amounts of food left in the food containers. In addition, although the biomarkers for liver and kidney function were in the normal range, the average BUN was significantly higher (P < 0.05) for mice given 0.1% Enigmol versus the control. Total serum protein and albumin levels were somewhat higher for the Enigmol treated mice compared to the control, which is generally not thought to be an indication of toxicity, but may indicate dehydration (Fig. 5C, right panels). All together, these results establish that Enigmol can reduce intestinal tumorigenesis in this mouse model with no evidence of host toxicity at 0.025% of the diet, but the higher level of 0.1% may be more problematic.
In vivo antitumor activity and toxicity of Enigmol in mouse xenografts for prostate cancer
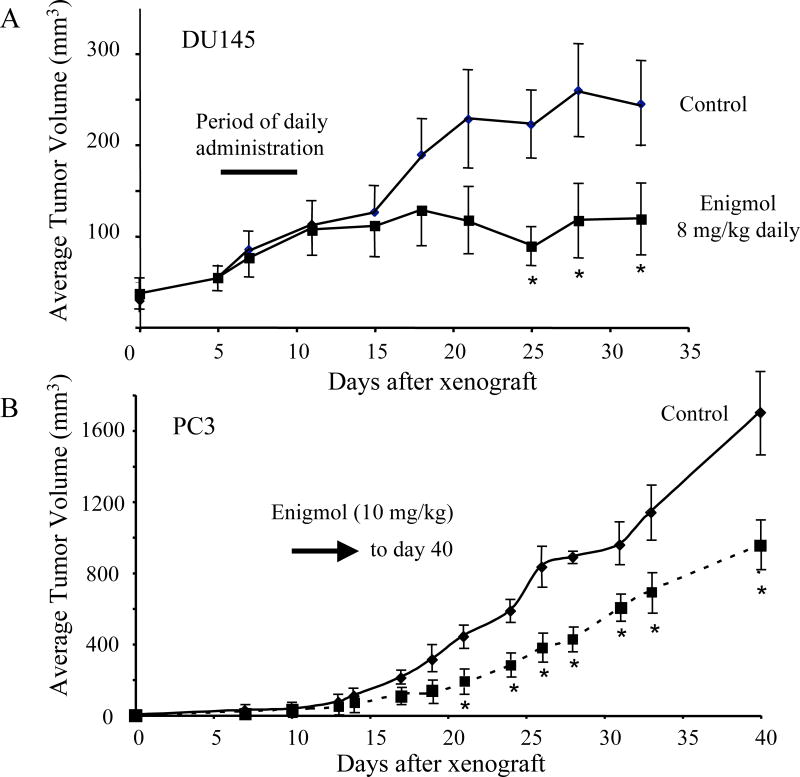
Although the focus of our studies has been on colon cancer due to the relatively well established link for this cancer (18–21), the screening of Enigmol against other cancer cell lines indicates that it might have broader efficacy (Figure 1C and supplementary data). To test this possibility, Enigmol was tested against the prostate cancer line DU145 in a mouse xenograft model (nu/nu BALBc mice) since it was toxic for these cells in culture (Figure 1C). As shown in Figure 6A, Enigmol significantly suppressed the growth (i.e., a ≥50% reduction in tumor size compared to the control) of tumors from these cells when injected i.p. at 8 mg/kg body weight for 5 d. At this dosage, there was no sign of host toxicity by histopathology nor clinical pathology analysis for either study; the Mean ± SD for 3 randomly selected male mice given Enigmol at this dosage were: BUN (25 ± 2 mg/dL), creatinine (0.65 ± 0.49 mg/dL), ALT (33 ± 9 U/L), albumin (2.6 ± 0.4 g/dL) and total serum protein (4.8 ± 0.3 g/dL) all within normal values.
Figure 6.
Evaluation of Enigmol in a nude mouse xenograft models for human prostate cancer. In panel A, DU145 cells were injected in both flanks of athymic nu/nu BALB/c mice (female) at 32 d of age and after tumors were palpable (63 ± 8 and 58 ± 7 mm3 for the control and treated mice, respectively, Mean ± SE for n=10/group), the control and treatment groups were given daily intraperitoneal injections of 200 µl olive oil or Enigmol at 8 mg/kg mouse body weight in 200 µl olive oil for 5 days (designated by the bar). Twice per week, the tumors were measured with calipers and tumor volume was calculated as the greatest diameter × smallest diameter2)/2. In panel B, PC-3 cells were injected in male, athymic (nu/nu) mice and when tumors became palpable (day 10), mice were assigned to groups (33 ± 5 and 30 ± 7 mm3 for the control and treated mice, respectively, Mean ± SE for n=15) that receive daily gavage with the vehicle (200 µl of olive oil) or vehicle containing 10 mg of Enigmol/kg body weight until termination of the experiment. Tumor volumes were measured as described above. For both graphs, the asterisk represents time points where the differences between the tumor volumes for the treated mice were statistically different from the control by P < 0.05 by a two-tailed unpaired t-test.
In a second study (Fig. 6B), BALB/c nu/nu mice implanted with PC-3 cells were assigned to three groups (n=15) that received a daily oral gavage with vehicle alone (200 µl of olive oil) or 10 mg/kg Enigmol in 200 µl of olive oil until termination of the experiment. This also resulted in an approximately 50% reduction in tumor size for the Enigmol group versus the vehicle control. The animals in these groups displayed similar changes in weight during the treatment period; i.e., the starting/ending weights were: 22.8 ± 2.4 g/19.5 ± 2.4 g (loss of 3.3 g) for the controls versus 23.5 ± 3.5 g/20.8 ± 2.9 g (loss of 2.7 g) for the animals administered Enigmol at 10 mg/kg.
Therefore, these studies show that Enigmol has anti-cancer activity against two human prostate cancer cell lines in a mouse xenograft model; and the latter study shows that an effect can be seen when Enigmol is administered orally.
Blood and tissue levels of Enigmol
An analysis of the pharmacokinetics of Enigmol uptake and elimination will be published separately, however, it is worth mentioning here that the amounts of Enigmol in blood on the fourth day of administration of 4 mg Enigmol/kg i. p. was 0.41 ± 0.20 µM (n=4), following the protocol used for Fig. 6A. In the study using PC-3 cells to prepare the xenografts (Fig. 6B), analysis of 3 of the tumors at the end of the study found that they contained 0.18 ± 0.07 nmol of Enigmol/g tissue. These results confirm that Enigmol appears in both blood and the tumors.
Discussion
These studies have established that Enigmol is toxic for numerous human cancer cell lines in the NCI-60 screen and suppressed tumor growth in mouse models for colon and prostate cancer. The toxicity for so many cell lines in the NCI-60 screen raised concern that Enigmol might be toxic for host and cancer cells, however, the in vivo studies suggested that tumors are most affected.
It is usually difficult to predict if compounds that display potential anti-cancer activity in studies of cells in culture will have efficacy in vivo, and this can be especially problematic when the agent is hydrophobic and its delivery to the target site might be limiting. Therefore, on a practical level, the most remarkable findings of these studies were that Enigmol suppresses tumor growth in not only a colon cancer model (Min mice), as has been seen before for dietary sphingolipids (20), but also in mouse xenografts with two prostate cancer cell lines—thereby extending the types of cancer that might be affected by this category of compounds; and, that orally administered Enigmol was able to affect the PC3 cell xenografts. These properties, plus the apparently low host toxicity of Enigmol when fed to animals at levels that decrease tumor growth, suggest that this compound has promise for cancer control.
The rationale behind the selection of this type of analog was that sphingoid bases lacking the 1-hydroxy group cannot be metabolized by sphingosine kinase(s) and the 2S,3S stereochemistry (compared to 2S,3R for sphingosine) also makes Enigmol a poorer substrate for N-acylation (24, 37); therefore, Enigmol would be predicted to be more persistent as the free sphingoid base, which was borne out by the cell culture studies with HT29 cells and the appearance of free Enigmol in tumors in the mouse xenograft studies. A longer persistence of the free sphingoid base might also contribute to the cytotoxicity of the synthetic analogs safingol (38), N,N-dimethylsphingosine (39) and naturally occurring sphingoid base variants (40, 41), including one recently evaluated in a Phase I trial (42).
The inability of Enigmol to undergo phosphorylation appears to be especially important because sphingosine kinase and S1P clearly play important roles in colon tumorigenesis (43, 44). Nonetheless, it does not appear that Enigmol acts via elimination of endogenous S1P (although it can inhibit sphingosine kinases in vitro) based on no measurable reduction in S1P in the cells (Fig. 2). This warrants further study, however, because the pertinent S1P might be localized in a specific subcompartment, secreted from the cells, and/or involved in receptor cross-talk (45) that would not have been detected in our analysis. It was striking that So addition to HT29 cells elevated S1P by >100-fold, which might be a major factor in its lower toxicity since S1P can sometimes protect cells against apoptosis. So addition also elevated Cer by over 10-fold and SM and glycosphingolipids by smaller but detectable amounts (Supplemental Fig. 2 and 3), which illustrates the complexity of studies of naturally occurring sphingolipids since the added compound undergoes extensive metabolism to multiple bioactive species.
The mechanism(s) for the tumor suppression by Enigmol have been only partially defined by these experiments; nonetheless, Enigmol is able to “normalize” one of the defects that is important in colon cancer--the aberrant appearance of β-catenin in the nucleus (20, 21) where it plays a major role in regulating proliferation via the TCF/Lef transcription factor (46). Furthermore, these studies have established that Enigmol and, to a lesser extent So, increase proteasomal turnover of β-catenin, apparently by increasing its phosphorylation by GSK-3β (and possibly CKI-α, although this was not tested), which precedes the polyubiquitination and degradation of β-catenin. The immediate target of Enigmol is not known, but might include protein kinase C, which is elevated in colon tumors (47) and is known to be inhibited by sphingoid bases (36); however, other protein kinases, such as PKB/Akt (48–50), might also play a roll because they are also known to be affected by sphingoid bases. It is noteworthy that interference with these mitogenic signaling pathways might also provide a link between Enigmol and cell death, since Akt and other signaling pathways influence caspase activation and apoptotic cell death.
All in all, these studies suggest that Enigmol represents a novel category of sphingoid base analog that is orally bioavailable and has the potential to be effective against multiple types of cancer.
Supplementary Material
Acknowledgments
Grant Support: NIH grant U19-CA87525 (all authors) and funds from the Smithgall Institute Chair in Molecular and Cell Biology at Georgia Tech (A. Merrill).
We thank David Menaldino, Kena Desai, Trey Perkins and Carrie Pack for conducting some of the initial experiments of this study, and Selwyn Hurwitz, David Pallas and Frank McDonald for many useful discussions.
Abbreviations
- ACF
aberrant crypt foci
- C2-Cer
N-acetylsphingosine
- C8-Cer
N-octanoylsphingosine
- Cer
ceramide
- DMH
dimethylhydrazine
- DMS
dimethylsphingosine
- Enigmol
(2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane
- Min
Multiple intestinal neoplasia
- S1P
sphingosine 1-phosphate
- Sa
sphinganine
- So
sphingosine
Footnotes
Potential conflicts of interest: None
References
- 1.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–40. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–95. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, et al. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006;5:1520–9. doi: 10.1158/1535-7163.MCT-05-0513. [DOI] [PubMed] [Google Scholar]
- 4.Heakal Y, Kester M. Nanoliposomal short-chain ceramide inhibits agonist-dependent translocation of neurotensin receptor 1 to structured membrane microdomains in breast cancer cells. Mol Cancer Res. 2009;7:724–34. doi: 10.1158/1541-7786.MCR-08-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 6.Borek C, Ong A, Stevens VL, Wang E, Merrill AH., Jr Long-chain (sphingoid) bases inhibit multistage carcinogenesis in mouse C3H/10T1/2 cells treated with radiation and phorbol 12-myristate 13-acetate. Proc Natl Acad Sci U S A. 1991;88:1953–7. doi: 10.1073/pnas.88.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borek C, Merrill AH., Jr Sphingolipids inhibit multistage carcinogenesis and protein kinase C. Basic Life Sci. 1993;61:367–71. doi: 10.1007/978-1-4615-2984-2_34. [DOI] [PubMed] [Google Scholar]
- 8.Stevens VL, Owens NE, Winton EF, Kinkade JM, Jr, Merrill AH., Jr Modulation of retinoic acid-induced differentiation of human leukemia (HL-60) cells by serum factors and sphinganine. Cancer Res. 1990;50:222–6. [PubMed] [Google Scholar]
- 9.Jarvis WD, Fornari FA, Traylor RS, Martin HA, Kramer LB, Erukulla RK, et al. Induction of apoptosis and potentiation of ceramide-mediated cytotoxicity by sphingoid bases in human myeloid leukemia cells. J Biol Chem. 1996;271:8275–84. doi: 10.1074/jbc.271.14.8275. [DOI] [PubMed] [Google Scholar]
- 10.Auzenne E, Leroux ME, Hu M, Pollock RE, Feig B, Klostergaard J. Cytotoxic effects of sphingolipids as single or multi-modality agents on human melanoma and soft tissue sarcoma in vitro. Melanoma Res. 1998;8:227–39. doi: 10.1097/00008390-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Schmelz EM, Dombrink-Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T, Merrill AH., Jr Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol Appl Pharmacol. 1998;148:252–60. doi: 10.1006/taap.1997.8356. [DOI] [PubMed] [Google Scholar]
- 12.Desai K, Sullards MC, Allegood J, Wang E, Schmelz EM, Hartl M, et al. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim Biophys Acta. 2002;1585:188–92. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, et al. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66:358–66. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–84. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, et al. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–93. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- 16.Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill AH., Jr Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr. 1994;124:702–12. doi: 10.1093/jn/124.5.702. [DOI] [PubMed] [Google Scholar]
- 17.Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res. 2009;48:62–72. doi: 10.1016/j.plipres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH., Jr Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56:4936–41. [PubMed] [Google Scholar]
- 19.Symolon H, Schmelz EM, Dillehay DL, Merrill AH., Jr Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutr. 2004;134:1157–61. doi: 10.1093/jn/134.5.1157. [DOI] [PubMed] [Google Scholar]
- 20.Schmelz EM, Roberts PC, Kustin EM, Lemonnier LA, Sullards MC, Dillehay DL, et al. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001;61:6723–9. [PubMed] [Google Scholar]
- 21.Simon KW, Roberts PC, Vespremi MJ, Manchen S, Schmelz EM. Regulation of beta-catenin and connexin-43 expression: targets for sphingolipids in colon cancer prevention. Mol Nutr Food Res. 2009;53:332–40. doi: 10.1002/mnfr.200800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergelin N, Blom T, Heikkila J, Lof C, Alam C, Balthasar S, et al. Sphingosine kinase as an oncogene: autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase C-alpha and ERK1/2. Endocrinology. 2009;150:2055–63. doi: 10.1210/en.2008-0625. [DOI] [PubMed] [Google Scholar]
- 23.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humpf HU, Schmelz EM, Meredith FI, Vesper H, Vales TR, Wang E, et al. Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase. Formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. J Biol Chem. 1998;273:19060–4. doi: 10.1074/jbc.273.30.19060. [DOI] [PubMed] [Google Scholar]
- 25.Bushnev AS, Baillie MT, Holt JJ, Menaldino DS, Merrill AH, Jr, Liotta DC. An efficient asymmetric synthesis of Enigmols (1-deoxy-5-hydroxysphingoid bases), an important class of bioactive lipid modulators. ARKIVOC. 2010;2010(viii):263–77. [Google Scholar]
- 26.Hannun YA, Merrill AH, Jr, Bell RM. Use of sphingosine as inhibitor of protein kinase C. Methods Enzymol. 1991;201:316–28. doi: 10.1016/0076-6879(91)01028-z. [DOI] [PubMed] [Google Scholar]
- 27.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–24. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Dillehay DL, Webb SK, Schmelz EM, Merrill AH., Jr Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 mice. J Nutr. 1994;124:615–20. doi: 10.1093/jn/124.5.615. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–23. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 30.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (Longevity Assurance Genes) become CerS (Ceramide Synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–5. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 31.Morales PR, Dillehay DL, Moody SJ, Pallas DC, Pruett S, Allgood JC, et al. Safingol toxicology after oral administration to TRAMP mice: demonstration of safingol uptake and metabolism by N-acylation and N-methylation. Drug Chem Toxicol. 2007;30:197–216. doi: 10.1080/01480540701375018. [DOI] [PubMed] [Google Scholar]
- 32.Sullards MC, Merrill AH., Jr Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci STKE. 2001;2001(67):PL1. doi: 10.1126/stke.2001.67.pl1. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Zhang W, Evans PM, Chen X, He X, Liu C. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 2006;281:17751–7. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 34.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–8. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1–8. doi: 10.1016/j.ctrv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Smith ER, Merrill AH, Obeid LM, Hannun YA. Effects of sphingosine and other sphingolipids on protein kinase C. Methods Enzymol. 2000;312:361–73. doi: 10.1016/s0076-6879(00)12921-0. [DOI] [PubMed] [Google Scholar]
- 37.Seiferlein M, Humpf HU, Voss KA, Sullards MC, Allegood JC, Wang E, et al. Hydrolyzed fumonisins HFB1 and HFB2 are acylated in vitro and in vivo by ceramide synthase to form cytotoxic N-acyl-metabolites. Mol Nutr Food Res. 2007;51:1120–30. doi: 10.1002/mnfr.200700118. [DOI] [PubMed] [Google Scholar]
- 38.Ling LU, Lin H, Tan KB, Chiu GN. The role of protein kinase C in the synergistic interaction of safingol and irinotecan in colon cancer cells. Int J Oncol. 2009 Dec;35(6):1463–71. doi: 10.3892/ijo_00000465. [DOI] [PubMed] [Google Scholar]
- 39.Kim HL, Im DSN. N-dimethyl-D-erythro-sphingosine increases intracellular Ca2+ concentration via Na+-Ca2+-exchanger in HCT116 human colon cancer cells. Arch Pharm Res. 2008;31:54–9. doi: 10.1007/s12272-008-1120-y. [DOI] [PubMed] [Google Scholar]
- 40.Padron JM, Peters GJ. Cytotoxicity of sphingoid marine compound analogs in mono- and multilayered solid tumor cell cultures. Invest New Drugs. 2006;24:195–202. doi: 10.1007/s10637-005-3691-5. [DOI] [PubMed] [Google Scholar]
- 41.Sugawara T, Zaima N, Yamamoto A, Sakai S, Noguchi R, Hirata T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci Biotechnol Biochem. 2006;70:2906–12. doi: 10.1271/bbb.60318. [DOI] [PubMed] [Google Scholar]
- 42.Baird RD, Kitzen J, Clarke PA, Planting A, Reade S, Reid A, et al. Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Mol Cancer Ther. 2009;8:1430–7. doi: 10.1158/1535-7163.MCT-08-1167. [DOI] [PubMed] [Google Scholar]
- 43.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–7. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 44.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–14. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, et al. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–77. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 47.Davidson LA, Jiang YH, Derr JN, Aukema HM, Lupton JR, Chapkin RS. Protein kinase C isoforms in human and rat colonic mucosa. Arch Biochem Biophys. 1994;312:547–53. doi: 10.1006/abbi.1994.1344. [DOI] [PubMed] [Google Scholar]
- 48.Ahn EH, Schroeder JJ. Sphinganine causes early activation of JNK and p38 MAPK and inhibition of AKT activation in HT-29 human colon cancer cells. Anticancer Res. 2006;26:121–7. [PubMed] [Google Scholar]
- 49.King CC, Zenke FT, Dawson PE, Dutil EM, Newton AC, Hemmings BA, et al. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J Biol Chem. 2000;275:18108–13. doi: 10.1074/jbc.M909663199. [DOI] [PubMed] [Google Scholar]
- 50.Fyrst H, Oskouian B, Bandhuvula P, Gong Y, Byun HS, Bittman R, et al. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res. 2009;69:9457–64. doi: 10.1158/0008-5472.CAN-09-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.