Abstract
Using combined endpoints to define no evident disease activity (NEDA) is becoming increasingly common when setting targets for treatment outcomes in multiple sclerosis (MS). Historically, NEDA has taken account of the occurrence of relapses, brain magnetic resonance imaging (MRI) lesions and disability worsening, but this approach places emphasis on inflammatory activity in the brain and mostly overlooks ongoing neurodegenerative damage. Combined assessments of NEDA which take account of changes in brain volume or neuropsychological outcomes such as cognitive function may begin to address this imbalance, and such assessments may also consider blood or spinal-fluid neurofilament levels or patient-reported outcomes and quality of life measures. If a combined NEDA assessment can be validated in prospective studies as indicative of long-term disease remission at the individual patient level, treating to achieve NEDA could become the goal of clinical practice and achieving NEDA may become the “new normal” state of disease control for patients with MS.
Keywords: Brain volume loss, cognition, combined assessments, no evident disease activity, NEDA-4, treatment algorithms
Introduction
The increase in disease-modifying therapies (DMTs) that are effective at different stages of multiple sclerosis (MS) has broadened treatment options for patients and provided new disease management challenges for clinicians. Since the approval of interferons in the 1990s, several DMTs have been introduced into clinical practice, each with a different mode of action and with characteristic effects on clinical or radiological disease markers.1,2 These additional therapeutic options have been accompanied by the evolution of treatment goals in MS, and some aspects of MS management now follow a more comprehensive approach to improving long-term outcomes, an approach already well established in the management of chronic immune-mediated inflammatory diseases (e.g. treat-to-target strategies in rheumatoid arthritis).1,3
Clinical trials typically report MRI activity as well as changes in relapse rates and disability worsening in MS; however, routine practice has relied heavily on the latter two outcomes (often termed clinical disease activity), which provide only a partial assessment of treatment effects.4 Combined assessments that encompass all three of the above outcomes were developed to examine treatment effects more comprehensively, and patients with no activity in these three domains have been categorized as “disease activity free” (DAF) or, more recently, as achieving “no evident disease activity” (NEDA) or NEDA-3.5–7 However, such three-domain combined assessments may provide an incomplete picture of disease activity. Observations such as cognitive deterioration among patients achieving three-domain NEDA provide some direct evidence for this,8 and in addition to the difficulty of sustaining three-domain NEDA status in the long-term9,10 also suggest that there may be underlying disease progression not captured by three-domain NEDA.
Collectively, relapses, MRI-lesion activity, and disability worsening provide useful information about inflammatory activity in the brain but may not adequately account for neurodegenerative disease progression.1 Although neurodegenerative damage may be captured in part by assessment of disability worsening, measurement of disability status is typically based on changes in Expanded Disability Status Scale (EDSS) score, which incompletely reflects other aspects of disease progression such as cognitive decline and fatigue. One proposal to address these limitations is the measurement of pathological brain volume loss (BVL) in addition to the three-domain assessment; this four-domain combined assessment has been termed NEDA-4.7 Another four-domain assessment, the multiple sclerosis decision model (MSDM), has also been proposed, which assesses neuropsychological outcomes (fatigue, depression, and quality of life) in addition to relapses, disability worsening, and MRI-lesion activity. It also deploys a rating system within each domain to help physicians assess the need for treatment review.4 Measures such as levels of neurofilament light chain might also be considered, as these reflect axonal injury, correlate with MS disease activity, and can now be quantified in blood.11,12 Here, we summarize developments in the use of combined assessments of disease activity in MS and explore their prognostic value. We also examine how combined assessments of disease activity might be used in treatment algorithms to signpost treatment review.
Combined assessment as a treatment goal
The aspirational goal of treating to NEDA in MS has been compared with achieving disease remission with biological DMTs in patients with rheumatoid arthritis.1 As medical practice advanced in rheumatology, long-term remission from disease started to become the “new normal,”13 and combinations of clinical and laboratory assessments became accepted as measures of treatment success.3 Notably, the inflammatory basis of rheumatoid arthritis was relatively well understood, so using potent anti-inflammatory drugs in a “treat-to-target” strategy, guided by composite clinical measures that defined disease remission, made conceptual sense.3 In contrast, uncertainty about the inflammatory and neurodegenerative disease processes in MS complicates the selection of appropriate clinical measures for inclusion in an equivalent definition.1
Combined assessments based on relapses, radiological outcomes, and disability worsening
Since the first clinical study that used combined assessments in MS,14 the definitions of DAF and NEDA (and of their component measures) used in clinical trials have varied.15–20 Generally, assessments of relapse rate, gadolinium-enhancing (Gd+) and new or enlarged T2 lesions, and confirmed disability worsening (CDW (previously termed confirmed disability progression (CDP))) at either 3 or 6 months have been combined to define disease activity (Table 1), and these measures have been assessed over the duration of a trial (typically 1 or 2 years).14–20 The use of such combined assessments has also been reported for patients treated with natalizumab in real-world observational studies such as BIONAT and NALTET.21,22
Table 1.
Combined assessments and their component definitions, used to define disease activity status in clinical trials.
| Study details | Relapse activity | MRI-lesion activity | Confirmed disability worsening | Disease-activity descriptor |
|---|---|---|---|---|
| AFFIRM study of natalizumab versus placebo14 | New or recurrent neurological symptoms not associated with fever or infection lasting for ⩾24 hours and accompanied by new neurological signs | Gadolinium-enhancing lesions; new or enlarging T2-hyperintense lesions |
1.5-point increase if EDSS = 0 at baseline, or 1.0-point increase if EDSS ⩾ 1.0 at baseline, confirmed at 3 months | Absence of disease activity |
| CLARITY study of cladribine versus placebo15 | An increase of two points in ⩾1 Kurtzke Functional System (KFS) or an increase of one point in ⩾2 KFS (except changes in bowel or bladder function, or cognition), in the absence of fever, lasting for ⩾24 hours, preceded by ⩾30 days of clinical stability or improvement | New T1 gadolinium-enhancing lesions and active T2 lesions on cranial MRI | ⩾1.5-point increase if EDSS = 0 at baseline, or ⩾1.0-point increase if EDSS = 0.5–4.5 at baseline, or ⩾0.5-point increase if EDSS ⩾ 5.0 at baseline, confirmed at 3 months | Freedom from disease activity |
| Escalation to natalizumab from interferon-beta or glatiramer acetate16 | ⩾1 new symptom, or worsening of pre-existing symptoms related to MS, accompanied by objective deterioration on neurological examination lasting for ⩾24 hours, in the absence of fever and preceded by neurological stability for ⩾30 days | Contrast-enhanced lesions or the appearance of new T2-hyperintense lesions, compared with the previous scan | ⩾1.0-point increase if EDSS < 5.0 at baseline, or 0.5-point increase if EDSS ⩾ 5.5 at baseline, confirmed at 6 months | Free from disease activity |
| Cross comparison of interferon, glatiramer acetate or both in combination17 | Symptoms attributable to MS, preceded by 30 days of stability, including ⩾0.5-point increase in EDSS score over prior visit or ⩾2-point increase in one functional system (FS) or a ⩾1-point increase in two FSs, except bladder/cognitive changes | Combined unique lesion activity, defined as the sum of the number of new enhanced lesions and the number of new unenhanced T2 and substantially enlarged unenhanced T2 lesions | 1.0-point increase if EDSS ⩽ 5.0 at baseline, or 0.5-point increase if EDSS ⩾ 5.5 at baseline, confirmed at 6 months | Disease activity free status (DAFS) |
| Indirect comparison of dimethyl fumarate, fingolimod, and teriflunomide18 | Not reported | Gadolinium-enhancing T1 lesions and new or newly enlarged T2 lesions | One-point increase in EDSS for fingolimod and teriflunomide, same for dimethyl fumarate, with a 1.5-point increase if EDSS = 0, confirmed at 3 months | No evident disease activity (NEDA) |
| SELECT study of daclizumab-HYP versus placebo19 | Relapses confirmed by Relapse Adjudication Committee | New or newly enlarging T2-hyperintense lesions and new Gd+ lesions | One-point increase in EDSS or 1.5-point increase if EDSS = 0, confirmed at 3 months | Disease activity free |
| ADVANCE study of peginterferon-beta-1a versus placebo (1-year interim)20 | Not reported | Gd+ lesions and new or newly enlarging T2-hyperintense lesions | 1.5-point increase if EDSS = 0 at baseline, 1.0-point if EDSS ⩾ 1.0 at baseline, confirmed at 3 months | NEDA |
AFFIRM: natalizumab safety and efficacy in relapsing–remitting multiple sclerosis; CLARITY: cladribine tablets treating multiple sclerosis orally; SELECT, safety and efficacy study of daclizumab high yield process (DAC HYP) to treat relapsing-remitting multiple sclerosis; ADVANCE: efficacy and safety study of peginterferon beta-1a in participants with relapsing multiple sclerosis; MRI: magnetic resonance imaging; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; HYP: high-yield process.
Selection and definition of component disease-activity assessments
Currently, there is no consensus regarding the definitions of the different components that constitute three-domain NEDA. Relapse definitions are broadly consistent among the trials summarized in Table 1, but can include, for example, a confirmed change in EDSS score. When assessing MRI-lesion activity, both new Gd+ and new or enlarged T2 lesions are usually considered, but a recent study found that assessment of both lesion types may be unnecessary. Hierarchical analyses of component NEDA measures within a large population of patients receiving fingolimod or placebo pooled from the two FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) trials indicated that T2 lesion changes almost invariably coincide with Gd+ lesion activity.7 In terms of disability assessment, differences exist between studies both in the change in EDSS score and in the observation period used to confirm worsening disability; 6-month CDW based on EDSS score is regarded as more indicative of a permanent change in disability status than is 3-month CDW,23 and 6-month CDW status at 2 years has been shown to be a good predictor of long-term disability status.24 However, use of the Multiple Sclerosis Functional Composite (MSFC) instead of EDSS score in combined assessments has been proposed, owing to the comparatively poor sensitivity of EDSS early in the disease course and because (unlike the EDSS) the MSFC assesses changes in both physical and cognitive functions.4
Sustainability of three-domain NEDA
Studies that have examined whether three-domain NEDA status is sustained once achieved tend to suggest that outcomes differ among DMTs. Long-term follow-up of a cohort of patients receiving natalizumab found that 34% (52/152) were classified as having NEDA-3 status at 7 years.25 In contrast, studies relating mainly to DMTs of lower efficacy than natalizumab have observed that few patients sustain three-domain NEDA status, even if a relatively large proportion of patients are initially categorized as such. Among 219 patients in the Comprehensive Longitudinal Investigation of Multiple Sclerosis at Brigham and Women’s Hospital (CLIMB) study, the proportions of patients attaining NEDA were 46% at 1 year, 27.5% at 2 years, but only 7.9% at 7 years (on average, patients received a DMT for 75% of the 7-year follow-up, and most received a first-line injectable DMT).9 In a preliminary analysis of the Study of Ocrelizumab in Comparison with Interferon Beta-1a [Rebif] in Participants with Relapsing Multiple Sclerosis (OPERA) trials of ocrelizumab, a similar proportion (25%–29%) of patients receiving subcutaneous interferon-beta-1a attained three-domain NEDA status at 2 years compared with 48% of patients receiving ocrelizumab.26 Loss of NEDA-3 status over time was also seen in a study of patients receiving intramuscular interferon-beta-1a in the Avonex-Steroids-Azathioprine (ASA) trial;10 among 162 patients with relapsing-remitting multiple sclerosis (RRMS), 33 (20.4%) attained NEDA-3 status at 1 year, 12 (7.6%) at 2 years, 5 (3.3%) at 4 years, but none beyond 6 years.10 Although differences in study design and in three-domain NEDA definitions hinder comparison of these study outcomes, the failure of lower efficacy DMTs to sustain three-domain NEDA when higher efficacy DMTs can, could be attributable to elements of disease progression targeted by higher efficacy DMTs but not captured by three-domain NEDA. It is also possible in the context of a treat-to-target strategy (in which treatment is switched or escalated) that long-term three-domain NEDA rates might have been higher than were seen in CLIMB, which analyzed routine clinical practice in an era when treat-to-target was not necessarily practiced owing to the lack of higher efficacy drugs.
The prognostic value of three-domain NEDA
The prognostic value of assessments based on relapses, disability worsening, and MRI findings has been investigated in real-world studies.9,27–29 Patients receiving interferon-beta who experienced MRI-lesion activity and clinical disease activity during the first year of treatment were at significantly increased risk of further relapses and/or sustained disability worsening in the subsequent 2 years of treatment.27 Based on this analysis, the “modified Rio” scoring system was validated to identify likely non-responders among patients receiving interferon-beta.30 Subsequent evaluation of the original Rio scoring system in patients receiving glatiramer acetate revealed a similar prognostic pattern to that seen with interferon-beta. However, patients treated with interferon-beta with isolated MRI-lesion activity in year 1 were at risk of subsequent disease progression, unlike those with MRI-lesion activity receiving glatiramer acetate. This difference may be attributable to the different mechanisms and speed with which interferon-beta and glatiramer acetate act.27,28 In CLIMB, the ability of NEDA status at different times to predict NEDA status at 7 years was also examined. As expected, the predictive power of NEDA increased with the observation period (by definition, 100% at 7 years) but reached almost 80% at 2 years, suggesting that this observation period may be optimal, and that waiting for more than 2 years to assess NEDA status is of little additional prognostic value.9 These studies highlight the fact that if combined measures such as NEDA are to be used to inform treatment decisions in routine clinical practice, it is critical to understand whether, and how, this relationship between observation period and prognostic power varies among different DMTs.
Aspects of disease progression overlooked by three-domain NEDA
Potential limitations to using MRI-lesion and clinical disease activity as the basis for NEDA have been revealed in a small study (n = 42) of patients with RRMS, in which 7 of the 12 individuals who sustained NEDA status at 2 years showed worsening in at least two cognitive domains.8 Combined assessments based only on relapse rate, disability worsening, and focal MRI activity track inflammatory activity in the central nervous system (CNS), but their ability to track other disease pathologies, including diffuse CNS damage and neurodegeneration, is perhaps more marginal. As these latter pathophysiological mechanisms contribute to BVL, which occurs in the earliest stages of MS and can be prognostic of disability worsening and cognitive decline,31–35 adding BVL measurement to three-domain NEDA seems a logical refinement that should yield a combined assessment that better reflects the underlying pathology of MS than three-domain NEDA.
Four-domain assessments: NEDA-4
Patients with MS experience a higher rate of BVL than do healthy individuals, and studies have shown that BVL in MS both correlates with inflammatory brain lesion activity and predicts long-term disability status.33–38 A meta-analysis of clinical trial outcomes (based on trials of interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, fingolimod, natalizumab, alemtuzumab, and cladribine) demonstrated that the effect of treatment on BVL over 2 years correlated with the effect of treatment on disability worsening.39 Taking together the observations that BVL predicts future disability status and that treatment to reduce BVL mitigates disability worsening suggests that identifying pathological changes in BVL should highlight the need for earlier treatment switch or escalation before future, and possibly permanent, changes in disability manifest.40 Key to this is the adoption of routine BV measurement and the determination of a rate of BVL that discriminates between normal and pathological atrophy.
A recent study determined that an annual threshold BVL rate of 0.4% achieves this discrimination with good specificity and sensitivity, and that significant worsening of disability is associated with annual BVL rates ⩾0.4%.40 Furthermore, reduction of BVL below this threshold is attainable: approximately 37% of patients from the pooled FREEDOMS trials had an annualized BVL rate below 0.4% at 2 years,7 and 5-year follow-up of the Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis (CARE MS) trials showed that median annual BVL rates associated with alemtuzumab were consistently below the 0.4% threshold from year 2 onward.41 However, the effect of alemtuzumab in reducing BVL seems to be delayed, as the median rate of BVL in year 1 of the CARE MS analysis was nearly 0.6%, with delayed, but subsequent fall in BVL below 0.3% from year 2 onward. In comparison, fingolimod’s effect in reducing BVL has been shown to occur earlier, within 6 months, which emphasizes the importance of determining for each specific DMT when assessment of disease activity status is of greatest prognostic value. Regarding the adoption of routine BV measurement, there is no doubt that determination of longitudinal changes in brain volume presents certain obstacles, such as accounting for pseudoatrophy, day-to-day physiological changes, and standardizing MRI acquisition and analysis parameters,42,43 and these obstacles will need to be overcome. Notably, blood levels of neurofilament light chain have recently been shown to correlate significantly with MRI-lesion activity, BVL rate, and relapse rates11 at a group level, so with appropriate validation these may offer an alternative to, or even supersede some components of a combined assessment in individual subjects.
The threshold annual BVL rate of 0.4% was incorporated in NEDA-4 (patients were dichotomized based on an annual rate <0.4% or ⩾0.4%) in a post hoc analysis of the two FREEDOMS trials and their extensions. The predictive values at 1 year of NEDA-3 and NEDA-4 status were compared at up to 6 years, and preliminary results showed that dichotomization based on NEDA-4 status at 1 year predicted long-term disability-related outcomes better than dichotomization based on NEDA-3 status.44 In terms of sustaining NEDA-4 status, the ASA study of patients with RRMS receiving interferon-beta also examined NEDA-4 status based on a threshold annual BVL rate of 0.4%.10 Only six patients (4.3%) attained NEDA-4 status at 2 years, only one (1.0%) at 4 years, and none thereafter.10 Post hoc analysis of the FREEDOMS trials found that 19.7% (139/706) of patients on fingolimod and 5.3% (38/721) of patients on placebo sustained NEDA-4 status over 2 years.7 A 7-year follow-up analysis of the FREEDOMS trials, in patients receiving fingolimod 0.5 mg or placebo who either continued receiving or switched to fingolimod 0.5 mg on entering the trial extensions at 2 years determined the proportion of patients achieving NEDA status in each year of the study.45 During year 7, approximately 45% of the 246 patients still on study had NEDA-4 status, and about 70% had NEDA-3 status.45 Notwithstanding differences between the two analyses, the greater propensity for pseudoatrophy in year 1 on interferon-beta, and potential drop-out of non-responders, these proportions appear greater than were seen in the ASA trial.10
Four-domain assessments: MSDM
The domains included in the MSDM are relapse activity, disability progression, MRI-lesion activity, and neuropsychological outcomes (fatigue, depression, anxiety, and quality of life). Instead of using the EDSS to track changes in disability, the MSDM adopts a modified version of the MSFC, which monitors changes in both cognitive and physical functioning. Cognitive changes can be detected in the earliest stages of MS,46 often before other disease-related changes are apparent,47 and cognitive impairment at diagnosis can also predict disability worsening.48 The modified MSFC proposed in the MSDM uses the Symbol Digit Modalities Test (SDMT) to follow cognitive changes instead of the Paced Auditory Serial Addition Test (PASAT),4 which can be limited by practice effects (patients’ performances improve upon repeated exposure to the test).49 The SDMT is simpler to administer and has slightly better predictive validity than the PASAT50 and has also been shown to correlate better with BVL.51 Other measures included in the MSDM include the Fatigue Scale for Motor and Cognitive Functions, the Hospital Anxiety and Depression Scale, and the Multiple Sclerosis Impact Scale (MSIS)-29 for evaluation of quality of life. A further refinement of the MSDM is the use of a traffic-light rating system in each domain, in contrast to the dichotomous classification evidence/no evidence of disease activity used in three- and four-domain NEDA. Finally, when using the MSDM, the timing of follow-up and therapy review are dictated by the combination of traffic-light ratings in each of its four domains.4
Clinical utility of combined assessments in treatment algorithms
It appears that greater proportions of patients sustained long-term three-domain NEDA status with natalizumab25 and with fingolimod45 than did with interferon-beta.9,10 Variation in trial design and in NEDA definitions notwithstanding, if this apparent difference in the performances of high and low efficacy DMTs is attributable to shortcomings of three-domain NEDA in assessing disease progression, then basing therapy decisions purely on MRI-lesion and clinical disease activity may never afford patients a state of long-term disease remission. The modified Rio scoring system essentially uses the three-domain NEDA measures, but tolerates low levels of MRI-lesion activity (like the recently coined treatment goal of “minimal evidence of disease activity” (MEDA)) among “responders.” Modified-Rio “responders” to interferon-beta have a low risk of progression at 3 years,30 but it is questionable whether their disease progression has been halted. For example, only a small proportion of patients achieving the more stringent NEDA-3 status with interferon-beta in year 1 of the ASA trial sustained NEDA-3 status at 4 years.10 Subject to determination of the time at which assessment is prognostically optimal, NEDA-4 seems to improve on three-domain NEDA. However, if ever adopted as a treatment target in clinic, it may be more informative for therapy review to rate progression in each domain of NEDA-4 (as proposed for MSDM) than to rely on dichotomous classification.
Treating to NEDA
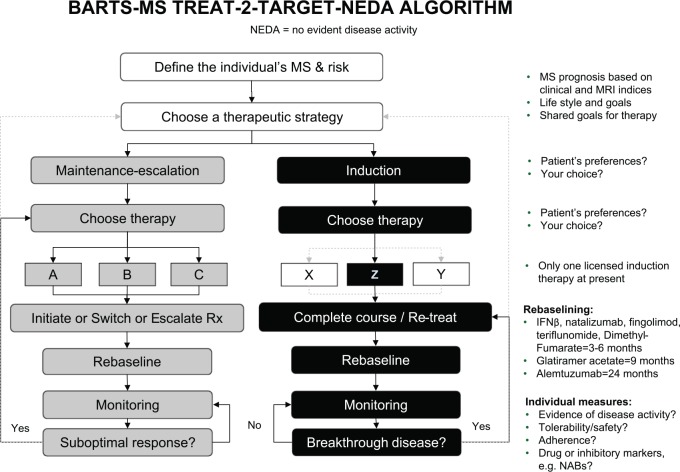
A possible treat-to-NEDA strategy is shown in Figure 1 (in this context NEDA is defined as the absence of relapses, MRI-lesion activity and disability worsening, but the authors acknowledge that this definition will evolve).2 To facilitate systematic monitoring of treatment effects, it is recommended that patients’ baseline disease activity is defined subsequent to initiation of a DMT, and that the timing of this re-baselining is tailored to the DMT administered. The differences between DMTs in the timing of treatment effects (as described above for alemtuzumab and fingolimod, and for interferon-beta and glatiramer acetate) illustrate why disease activity in this re-baselining period should not be regarded as treatment failure. Changes in treatment strategy, such as switching to higher efficacy DMTs, would be triggered when a predefined threshold in a particular NEDA component was reached.2 We anticipate that a quite a large proportion of subjects who are NEDA-3 will require switching to a higher efficacy DMT based on NEDA-4 status. It will be important to assess using real-life data if treating to a target of NEDA-4 improves long-term clinical outcomes compared to treating to a simpler target of NEDA-3.
Figure 1.
An example of a treat-to-target algorithm, based on NEDA, for the treatment of patients with active MS.
Source: Reproduced with permission from Giovannoni et al.2
MRI: magnetic resonance activity; MS: multiple sclerosis; NABs: neutralizing antibodies; NEDA: no evident disease activity; Rx: treatment; IFNβ: interferon-beta.
Conclusion and recommendations
Improvements in the efficacy of DMTs for MS offer clinicians the opportunity to adopt a “treat-to-target” approach, with the objective of halting disease progression. If achieved this would give patients a period of remission to adjust to a “new normal” state of disease control. Assessment based on MRI-lesion activity, relapses, and disability worsening is probably insufficient to realize this goal, and both the inclusion of other measures in combined assessments and their validation in prospective studies are warranted. How evidence of the utility of such combined assessments at the group level translates into routine clinical practice at the patient level will also need to be determined.
Acknowledgments
All authors critically reviewed the manuscript and approved the final version for submission. Medical writing services, provided by Oxford PharmaGenesis, were funded by Novartis Pharma AG.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.G. serves on the scientific advisory board for Abbvie, Biogen Idec, Canbex, Fiveprime, Genzyme, GW Pharma, Ironwood, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Synthon BV, Teva, and Vertex Pharmaceutical; has received speaker honoraria from Biogen Idec, Genzyme, GW Pharma, Merck-Serono, Novartis, Roche, and Teva; is an editor for Multiple Sclerosis and Related Disorders; has consulted for Abbvie, Biogen Idec, Canbex, Fiveprime, Genzyme, GW Pharma, Ironwood, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Synthon BV, Teva, and Vertex Pharmaceuticals; is on the speaker’s bureau for Novartis and Teva; and has received research support from Genzyme and Merck. D.T. is an employee of Novartis Pharma AG. J.R.B. is an employee of Oxford PharmaGenesis, which was funded by Novartis Pharma AG to provide medical writing support. E.H. serves on scientific advisory boards for Actelion, Biogen, Celgene, Genzyme, and Novartis; has received funding for travel or speaker honoraria from Actelion, Biogen, Celgene, Genzyme, Novartis, and Roche; serves as a consultant for Actelion, Biogen, Celgene, Genzyme, Novartis, and Roche; and receives research support from Biogen, Merck-Serono, the Czech Ministry of Education (PRVOUK P-26/LF1/4), and the European Commission.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Gavin Giovannoni, Centre for Neuroscience and Trauma, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK/Department of Neurology, Royal London Hospital, Barts Health NHS Trust, London, UK.
Davorka Tomic, Novartis Pharma AG, Basel, Switzerland.
Jeremy R Bright, Oxford PharmaGenesis, Oxford, UK.
Eva Havrdová, Department of Neurology and Center of Clinical Neuroscience, Charles University, Prague, Czech Republic/First Faculty of Medicine, Charles University, Prague, Czech Republic/General University Hospital, Prague, Czech Republic.
References
- 1. Bevan CJ, Cree BA. Disease activity free status: A new end point for a new era in multiple sclerosis clinical research? JAMA Neurol 2014; 71: 269–270. [DOI] [PubMed] [Google Scholar]
- 2. Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 2015; 4: 329–333. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014; 73: 492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of “no evidence of disease activity” in multiple sclerosis treatment: The multiple sclerosis decision model. Ther Adv Neurol Disord 2015; 8: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banwell B, Giovannoni G, Hawkes C, et al. Editors’ welcome and a working definition for a multiple sclerosis cure. Mult Scler Relat Disord 2013; 2: 65–67. [DOI] [PubMed] [Google Scholar]
- 6. Lublin FD. Disease activity free status in MS. Mult Scler Relat Disord 2012; 1: 6–7. [DOI] [PubMed] [Google Scholar]
- 7. Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016; 22(10): 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: Implications on cognition and brain atrophy. Mult Scler 2016; 22: 64–72. [DOI] [PubMed] [Google Scholar]
- 9. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 10. Uher T, Havrdova E, Sobisek L, et al. Is no evidence of disease activity an achievable goal in MS patients on intramuscular interferon beta-1a treatment over long-term follow-up? Mult Scler 2017; 23(2): 242–252. [DOI] [PubMed] [Google Scholar]
- 11. Kuhle J, Barro C, Brachat AH, et al. Blood neurofilament light chain levels are elevated in multiple sclerosis and correlate with disease activity. Mult Scler 2016; 22: 828. [Google Scholar]
- 12. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016; 54: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 13. Collins T. EULAR 2012: Remission the new normal for rheumatoid arthritis. The Rheumatologist, http://www.the-rheumatologist.org/article/eular-2012-remission-the-new-normal-for-rheumatoid-arthritis/ (2012, accessed 8 September 2016).
- 14. Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: A retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8: 254–260. [DOI] [PubMed] [Google Scholar]
- 15. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 16. Prosperini L, Gianni C, Leonardi L, et al. Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler 2012; 18: 64–71. [DOI] [PubMed] [Google Scholar]
- 17. Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol 2013; 73: 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nixon R, Bergvall N, Tomic D, et al. No evidence of disease activity: Indirect comparisons of oral therapies for the treatment of relapsing-remitting multiple sclerosis. Adv Ther 2014; 31: 1134–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Havrdova E, Giovannoni G, Stefoski D, et al. Disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with daclizumab high-yield process in the SELECT study. Mult Scler 2014; 20: 464–470. [DOI] [PubMed] [Google Scholar]
- 20. Arnold DL, Calabresi PA, Kieseier BC, et al. Effect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: Results from a randomized controlled trial in relapsing-remitting multiple sclerosis. BMC Neurol 2014; 14: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Outteryck O, Ongagna JC, Brochet B, et al. A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: Clinical, radiological and biological features and adverse events. The BIONAT cohort. Eur J Neurol 2014; 21: 40–48. [DOI] [PubMed] [Google Scholar]
- 22. Totaro R, Lugaresi A, Bellantonio P, et al. Natalizumab treatment in multiple sclerosis patients: A multicenter experience in clinical practice in Italy. Int J Immunopathol Pharmacol 2014; 27: 147–154. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. EMA/CHMP/771815/2011, Rev. 2, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf (2015, accessed 8 September 2016).
- 24. Rudick RA, Lee JC, Cutter GR, et al. Disability progression in a clinical trial of relapsing-remitting multiple sclerosis: Eight-year follow-up. Arch Neurol 2010; 67: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 25. Prosperini L, Fanelli F, Pozzilli C. Long-term assessment of no evidence of disease activity with natalizumab in relapsing multiple sclerosis. J Neurol Sci 2016; 364: 145–147. [DOI] [PubMed] [Google Scholar]
- 26. Traboulsee A, Arnold D, Bar-Or A, et al. Ocrelizumab no evidence of disease activity (NEDA) status at 96 weeks in patients with relapsing multiple sclerosis: Analysis of the phase III double-blind, double-dummy, interferon beta-1a-controlled OPERA I and OPERA II studies. Neurology 2016; 86(16 Suppl.): PL02.004. [Google Scholar]
- 27. Rio J, Castillo J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009; 15: 848–853. [DOI] [PubMed] [Google Scholar]
- 28. Rio J, Rovira A, Tintore M, et al. Evaluating the response to glatiramer acetate in relapsing-remitting multiple sclerosis (RRMS) patients. Mult Scler 2014; 20: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 29. Sormani MP, Rovaris M, Comi G, et al. A composite score to predict short-term disease activity in patients with relapsing-remitting MS. Neurology 2007; 69: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 30. Sormani MP, Rio J, Tintore M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 2013; 19: 605–612. [DOI] [PubMed] [Google Scholar]
- 31. De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147–156. [DOI] [PubMed] [Google Scholar]
- 32. Deloire MS, Ruet A, Hamel D, et al. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 2011; 76: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002; 59: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 34. Horakova D, Dwyer MG, Havrdova E, et al. Gray matter atrophy and disability progression in patients with early relapsing-remitting multiple sclerosis: A 5-year longitudinal study. J Neurol Sci 2009; 282: 112–119. [DOI] [PubMed] [Google Scholar]
- 35. Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84: 1082–1091. [DOI] [PubMed] [Google Scholar]
- 36. Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sastre-Garriga J, Tur C, Pareto D, et al. Brain atrophy in natalizumab-treated patients: A 3-year follow-up. Mult Scler 2015; 21: 749–756. [DOI] [PubMed] [Google Scholar]
- 38. Vollmer T, Signorovitch J, Huynh L, et al. The natural history of brain volume loss among patients with multiple sclerosis: A systematic literature review and meta-analysis. J Neurol Sci 2015; 357: 8–18. [DOI] [PubMed] [Google Scholar]
- 39. Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014; 75: 43–49. [DOI] [PubMed] [Google Scholar]
- 40. De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barkhof F, Cohen JA, Coles AJ, et al. Alemtuzumab slows brain volume loss over 5 years in patients with active relapsing-remitting multiple sclerosis with most patients not receiving treatment for 4 years: CARE MS I and II extension study. Mult Scler 2015; 21: 7–75. [Google Scholar]
- 42. Zivadinov R, Jakimovski D, Gandhi S, et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 2016; 16(7): 777–793. [DOI] [PubMed] [Google Scholar]
- 43. Azevedo CJ, Pelletier D. Whole-brain atrophy: Ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol 2016; 29: 237–242. [DOI] [PubMed] [Google Scholar]
- 44. Kappos L, De Stefano N, Chitnis T, et al. Predictive value of NEDA for disease outcomes over 6 years in patients with RRMS. Mult Scler 2015; 23(Suppl. 11): 33. [Google Scholar]
- 45. Cree BAC, Kappos L, Freedman MS, et al. Long-term effects of fingolimod on NEDA by year of treatment. Mult Scler 2015; 23(Suppl. 11): 302. [Google Scholar]
- 46. Amato MP, Portaccio E, Goretti B, et al. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci 2010; 31(Suppl. 2): S211–S214. [DOI] [PubMed] [Google Scholar]
- 47. Amato MP, Portaccio E, Goretti B, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: A 3-year follow-up study. Mult Scler 2010; 16: 1474–1482. [DOI] [PubMed] [Google Scholar]
- 48. Moccia M, Lanzillo R, Palladino R, et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult Scler 2016; 22: 659–667. [DOI] [PubMed] [Google Scholar]
- 49. Rogers JM, Fox AM. Event-related potential practice effects on the Paced Auditory Serial Addition Test (PASAT). Adv Cogn Psychol 2016; 8: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drake AS, Weinstock-Guttman B, Morrow SA, et al. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: Replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler 2010; 16: 228–237. [DOI] [PubMed] [Google Scholar]
- 51. Rao SM, Martin AL, Huelin R, et al. Correlations between MRI and information processing speed in MS: A meta-analysis. Mult Scler Int 2014; 2014: 975803. [DOI] [PMC free article] [PubMed] [Google Scholar]