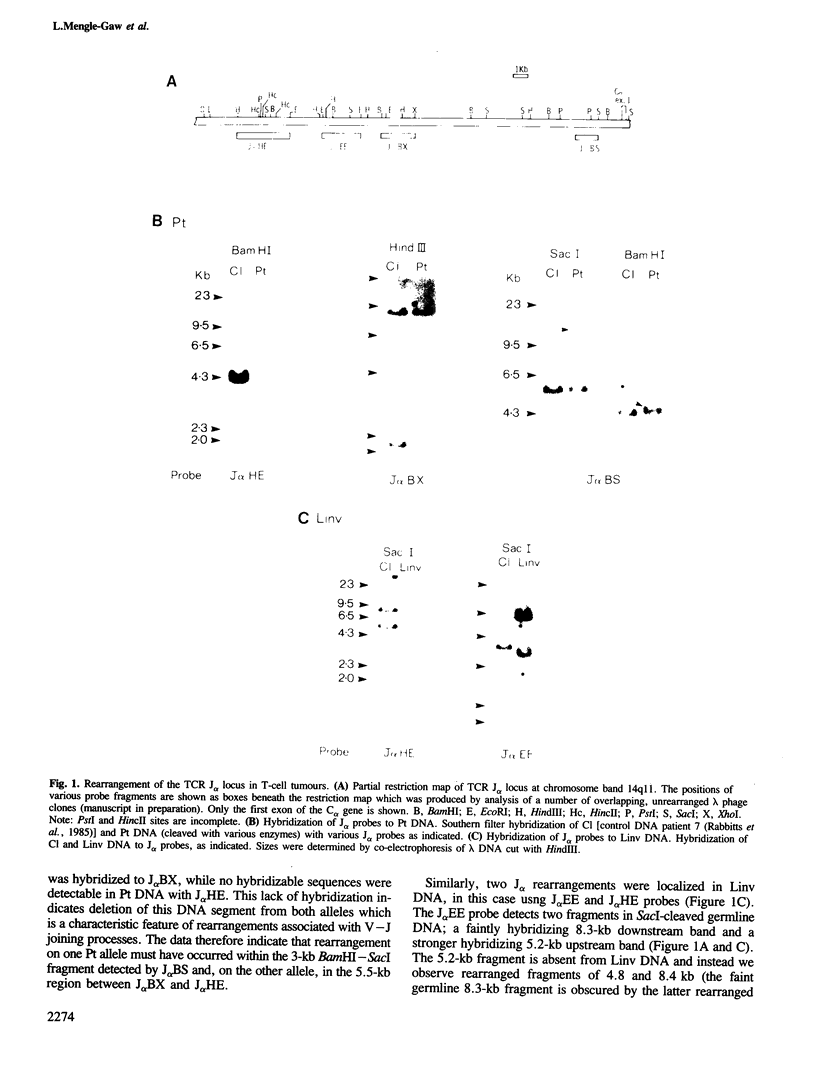
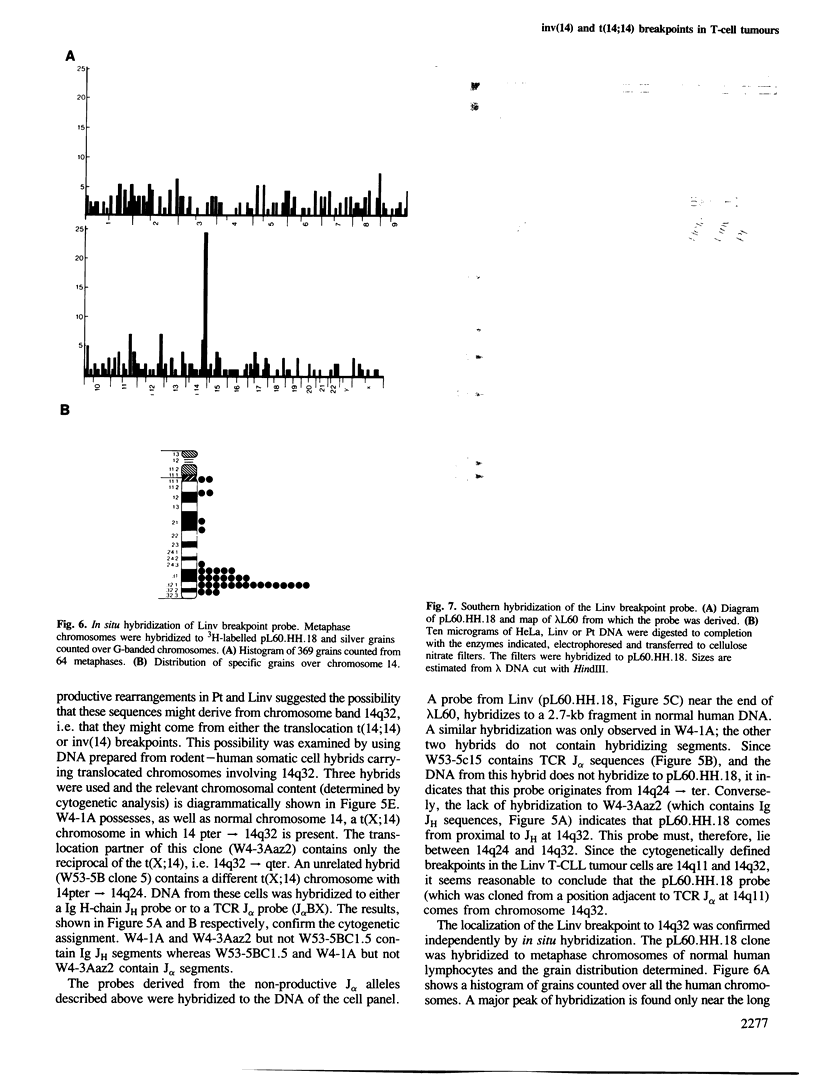
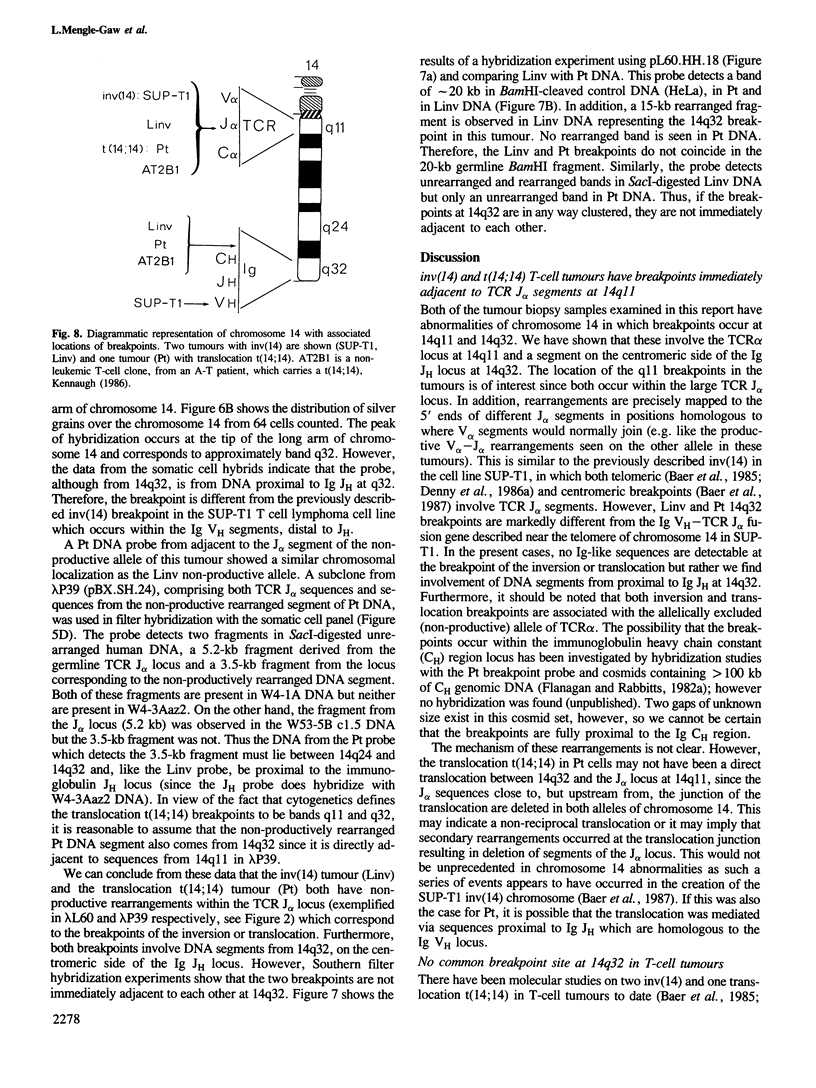
Abstract
T-cell tumours are frequently found to carry an inversion of chromosome 14 (inv(14)) (q11;q32) or more rarely a chromosome 14 translocation t(14;14) with the same cytogenetic breakpoints (q11;q32). We have examined the molecular junctions of an inv(14) and a translocation t(14;14) using T-cell receptor (TCR) alpha joining (J) region probes. Both of these chromosomal abnormalities have breakpoints within the TCR J alpha locus at 14q11 and both have breakpoints which are proximal (i.e. on the centromeric side) to the immunoglobulin heavy chain JH region at 14q32. The cloned segments corresponding to the junctions at 14q32 are not associated with obvious immunoglobulin-like sequences. This contrasts to the previously described inv(14) in the cell line SUP-T1 and places a potential cluster of chromosome 14 breakpoints downstream of the Ig JH locus. The possible role of the varying breakpoints in the development of these tumours is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurias A., Couturier J., Dutrillaux A. M., Dutrillaux B., Herpin F., Lamoliatte E., Lombard M., Muleris M., Paravatou M., Prieur M. Inversion (14)(q12qter) or (q11.2q32.3): the most frequently acquired rearrangement in lymphocytes. Hum Genet. 1985;71(1):19–21. doi: 10.1007/BF00295660. [DOI] [PubMed] [Google Scholar]
- Aurias A., Croquette M. F., Nuyts J. P., Griscelli C., Dutrillaux B. New data on clonal anomalies of chromosome 14 in ataxia telangiectasia: tct(14;14) and inv(14). Hum Genet. 1986 Jan;72(1):22–24. doi: 10.1007/BF00278811. [DOI] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Baer R., Lefranc M. P., Minowada J., Forster A., Stinson M. A., Rabbitts T. H. Organization of the T-cell receptor alpha-chain gene and rearrangement in human T-cell leukaemias. Mol Biol Med. 1986 Jun;3(3):265–277. [PubMed] [Google Scholar]
- Clare N., Boldt D., Messerschmidt G., Zeltzer P., Hansen K., Manhoff L. Lymphocyte malignancy and chromosome 14: structural aberrations involving band q11. Blood. 1986 Mar;67(3):704–709. [PubMed] [Google Scholar]
- Denny C. T., Hollis G. F., Hecht F., Morgan R., Link M. P., Smith S. D., Kirsch I. R. Common mechanism of chromosome inversion in B- and T-cell tumors: relevance to lymphoid development. Science. 1986 Oct 10;234(4773):197–200. doi: 10.1126/science.3092355. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Yoshikai Y., Mak T. W., Smith S. D., Hollis G. F., Kirsch I. R. A chromosome 14 inversion in a T-cell lymphoma is caused by site-specific recombination between immunoglobulin and T-cell receptor loci. Nature. 1986 Apr 10;320(6062):549–551. doi: 10.1038/320549a0. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. The sequence of a human immunoglobulin epsilon heavy chain constant region gene, and evidence for three non-allelic genes. EMBO J. 1982;1(5):655–660. doi: 10.1002/j.1460-2075.1982.tb01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M., Adams J. M. Chromosome 8 breakpoint far 3' of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986 Nov;5(11):2845–2851. doi: 10.1002/j.1460-2075.1986.tb04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Hecht F., Morgan R., Hecht B. K., Smith S. D. Common region on chromosome 14 in T-cell leukemia and lymphoma. Science. 1984 Dec 21;226(4681):1445–1447. doi: 10.1126/science.6438800. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- McCaw B. K., Hecht F., Harnden D. G., Teplitz R. L. Somatic rearrangement of chromosome 14 in human lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2071–2075. doi: 10.1073/pnas.72.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., Rabbitts T. H. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987 Jul;6(7):1959–1965. doi: 10.1002/j.1460-2075.1987.tb02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Baer R., Buluwela L., Mengle-Gaw L., Taylor A. M., Rabbitts P. H. Molecular genetics of antigen receptors and associated chromosomal abnormalities in human leukemias. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):923–930. doi: 10.1101/sqb.1986.051.01.105. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Stinson A., Forster A., Foroni L., Luzzatto L., Catovsky D., Hammarström L., Smith C. I., Jones D., Karpas A. Heterogeneity of T-cell beta-chain gene rearrangements in human leukaemias and lymphomas. EMBO J. 1985 Sep;4(9):2217–2224. doi: 10.1002/j.1460-2075.1985.tb03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sadamori N., Kusano M., Nishino K., Tagawa M., Yao E., Yamada Y., Amagasaki T., Kinoshita K., Ichimaru M. Abnormalities of chromosome 14 at band 14q11 in Japanese patients with adult T-cell leukemia. Cancer Genet Cytogenet. 1985 Jul;17(3):279–282. doi: 10.1016/0165-4608(85)90019-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Butterworth S. V. Clonal evolution of T-cell chronic lymphocytic leukaemia in a patient with ataxia telangiectasia. Int J Cancer. 1986 Apr 15;37(4):511–516. doi: 10.1002/ijc.2910370407. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Oxford J. M., Metcalfe J. A. Spontaneous cytogenetic abnormalities in lymphocytes from thirteen patients with ataxia telangiectasia. Int J Cancer. 1981 Mar 15;27(3):311–319. doi: 10.1002/ijc.2910270309. [DOI] [PubMed] [Google Scholar]
- Ueshima Y., Rowley J. D., Variakojis D., Winter J., Gordon L. Cytogenetic studies on patients with chronic T cell leukemia/lymphoma. Blood. 1984 May;63(5):1028–1038. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Holmes M. T. A sensitive and dependable assay for distinguishing hamster and human X-linked steroid sulfatase activity in somatic cell hybrids. Hum Genet. 1984;66(2-3):272–275. doi: 10.1007/BF00286615. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Meakin S. O., Tsui L. C., Breitman M. L. Assignment of human gamma crystallin multigene family to chromosome 2. Somat Cell Mol Genet. 1985 Sep;11(5):511–516. doi: 10.1007/BF01534846. [DOI] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Clark S. P., Taylor S., Sohn U., Wilson B. I., Minden M. D., Mak T. W. Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):837–840. doi: 10.1038/316837a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Kimura N., Toyonaga B., Mak T. W. Sequences and repertoire of human T cell receptor alpha chain variable region genes in mature T lymphocytes. J Exp Med. 1986 Jul 1;164(1):90–103. doi: 10.1084/jem.164.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- Zech L., Gahrton G., Hammarström L., Juliusson G., Mellstedt H., Robèrt K. H., Smith C. I. Inversion of chromosome 14 marks human T-cell chronic lymphocytic leukaemia. 1984 Apr 26-May 2Nature. 308(5962):858–860. doi: 10.1038/308858a0. [DOI] [PubMed] [Google Scholar]
- Zech L., Godal T., Hammarström L., Mellstedt H., Smith C. I., Tötterman T., Went M. Specific chromosome markers involved with chronic T lymphocyte tumors. Cancer Genet Cytogenet. 1986 Mar 1;21(1):67–77. doi: 10.1016/0165-4608(86)90201-3. [DOI] [PubMed] [Google Scholar]