Abstract
The prevalence of low vitamin D status in the elderly population of subtropical area and the potential risk/protective factors have not been addressed. This cross-sectional questionnaire-based study, which collected demographic/anthropometric data and information on diet habit and sun exposure, recruited 170 subjects with mean age 70.9±5.6 in rural areas of southern Taiwan. Serum 25-OH vitamin D, calcium, and intact parathyroid hormone were also measured. Using cut-off level of 30 ng/mL, subjects were divided into low (n = 95) and normal (n = 75) serum vitamin D groups. The results demonstrated a low vitamin D status in 30.6% of men and 57.7% of women. Dietary vitamin D intake was another factor associated with vitamin D status (p = 0.02). Logistic regression identified inadequate intake of vitamin D-rich food as the only risk factor for low vitamin D status in men (OR = 4.55, p = 0.01), whereas inadequate sun exposure was the only predictable risk with dose-response relationship in women (low vs. high sun exposure, OR = 6.84, p = 0.018; moderate vs. high sun exposure, OR = 6.67, p = 0.005). In conclusion, low vitamin D status was common in the elderly of subtropical rural areas. Low sun exposure and inadequate dietary vitamin D consumption were associated with a low vitamin D status in females and males, respectively.
Introduction
Vitamin D, which is one essential nutrient for intestinal calcium absorption to facilitate mineralization of bone [1], not only helps in maintaining muscle strength and function [2], but has also been reported to reinforce immunity [3, 4] as well as improve outcomes of cardiovascular diseases [5–7]. At the other end of the spectrum, vitamin D deficiency has been shown to be associated with rickets, osteomalacia, osteoporosis, risk of fracture, and cancers [1, 8–10]. It has also been reported to be associated with colorectal, lung, prostate, breast and ovarian cancer [11]. Consistently, vitamin D supplementation has been reported to reduce all-cause cancer mortality [12]. Moreover, vitamin D supplementation seems to lower the risk of acute respiratory infection and asthma exacerbation [13, 14].
Vitamin D can be produced by the skin through exposure to ultraviolet-B (UVB) or obtained from the diet. UVB converts 7-dehydrocholesterol to pre-vitamin D3 in the skin, followed by conversion to vitamin D3. On the other hand, vitamin D2 (ergocalciferol) is acquired exclusively from diet. Vitamin D (i.e., vitamin D2 and D3) is metabolized in the liver to 25-hydroxyvitamin D (25-(OH)D), which is often used as a biomarker to determine vitamin D status [15]. Although vitamin D production has been found to be adequate as long as there is sunlight exposure of arms and legs for 5 to 30 minutes twice weekly [16, 17], low vitamin D status remains a public health problem worldwide [18].
Although there is no definite consensus so far, vitamin D deficiency and insufficiency are usually defined as 25-hydroxyvitamin D < 20 ng/mL and 20–30 ng/mL, respectively [1, 2, 19–21]. According to large-scale cross-sectional studies in North America, one-third of all subjects was demonstrated to have 25-(OH)D level below 20 ng/mL, two-thirds of those below 30 ng/mL [22, 23]. In other words, low vitamin D status is a common condition in western populations. On the other hand, in addition to the dearth of study on the prevalence of low vitamin D status in Asia [18, 24, 25], there is no investigation addressing the issue in subtropical countries. Moreover, the target population in previous studies focused mainly on adolescents and young adults rather than the elderly whose population has been on the increase worldwide. Indeed, aging is known to be associated with decreased 7-dehydrocholesterol concentration in the skin, resulting in reduction of vitamin D3 production [17, 26, 27]. Interestingly, although previous studies carried out across different countries in Southern Asia revealed a high prevalence of low vitamin D status, no particular attention has been paid to the elderly population [28, 29]. Although risk factors for a low vitamin D status (i.e., age, gender, race, body mass index, latitude, season, nutritional intake, sun exposure, and physical activity) have been reported for the general population [23, 29–31], risk and protective factors contributing to the vitamin D status in the elderly have not be addressed. The purposes of the study, therefore, is to determine the prevalence of low vitamin D status in the elderly population living in a subtropical area and to explore the potential risk or protective factors.
Subjects and methods
Study population
Healthy volunteers aged 65 or older were randomly recruited from a tertiary referral hospital in a rural area in Southern Taiwan from February to August 2015. Community dwellers older than 65 years with written informed consent were eligible for the present study, whereas those with active cancer in previous 5 years, end-stage renal disease, liver cirrhosis, psychiatric disease, or disorders known to affect bone mineral metabolism including hyperthyroidism, hyperparathyroidism, osteomalacia, Crohn's disease, cystic fibrosis, or Paget’s disease were excluded.
Study design and parameters
This is a prospective cross-sectional study based on information acquired through questionnaires and blood samples. After signing the informed consent, each subject was required to complete a questionnaire for collecting information on age, gender, body height, body weight, past history, medical and drug history, status of vitamin D supplement, status of sun exposure, and consumption of vitamin D-rich food through a food diary. According to the total diet scores, the participants were classified into those with high vitamin D intake and those with relatively low intake. Blood samples were obtained from all subjects for the quantification of 25-hydroxyvitamin D (25-(OH)D), intact parathyroid hormone (iPTH), and serum calcium at the central laboratory of the institute. The whole study protocol was reviewed and approved by the Institutional Review Board of E-Da Hospital (EMRP-102-045). Informed consents were obtained from all subjects.
Questionnaire design
The instrument used for assessing the factors affecting an individual’s vitamin D status was a questionnaire containing four major items, including (1) basic demographic, anthropometric, and clinical information including medical history, drug history (2) consumption of vitamin D supplements, including type and dosage (3) extent of sun exposure and habits of sunscreen use (4) food frequency questionnaire (FFQ) regarding the consumption of vitamin D-rich food. To estimate the amount of vitamin D from food, a food frequency questionnaire (FFQ) was developed by an experienced dietician (S1 and S2 Tables). The vitamin D contents of the commonly available vitamin D-rich food items included in the questionnaire are summarized in supporting information S3 Table [1, 32]. In each item, consumption was recorded by frequency per week, which was transformed into scores (i.e., never = 0 point, 1–2 times/week = 1 point, more than 2 times/week = 2 points). The sum of total scores was used to estimate the intake during recent 3 months. Physical activity was measured by using Taiwanese version of the International Physical Activity Questionnaire, which has been shown to be a valid and reliable tool [33].
Blood tests
Five milliliters of blood was obtained from each subject between 10 a.m. and 11 a.m. in the morning. All subjects were refrained from calcium-containing food and beverage including calcium supplement tablets, and milk as well as caffeine- or alcohol-containing beverage for at least 12 hours before collection of blood samples. Serum levels of 25-hydroxyvitamin D (25-(OH)D), intact parathyroid hormone (iPTH) were determined at the central laboratory of the institute with standard methods using chemiluminescent immunoassay (Abbott ARCHITECT i2000, Illinois, U.S.A.). Serum calcium level was determined by o-cresolphthalein complexone (OCPC) method (Toshiba C, Tokyo, Japan). All analyses were completed within 12 hours of blood sample harvesting.
Definitions
Degree of sun exposure was divided into three categories according to the duration between 8 a.m. and 5 p.m.: Low (<1 hour/week), medium (1–2 hours/week), and high (>2 hours/week). Sun screen use was defined as the use of any sun screen products within 3 months prior to the study regardless of the frequency. Vitamin D supplements were defined as all oral supplements containing vitamin D other than food. A diet scoring system was developed based on the frequency of consumption of vitamin D-rich food products including fish, sardine, herring, salmon, oysters, eggs, fortified cereals, fortified soymilk, and fortified milk. The testing subjects were categorized into low (score 0—5) and high (score 6—10) diet scorers. The four seasons were defined as: Spring (March—May), summer (June—August), fall (September—November), and winter (December—February). Three levels of Physical activity, which were proposed by the scoring protocol in guidelines for data processing and analysis of the Taiwan version of International Physical Activity Questionnaire (IPAQ), were used to label participants as low, moderate, and high groups[33]. The definition of vitamin D deficiency and insufficiency are usually defined as 25-hydroxyvitamin D < 20ng/mL and 20-30ng/mL, respectively [1]. The testing subjects in our study were divided into low and normal vitamin D groups if their serum 25-OH vitamin D levels were < 30 ng/mL and ≧ 30 ng/mL, respectively.
Statistical analysis
All statistical analyses were carried out using the IBM SPSS for Windows software program version 22.0 (Armonk, NY: IBM Corp, USA). A p-value <0.05 was considered significant. Demographic and anthropometric data are expressed as mean ±standardized deviation (SD). Pearson correlation was adopted to explore the relationship between serum 25(OH)D and iPTH level. Chi-square test was used for determination of significance of difference between categorical variables. Multiple logistic regression analysis was performed to study the association between each presumed risk factor and vitamin D status, including age, gender, sun exposure, and vitamin D intake.
Results
Comparison of baseline characteristics between participants with low and normal serum vitamin D levels
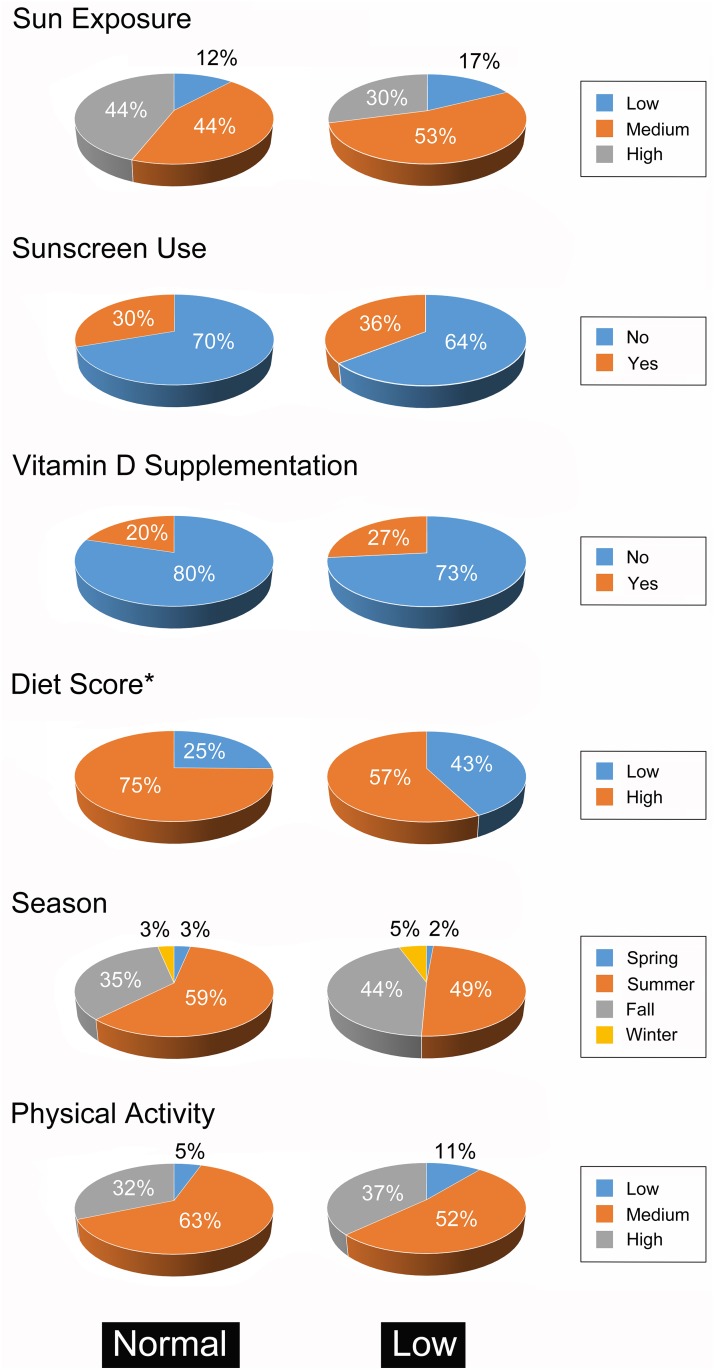
Totally 170 volunteers were recruited, including 85 men and 85 women with a mean age of 70.9 ± 5.6 years (range, 65–85) and a mean BMI of 24.0 ± 3.2 kg/m2 (range, 15.1–34.2) which was around the upper limit of normal (Table 1). Most participants had medium sun exposure (48.2%) without the habit of sunscreen use (67.1%) (Fig 1). In addition, most of them consumed adequate vitamin D through normal diets (67.1%) without the habit of taking vitamin D supplements (77.1%). According to the serum vitamin D levels, there were 75 (44.1%) and 95 (55.9%) testing subjects belonging to the low and normal vitamin D group, respectively. A low vitamin D status was demonstrated in 30.6% men and 57.7% of women. Gender was a significant indicator of vitamin D status. The majority of participants with low vitamin D status were women (65.3%) (p < 0.001). Dietary intake of vitamin D was another factor significantly associated with vitamin D status (p = 0.02). Age, BMI, sun exposure, use of sun screen, vitamin D supplement, season of blood withdrawal, or physical activity were not associated with vitamin D status.
Table 1. Comparison of demographic and anthropometric characteristics between low and normal vitamin D groups.
| Total (N = 170) | Low vitamin D group (N = 95) (25-OH vitamin D<30 ng/mL) |
Normal vitamin D group (N = 75) (25-OH vitamin D≧30 ng/mL) |
*P value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 70.9 ± 5.6 | 70.4 ± 5.7 | 71.2 ± 5.5 | 0.36 |
| Gender | ||||
| Male, N (%) | 85 (50%) | 26 (34.7%) | 59 (62.1%) | <0.001 |
| Female, N (%) | 85 (50%) | 49 (65.3%) | 36 (37.9%) | |
| Height, cm (mean ± SD) | 159.5 ± 11.7 | 158.8 ± 15.4 | 160.0 ± 8.0 | 0.51 |
| Weight, kg (mean ± SD) | 60.9 ± 9.6 | 60.0 ± 9.7 | 61.7 ± 9.5 | 0.25 |
| BMI, kg/m2 (mean ± SD) | 24.0 ± 3.2 | 23.9 ± 3.4 | 24.1 ± 3.1 | 0.81 |
*Significance of difference determined by Chi-square test
Fig 1. Comparison of factors affecting vitamin D level.
Factors affecting vitamin D level between subjects (n = 170) with normal (n = 75) and low (n = 95) serum vitamin D level. *p <0.005 between the two groups (Chi-square test).
Correlations among serum vitamin D, calcium, and iPTH concentrations
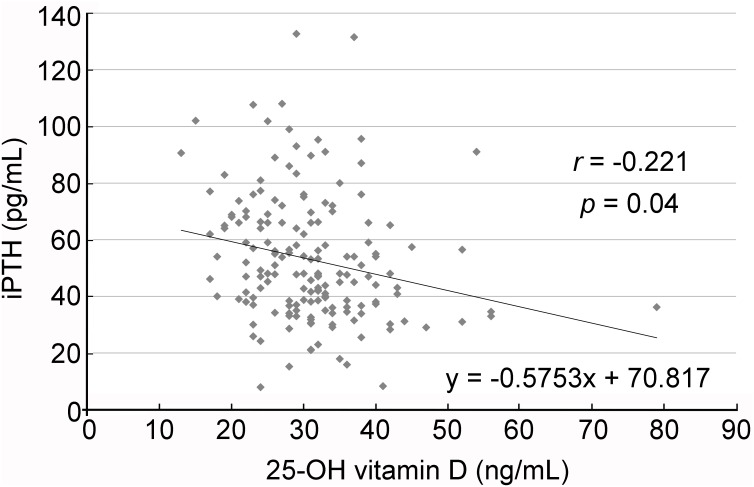
The participants showed a mean serum 25-OH vitamin D level of 30.9 ± 8.6 ng/mL, ranging from 13 to 79 ng/mL. On the other hand, their mean serum iPTH level was 53.0 ± 22.3 pg/mL, ranging from 7.9 to 132.7 pg/mL. Hyperparathyroidism was found in 21.76% of all participants (37/170) in whom the prevalence of hyperparathyroidism was higher in the low vitamin D group (29.33%, 22/75) than that in the normal vitamin D group (15.8%, 15/95). Scattered plot (Fig 2) showed a negative correlation between 25-OH vitamin D level and iPTH (r = -0.221, p = 0.004) after adjustment for age and sex. No significant correlation was noted between serum iPTH and serum calcium levels (r = 0.013, p = 0.865).
Fig 2. Relationship between serum parathyroid hormone (iPTH) concentration and 25-OH vitamin D level.
Regression analysis with scatter plot showing inverse relationship between serum iPTH concentration and that of 25-OH vitamin D.
Factor associated with low vitamin D status and gender differences
Chi-square test demonstrated significantly higher sun exposure in men than that in women (p = 0.003). The results of logistic regression of presumed risk factors for low vitamin D status were summarized in Table 2. Female gender was at higher risk of low vitamin D status (OR = 2.85, 95% CI: 1.39–5.85, p = 0.004) compared with that in their male counterparts. Low consumption of vitamin D-rich food was also significantly associated with an increased risk (OR = 2.09, 95% CI: 1.01–4.34, p = 0.049). In men (Table 3), inadequate intake of vitamin D-rich food was the only risk factor for a low vitamin D status (OR = 4.55, 95% CI: 1.43–14.43, p = 0.01, Nagelkerke r2 = 0.217). However, in women (Table 4), a lack of sun exposure was the only predictable risk with dose-response relationship. Compared with their female counterparts with high extent of sun exposure, elderly women with low sun exposure (OR = 6.84, 95% CI: 1.40–33.51, p = 0.018) and moderate sun exposure (OR = 6.67, 95% CI: 1.79–24.77, p = 0.005) were at greater risks of low vitamin D status (Nagelkerke r2 = 0.252).
Table 2. Logistic regression for risk factors of low vitamin D status.
| Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Age | 0.98 | 0.91 | 1.04 | 0.467 |
| Gender | ||||
| Female | 2.85 | 1.39 | 5.85 | 0.004 |
| Male | 1.00 | |||
| BMI | 0.96 | 0.86 | 1.07 | 0.445 |
| Sun exposure | ||||
| Low | 1.39 | 0.46 | 4.19 | 0.561 |
| Medium | 1.71 | 0.79 | 3.70 | 0.176 |
| High | 1.00 | |||
| Sun screen | ||||
| No | 1.28 | 0.58 | 2.84 | 0.541 |
| Yes | 1.00 | |||
| Vitamin D supplement | ||||
| No | 0.75 | 0.32 | 1.75 | 0.504 |
| Yes | 1.00 | |||
| Diet score | ||||
| Low | 2.09 | 1.01 | 4.34 | 0.049 |
| High | 1.00 | |||
| Season | ||||
| Spring | 0.20 | 0.01 | 3.66 | 0.277 |
| Summer | 0.59 | 0.11 | 3.23 | 0.541 |
| Fall | 0.68 | 0.12 | 3.78 | 0.662 |
| Winter | 1.00 | |||
| Physical activity | ||||
| Low | 1.14 | 0.27 | 4.77 | 0.859 |
| Medium | 0.53 | 0.25 | 1.11 | 0.092 |
| High | 1.00 | |||
Table 3. Logistic regression for risk factors of low vitamin D status (Male).
| Odds ratio | 95%CI | P value | ||
|---|---|---|---|---|
| Age | 0.99 | 0.89 | 1.10 | 0.825 |
| BMI | 0.91 | 0.77 | 1.08 | 0.292 |
| Sun exposure | ||||
| Low | 0.24 | 0.02 | 2.98 | 0.264 |
| Medium | 0.57 | 0.18 | 1.80 | 0.335 |
| High | 1.00 | |||
| Sun screen | ||||
| No | 0.91 | 0.26 | 3.16 | 0.884 |
| Yes | 1.00 | |||
| Vitamin D supplement | ||||
| No | 0.73 | 0.18 | 3.03 | 0.663 |
| Yes | 1.00 | |||
| Diet score | ||||
| Low | 4.55 | 1.43 | 14.43 | 0.010 |
| High | 1.00 | |||
| Season | ||||
| Spring | 0.16 | 0.01 | 5.46 | 0.310 |
| Summer | 0.14 | 0.01 | 1.67 | 0.119 |
| Fall | 0.26 | 0.02 | 3.41 | 0.307 |
| Winter | 1.00 | |||
| Physical activity | ||||
| Low | 0.47 | 0.44 | 0.05 | 4.125 |
| Medium | 0.53 | 0.70 | 0.22 | 2.160 |
| High | 1.00 | |||
Table 4. Logistic regression for risk factors of low vitamin D status (Female).
| Odds ratio | 95%CI | P value | ||
|---|---|---|---|---|
| Age | 0.95 | 0.85 | 1.07 | 0.371 |
| BMI | 0.93 | 0.78 | 1.11 | 0.421 |
| Sun exposure | ||||
| Low | 6.84 | 1.40 | 33.51 | 0.018 |
| Medium | 6.67 | 1.79 | 24.77 | 0.005 |
| High | 1.00 | |||
| Sun screen | ||||
| No | 1.78 | 0.52 | 6.03 | 0.357 |
| Yes | 1.00 | |||
| Vitamin D supplement | ||||
| No | 1.00 | 0.29 | 3.44 | 1.000 |
| Yes | 1.00 | |||
| Diet score | ||||
| Low | 1.16 | 0.40 | 3.33 | 0.788 |
| High | 1.00 | |||
| Season | ||||
| Spring | 0.000 | 0.00 | 1.000 | |
| Summer | 1.22 | 0.07 | 20.78 | 0.891 |
| Fall | 0.68 | 0.05 | 9.09 | 0.769 |
| Winter | 1.00 | |||
| Physical activity | ||||
| Low | 2.88 | 0.23 | 36.01 | 0.412 |
| Medium | 0.65 | 0.19 | 2.24 | 0.498 |
| High | 1.00 | |||
Discussion
Although vitamin D deficiency and insufficiency are common worldwide, only a few studies have focused on the vitamin D status of the elderly. Moreover, limited surveys were completed in Asian countries so far. One multicenter survey showed a high prevalence of vitamin D deficiency in large cities in China, but no associated risk factors were reported [30]. Accordingly, our survey was designed to explore the risk factors of low vitamin D status in the elderly living in rural areas of Taiwan. Our survey demonstrated an incidence of low vitamin D status up to 44.1% in this population with female predominance (women: 57.7%, men: 30.6%).
Geographically, the percentage of vitamin D deficiency and insufficiency was higher than expectation in Asian countries, especially in South Asia [18]. In a multicenter vitamin D survey in China, the prevalence of vitamin D deficiency (<20 ng/mL) and vitamin D insufficiency (20–30 ng/mL) was 55.9% and 38.7%, respectively [30]. Another national survey in temperate areas of China reported the prevalence of vitamin D deficiency and insufficiency was 69.2 and 24.4%, respectively [25]. Based on the Korea National Health and Nutrition Examination Survey (KNHANES) 2008, Fourth Korea National Health and Nutrition Examination Surveys showed the prevalence of vitamin D deficiency was 47% in the males and 65% in the females [24]. As sun exposure is an essential source of vitamin D production, people living in low-latitude countries might be expected to have higher vitamin D levels than those living at high latitudes [1]. In contrast with China and Korea, Thailand, Taiwan and India are located at a lower latitude. However, the prevalence of low vitamin D status in people living in urban areas was reported to be up to 46.7% in one Thai study. For instance, the situation was more common in Bangkok (64.6%) than that in rural areas (33.5%), highlighting the impact of lifestyle and environmental factors on ultraviolet exposure and vitamin D synthesis [28]. In Taiwan, a survey including 215 participants living in Northern island demonstrated an incidence of 25-(OH)D deficiency up to 31% [34]. Even in tropical regions with plenty of sunshine such as India, more than 70% of the population suffered from vitamin D deficiency in all age groups and both genders [35].
Generally speaking, individual differences are significant contributors to vitamin D status. Vitamin D deficiency has been reported to be highly prevalent in pregnant women, children, older people, and residents of institutions [36]. Besides, more than 50% of postmenopausal women taking medication for osteoporosis also had 25-(OH)D ≤ 30ng/mL [37, 38]. Despite being a notorious culprit responsible for skin pigmentation, ultraviolet B triggers vitamin D production [16, 39]. Several studies have already revealed a lower vitamin D status in Asian women than that in their male counterparts [25, 40–42]. The finding may be explained by the observation that Asian women generally prefer indoor activities and take more precautions against sunlight exposure owing to aesthetic concern. The more frequent use of sunscreens in females than in males may also contribute to the difference [43]. Our research also identified the female gender as a risk factor for a low vitamin D status. Although use of sunscreen was not a predictive factor in our survey, the extent of sun exposure was shown to be positively associated with a high vitamin D status in females. In comparison to the high sun exposure group, the medium sun exposure group (OR = 6.67) and low sun exposure group (OR = 6.84) had significantly increased risks for low vitamin D status. On the other hand, the association was not shown among men. One reason may be that more men obtained sufficient sun exposure in contrast to women (92.94% v.s.78.83%), which implied the influence of sun exposure on vitamin D status of men was not as crucial as that of women.
Although exposure to sunlight for 5–30 minutes daily was considered adequate for the production of pre-vitamin D3 in adults, old age is a significant predictor of low vitamin D status regardless of seasons or the extent of sun exposure [1, 44]. Indeed, based on skin biopsy samples, the capacity of production of pre-vitamin D3 in the elderly was less than half of that in young adults [26]. Besides, it has been reported that a 70-year-old person only produced one-fourth of the amount of vitamin D compared with that of a 20-year-old through cutaneous synthesis [27]. This may partly account for the high prevalence of low vitamin D status in the rural areas of Taiwan, which is located in the subtropical region. Although the present study showed that the majority of the elderly population (85.89%) had adequate sun exposure because of farming, gardening, and other outdoor activities, they were not exempted from vitamin D sufficiency. The finding, therefore, may suggest a more detrimental influence of age on vitamin D status compared to that of inadequate sun exposure.
Consumption of vitamin D-rich food, such as fortified milk, meat, oily fish, eggs, has been demonstrated to be limited in the diets of adolescents, regardless of races and genders [45–47]. Similarly, it has been reported that the elderly seldom take adequate daily amount to meet recommended daily allowance [1]. Moreover, for the aged population, in addition to the reduced absorption of dietary vitamin D associated with aging, polypharmacy commonly seen has also been shown to reduce absorption of vitamin D because of drug-food interaction [48]. Therefore, the importance of vitamin D supplementation increases by age. In our logistic regression model, only elderly men benefited from vitamin D-rich food to maintain adequate vitamin D status. The odds ratio of having a low vitamin D status in men with low diet score was 4.55 times higher than that in those with a high score. However, our results failed to show significant association between diet habits and serum vitamin D level among women. One possible explanation is that, unlike the case in men, factors other than diet such as menopausal status, lifestyle, and environmental influence may play a more important role in determining vitamin D status in women. [23, 49]
Predictors of vitamin D status were introduced in several large cohort studies, such as race, gender, body mass index, residential area, vitamin D supplement, dietary intake, and season [50, 51]. These possible indicators were all included in our logistic regression. In addition, gender was divided in subgroups to explore the difference between male and female living in the rural areas. Some previous studies, but not all, showed gender differences consistent with our results [52] [53]. Our data further revealed gender differences in predictors of low vitamin D status in the elderly. Moreover, the present study demonstrated that inadequate sun exposure and insufficient dietary vitamin D intake were significantly associated with low vitamin D status in women and men, respectively. The association between a low vitamin D status and malignancy has also been reported. Previous reviews [54, 55] showed a significant inverse relationship between colorectal cancer and vitamin D level, while the association in other malignancies including breast cancer, prostate cancer are less well defined [56, 57]. Another Asian study also supported the inverse relationship between vitamin D level and risk of rectal cancer [58]. In addition, previous epidemiological studies also showed that vitamin D level was negatively associated with cardiovascular risks, although benefit of vitamin D supplementation was not demonstrated in interventional studies [59]. On the other hand, another study reported an association between a low vitamin D status and the prevalence of osteoporosis, which was shown to improve with oral vitamin D supplementation [60]. The importance of gender influence on vitamin D status in the elderly as well as the role of vitamin D in the prevention and therapy of cancer, osteoporosis, cardiovascular diseases warrant further exploration [56, 61, 62].
Regarding the role of vitamin D in specific Taiwanese patient populations, there have been two studies investigating the impact of vitamin D level on disease progression. One of the studies investigated the influence of vitamin D status on bone complications in post-menopausal women [63], while the other studied the clinical significance of vitamin D level in patients with chronic hepatitis B [64]. The study focusing on vitamin D status and osteoporosis in 199 middle-aged to elderly postmenopausal women demonstrated a prevalence of vitamin D inadequacy (i.e., insufficiency and deficiency) of 86.6% [63]. Moreover, the other study on relatively young patients aged around 37 with chronic hepatitis B revealed a prevalence of vitamin D insufficiency and deficiency of 35% and 58%, respectively, both of which were shown to adversely affect prognostic outcome [64]. The two studies, therefore, highlight the high prevalence of vitamin D inadequacy in the diseased populations regardless of age and gender.
There are some limitations in the current study. Firstly, relatively young individuals were not recruited in the present study for evaluation of the impact of age on vitamin D status. Secondly, only subjects in rural areas were studied so that the vitamin D status of their counterparts in urban areas was unavailable for comparison. Thirdly, the information on some predictors that cannot be accurately quantitated, including dietary intake of vitamin D and sun exposure, was obtained through questionnaires that may have caused reporting and recall bias. Finally, the cross-sectional design of this study precluded the investigation of causative effects of demographic and lifestyle factors on vitamin D status. Further cohort studies, therefore, are warranted to address these issues.
Conclusions
In conclusion, low vitamin D status, including vitamin D insufficiency and deficiency, was common in the aged population living in subtropical rural areas. Low sun exposure and inadequate dietary vitamin D consumption were associated with a status of low vitamin D in females and males, respectively.
Supporting information
English version of FFQ estimating oral vitamin D intake through assessing the frequency of vitamin D-rich food consumption in recent 3 months.
(DOCX)
Original Chinese version of FFQ estimating oral vitamin D intake through assessing the frequency of vitamin D-rich food consumption in recent 3 months.
(DOCX)
Vitamin D contents of commonly available vitamin D-rich food items included in FFQ. IU: International unit.
(DOCX)
Data Availability
Data are available from doi:10.6084/m9.figshare.5162119.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357(3):266–81. Epub 2007/07/20. doi: 10.1056/NEJMra070553 . [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. The American journal of clinical nutrition. 2006;84(1):18–28. . [DOI] [PubMed] [Google Scholar]
- 3.DeLuca HF. Overview of general physiologic features and functions of vitamin D. The American journal of clinical nutrition. 2004;80(6 Suppl):1689S–96S. . [DOI] [PubMed] [Google Scholar]
- 4.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–7. doi: 10.1182/blood-2005-05-2044 . [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. The Journal of clinical endocrinology and metabolism. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393 . [DOI] [PubMed] [Google Scholar]
- 6.Myrup B, Jensen GF, McNair P. Cardiovascular risk factors during estrogen-norethindrone and cholecalciferol treatment. Archives of internal medicine. 1992;152(11):2265–8. . [PubMed] [Google Scholar]
- 7.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. European journal of clinical nutrition. 1995;49(9):640–6. . [PubMed] [Google Scholar]
- 8.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinology and metabolism clinics of North America. 2010;39(2):321–31, table of contents. doi: 10.1016/j.ecl.2010.02.001 . [DOI] [PubMed] [Google Scholar]
- 9.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Resurrection of vitamin D deficiency and rickets. The Journal of clinical investigation. 2006;116(8):2062–72. doi: 10.1172/JCI29449 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wranicz J, Szostak-Wegierek D. Health outcomes of vitamin D. Part II. Role in prevention of diseases. Rocz Panstw Zakl Hig. 2014;65(4):273–9. . [PubMed] [Google Scholar]
- 12.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470 doi: 10.1002/14651858.CD007470.pub2 . [DOI] [PubMed] [Google Scholar]
- 13.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511 doi: 10.1002/14651858.CD011511.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ (Clinical research ed). 2017;356:i6583 doi: 10.1136/bmj.i6583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. The American journal of the medical sciences. 2009;338(1):40–4. Epub 2009/07/14. doi: 10.1097/MAJ.0b013e3181aaee91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic proceedings. 2006;81(3):353–73. doi: 10.4065/81.3.353 . [DOI] [PubMed] [Google Scholar]
- 17.Reid IR, Gallagher DJ, Bosworth J. Prophylaxis against vitamin D deficiency in the elderly by regular sunlight exposure. Age and ageing. 1986;15(1):35–40. . [DOI] [PubMed] [Google Scholar]
- 18.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(11):1807–20. doi: 10.1007/s00198-009-0954-6 . [DOI] [PubMed] [Google Scholar]
- 19.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–6. . [DOI] [PubMed] [Google Scholar]
- 20.Adams JS, Hewison M. Update in vitamin D. The Journal of clinical endocrinology and metabolism. 2010;95(2):471–8. doi: 10.1210/jc.2009-1773 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannucci E. Expanding roles of vitamin D. The Journal of clinical endocrinology and metabolism. 2009;94(2):418–20. doi: 10.1210/jc.2008-2695 . [DOI] [PubMed] [Google Scholar]
- 22.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS data brief. 2011;(59):1–8. . [PubMed] [Google Scholar]
- 23.Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012;18(6):914–23. doi: 10.4158/EP12072.OR . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. The Journal of clinical endocrinology and metabolism. 2011;96(3):643–51. doi: 10.1210/jc.2010-2133 . [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes care. 2009;32(7):1278–83. doi: 10.2337/dc09-0209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. The Journal of clinical investigation. 1985;76(4):1536–8. doi: 10.1172/JCI112134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2(8671):1104–5. . [DOI] [PubMed] [Google Scholar]
- 28.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC public health. 2011;11:853 doi: 10.1186/1471-2458-11-853 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimitphong H, Holick MF. Vitamin D status and sun exposure in southeast Asia. Dermato-endocrinology. 2013;5(1):34–7. doi: 10.4161/derm.24054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S, Fang H, Han J, Cheng X, Xia L, Li S, et al. The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine. 2015;94(8):e585 doi: 10.1097/MD.0000000000000585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Archives of pediatrics & adolescent medicine. 2004;158(6):531–7. Epub 2004/06/09. doi: 10.1001/archpedi.158.6.531 . [DOI] [PubMed] [Google Scholar]
- 32.Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynologia Polska. 2013;64(4):319–27. doi: 10.5603/ep.2013.0012 [DOI] [PubMed] [Google Scholar]
- 33.Liou YM, Jwo CJ, Yao KG, Chiang LC, Huang LH. Selection of appropriate Chinese terms to represent intensity and types of physical activity terms for use in the Taiwan version of IPAQ. J Nurs Res. 2008;16(4):252–63. . [DOI] [PubMed] [Google Scholar]
- 34.Chang CI, Chan DC, Kuo KN, Hsiung CA, Chen CY. Vitamin D insufficiency and frailty syndrome in older adults living in a Northern Taiwan community. Arch Gerontol Geriatr. 2010;50 Suppl 1:S17–21. doi: 10.1016/S0167-4943(10)70006-6 . [DOI] [PubMed] [Google Scholar]
- 35.Babu US, Calvo MS. Modern India and the vitamin D dilemma: evidence for the need of a national food fortification program. Molecular nutrition & food research. 2010;54(8):1134–47. doi: 10.1002/mnfr.200900480 . [DOI] [PubMed] [Google Scholar]
- 36.van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25(4):671–80. doi: 10.1016/j.beem.2011.06.007 . [DOI] [PubMed] [Google Scholar]
- 37.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. The Journal of clinical endocrinology and metabolism. 2005;90(6):3215–24. doi: 10.1210/jc.2004-2364 . [DOI] [PubMed] [Google Scholar]
- 38.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. Journal of internal medicine. 2006;260(3):245–54. doi: 10.1111/j.1365-2796.2006.01685.x . [DOI] [PubMed] [Google Scholar]
- 39.Holick MF. Vitamin D: its role in cancer prevention and treatment. Progress in biophysics and molecular biology. 2006;92(1):49–59. Epub 2006/03/29. doi: 10.1016/j.pbiomolbio.2006.02.014 . [DOI] [PubMed] [Google Scholar]
- 40.Robien K, Butler LM, Wang R, Beckman KB, Walek D, Koh WP, et al. Genetic and environmental predictors of serum 25-hydroxyvitamin D concentrations among middle-aged and elderly Chinese in Singapore. Br J Nutr. 2013;109(3):493–502. doi: 10.1017/S0007114512001675 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moy FM. Vitamin D status and its associated factors of free living Malay adults in a tropical country, Malaysia. J Photochem Photobiol B. 2011;104(3):444–8. doi: 10.1016/j.jphotobiol.2011.05.002 . [DOI] [PubMed] [Google Scholar]
- 42.Kung AW, Lee KK. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: a population survey in Hong Kong. BMC public health. 2006;6:226 doi: 10.1186/1471-2458-6-226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holick MF. McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. The American journal of clinical nutrition. 1994;60(4):619–30. . [DOI] [PubMed] [Google Scholar]
- 44.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2002;166(12):1517–24. Epub 2002/06/21. . [PMC free article] [PubMed] [Google Scholar]
- 45.Yu A, Kim J, Kwon O, Oh SY, Kim J, Yang YJ. The association between serum 25-hydroxyvitamin d concentration and consumption frequencies of vitamin d food sources in korean adolescents. Clin Nutr Res. 2013;2(2):107–14. doi: 10.7762/cnr.2013.2.2.107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Horn LV, Bausermann R, Affenito S, Thompson D, Striegel-Moore R, Franko D, et al. Ethnic differences in food sources of vitamin D in adolescent American girls: the National Heart, Lung, and Blood Institute Growth and Health Study. Nutr Res. 2011;31(8):579–85. doi: 10.1016/j.nutres.2011.07.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. The New England journal of medicine. 1992;326(18):1178–81. doi: 10.1056/NEJM199204303261802 . [DOI] [PubMed] [Google Scholar]
- 48.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. The American journal of clinical nutrition. 1985;42(4):644–9. . [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal S, Nityanand. Calcium and vitamin D in post menopausal women. Indian J Endocrinol Metab. 2013;17(Suppl 3):S618–20. doi: 10.4103/2230-8210.123549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101 . [DOI] [PubMed] [Google Scholar]
- 51.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. The American journal of clinical nutrition. 2010;91(6):1627–33. doi: 10.3945/ajcn.2009.28441 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vierucci F, Del Pistoia M, Fanos M, Erba P, Saggese G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Italian journal of pediatrics. 2014;40:54 doi: 10.1186/1824-7288-40-54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Musharaf S, Al-Othman A, Al-Daghri NM, Krishnaswamy S, Yusuf DS, Alkharfy KM, et al. Vitamin D deficiency and calcium intake in reference to increased body mass index in children and adolescents. Eur J Pediatr. 2012;171(7):1081–6. doi: 10.1007/s00431-012-1686-8 . [DOI] [PubMed] [Google Scholar]
- 54.Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735–43. doi: 10.1158/1940-6207.CAPR-10-0289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29(28):3775–82. doi: 10.1200/JCO.2011.35.7566 . [DOI] [PubMed] [Google Scholar]
- 56.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. American journal of public health. 2006;96(2):252–61. Epub 2005/12/29. doi: 10.2105/AJPH.2004.045260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol Rev. 2017:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S, Japan Public Health Center-Based Prospective Study G. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97(3):446–51. doi: 10.1038/sj.bjc.6603892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grubler MR, Marz W, Pilz S, Grammer TB, Trummer C, Mullner C, et al. Vitamin-D concentrations, cardiovascular risk and events—a review of epidemiological evidence. Rev Endocr Metab Disord. 2017. doi: 10.1007/s11154-017-9417-0 . [DOI] [PubMed] [Google Scholar]
- 60.Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(1):367–76. doi: 10.1007/s00198-015-3386-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. The New England journal of medicine. 1992;327(23):1637–42. Epub 1992/12/03. doi: 10.1056/NEJM199212033272305 . [DOI] [PubMed] [Google Scholar]
- 62.Bulathsinghala P, Syrigos KN, Saif MW. Role of vitamin d in the prevention of pancreatic cancer. Journal of nutrition and metabolism. 2010;2010:721365 Epub 2011/01/29. doi: 10.1155/2010/721365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang JS, Tsai KS, Cheng YM, Chen WJ, Tu ST, Lu KH, et al. Vitamin D status in non-supplemented postmenopausal Taiwanese women with osteoporosis and fragility fracture. BMC Musculoskelet Disord. 2014;15:257 doi: 10.1186/1471-2474-15-257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan HL, Elkhashab M, Trinh H, Tak WY, Ma X, Chuang WL, et al. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J Hepatol. 2015;63(5):1086–92. doi: 10.1016/j.jhep.2015.06.025 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
English version of FFQ estimating oral vitamin D intake through assessing the frequency of vitamin D-rich food consumption in recent 3 months.
(DOCX)
Original Chinese version of FFQ estimating oral vitamin D intake through assessing the frequency of vitamin D-rich food consumption in recent 3 months.
(DOCX)
Vitamin D contents of commonly available vitamin D-rich food items included in FFQ. IU: International unit.
(DOCX)
Data Availability Statement
Data are available from doi:10.6084/m9.figshare.5162119.