Abstract
Objective
The purpose of this study is to compare the characteristics of those ertapenem-treated adult patients with and without development of seizures, and identify the associated factors for the development of seizures.
Methods
This retrospective study was conducted at Chia-Yi Christian Hospital from January 2012 to December 2014. Patients developing seizures during their ertapenem treatment course were identified as case patients. Those without seizures who had received ertapenem for at least five days were considered as the pool of control patients. For each case patient, four matched patients from the control pool were randomly selected as the final control group, based on age, gender, and the date of ertapenem prescription.
Results
A total of 1706 ertapenem-treated patients were identified, 33 (1.9%) individuals developed seizures with the enrollment of 132 matched control patients. Among these 33 patients, the average age was 79.3 ± 7.5 years, and 20 (60.6%) were male. The mean Charlson co-morbidity score was 4.5 ± 2.4, and the first episode of seizure happened 3.3 ± 2.6 days after receiving ertapenem. In multivariate logistic regression analysis, the independent predictors associated with the development of ertapenem-associated seizures were old stroke (OR, 14.36; 95% CI, 4.38–47.02; p < 0.0001), undergoing brain images within one year prior to the admission (OR, 5.73; 95% CI, 1.78–18.43; p = 0.0034), low hemoglobin level (OR, 3.88; 95% CI, 1.28–12.75; p = 0.0165) and low platelet count (OR, 4,94; 95% CI, 1.56–15.68; p = 0.0067) at presentations, and protective factors against the development of seizures were heart failure (OR, 0.04; 95% CI, 0.00–0.63; p = 0.0222), concomitant use of steroids (OR, 0.19; 95% CI, 0.05–0.77; p = 0.0201), or antiplatelet agents (OR, 0.12; 95% CI, 0.02–0.63, p = 0.0123) with ertapenem.
Conclusions
The development of ertapenem-associated seizures may occur more frequently and much earlier due to its widespread use in treating drug-resistant pathogens, especially when these pathogens emerged worldwide.Our study would help physician to estimate the risk of developing seizure among patients receiving ertapenem.
Introduction
Carbapenems possess greatest broad-spectrum activity against gram-positive and gram-negative aerobic and anaerobic bacteria. Among them, imipenem, meropenem, ertapenem, and doripenem have been approved for clinical use in various countries, including Taiwan [1]. They have been proven to be effective against serious infections, including bloodstream infections, nosocomial pneumonia, intra-abdominal infections, and complicated urinary tract infections [2–5].
Although good tolerability has been observed, the most common side effects of carbapenems were gastrointestinal tract upset with an estimated incidence of around 1% to 5% [6–9]. In addition, seizures (abnormal excessive or synchronous electrical discharge in the brain with clinical presentations of involuntary motor movements) have been associated with all carbapenems [6–9]. The mechanism of carbapenem-associated seizures has been thought to be directly associated with the resemblance of the β-lactam ring with the conformation of the γ-aminobutyric acid (GABA) neurotransmitter and antagonism at the receptor site [10,11]. Although such an adverse reaction happens rarely, uncontrolled seizures could lead to major injury or even increased mortality due to impaired level of consciousness or loss of motor control [12].
In general, the incidence of seizures associated with carbapenems is as follows, in order of decreasing frequency: Meropenem (0.7%) [6], ertapenem (0.5%) [7], and imipenem (0.4%) [8], according to FDA-approved labeling; but compared to other carbapenems, the highest seizure rate has occurred with the use of imipenem (3.8%), in clinical trials [13]. However, in a meta-analysis study directly comparing imipenem and meropenem, no difference existed in the rate of seizures in pooled OR analyses [14]. Among those imipenem-treated patients, important risk factors for seizures identified were high dose therapy (> 25 mg/kg), renal impairment, and preexisting neurologic disorders [15].
Nevertheless, only a few case reports or case series discussing the association between ertapenem use and seizures have been published [16–18,19–23]. Thus, we designed a case-control study to investigate the factors associated with seizures in those adult patients receiving ertapenem therapy to provide much safer prescription of the antimicrobial agent.
Methods
Ethics statement
This study was reviewed and approved by the Institutional Review Board of Chia-Yi Christian Hospital (CYCH), a 1000-bed regional teaching hospital in southern Taiwan (Approval # CYCH-IRB-105066). The IRB waived informed consent due to the retrospective study design and the research posing no more than minimal risk. All primary data were collected according to procedures outlined in epidemiology guidelines to strengthen the reporting of observational studies.
Study design and settings
In CYCH, ertapenem was introduced in 2008. Physicians became aware of the growing number of patients developing seizures associated with the use of ertapenem thereafter. Accordingly, this retrospective 1:4 case-control study was carried out at CYCH to identify the associated factors for seizures in patients receiving ertapenem. The list of all patients aged 18 years or above admitted at CYCH and receiving at least one dose of ertapenem to treat their bacterial infections between January 2012 and December 2014 was retrieved from the computerized database of the Department of Pharmacy. For patients in the list, case patients were defined as those developing a seizure during the course of ertapenem treatment. The pool of control patients were those who did not develop seizures while receiving ertapenem and who were administered at least five days of ertapenem, as previous case series revealed that the first seizure episode occurred a mean of 6.7 days after start of ertapenem therapy [24]. For each case patient, 4 non-seizure controls with matching criteria such as sex, age (± 5 years), and the date of ertapenem prescription (± 30 days) were randomly selected from the control pool.
Data collection
Electronic and written medical records for all enrolled patients were reviewed. A standardized case report form was utilized to collect information on their demographics and clinical characteristics such as age, gender, underlying diseases, undergoing brain images due to neurologic symptoms or signs within one year prior to this admission, previous use of medications within one month prior to this hospitalization, initial vital signs and laboratory data before the use of ertapenem, intensive care unit (ICU) admission, infection syndromes treated by ertapenem, dosage and treatment duration of ertapenem, concomitant medications during the treatment course of ertapenem, length of hospital stay, and in-hospital mortality. Underlying diseases included neurologic comorbidities, such as old stroke, parkinsonism, dementia and epilepsy. For patients with seizures, the onset date of seizures, results of computed tomography (CT) scan of brain performed for the seizure, electroencephalograph (EEG) performed for the seizure, and management of seizures were also recorded.
Definitions
Seizures were defined as any abnormal motor movements with or without dyscognitive features, including focal and generalized type [12], and every episode of seizure was documented in medical records by the primary care physicians. Patients were considered to have chronic kidney disease if their baseline estimated glomerular filtration rate (eGFR) calculated by Modification of Diet in Renal Disease (MDRD) formula was less than 45 ml/min/1.73 m2. Utilization of some specific medications was categorized into the following: calcium channel blockers including amlodipine, verapamil, and diltiazem; angiotensin-converting enzyme inhibitors or angiotensin receptor blockers including captopril, valsartan, and losartan; diuretics including spironolactone, hydrochlorothiazide, trichlormethiazide, and furosemide; beta-blockers including propranolol, bisoprolol, carvedilol, atenolol, and labetalol; alpha-blockers including terazosin, and doxazosin; nitrates including isosorbide mononitrate, and isosorbide dinitrate; antimicrobial agents including levofloxacin, ceftibuten, amoxicillin, cefuroxime, amoxicillin/clavulanate, azithromycin, trimethoprim/sulfamethoxazole, ciprofloxacin, clarithromycin, norfloxacin, ceftazidime, fluconazole, vancomycin, aminoglycoside, metronidazole, cefotaxime, ampicillin, colimycin, oxacillin, micafungin, daptomycin, and fosfomycin; antiplatelet agents including aspirin, clopidogrel, dipyridamole and ticlopidine; prokinetic agents including metoclopramide, domperidone, and mosapride; sedative-hypnotics including fludiazepam, midazolam, diazepam, lorazepam, clonazepam, alprazolam, zopiclone, estazolam, and zolpidem; opioids including morphine, tramadol, meperidine, and fentanyl; non-steroid anti-inflammatory drugs including ketorolac, diclofenac, etoricoxib, and celecoxib. During the study period, the recommended ertapenem doses were as described in the package insert of ertapenem [7], i.e., the dose of ertapenem given was one gram once a day in those patients with normal renal function and 500 mg daily if their creatinine clearance ≦ 30 ml/min/1.73m2, calculated by Cockcroft & Gault equation.
Statistical analysis
Continuous variables were described as means and standard deviations, and analyzed using a Mann-Whitney U-test. Categorical variables were expressed as frequency and proportions, and compared with a chi-square test or Fisher’s exact test if the expected number was less than or equal to 10. Factors associated with the development of seizure were identified using a conditional logistic regression model. All potential variables associated were tested using univariate analysis first. Factors with a p value less than 0.2 were then included in the multivariate analysis using the backward stepwise method. All tests were two-tailed and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 software (International Business Machines Corp., Armonk, NY, USA).
Results
Baseline characteristics of enrolled patients
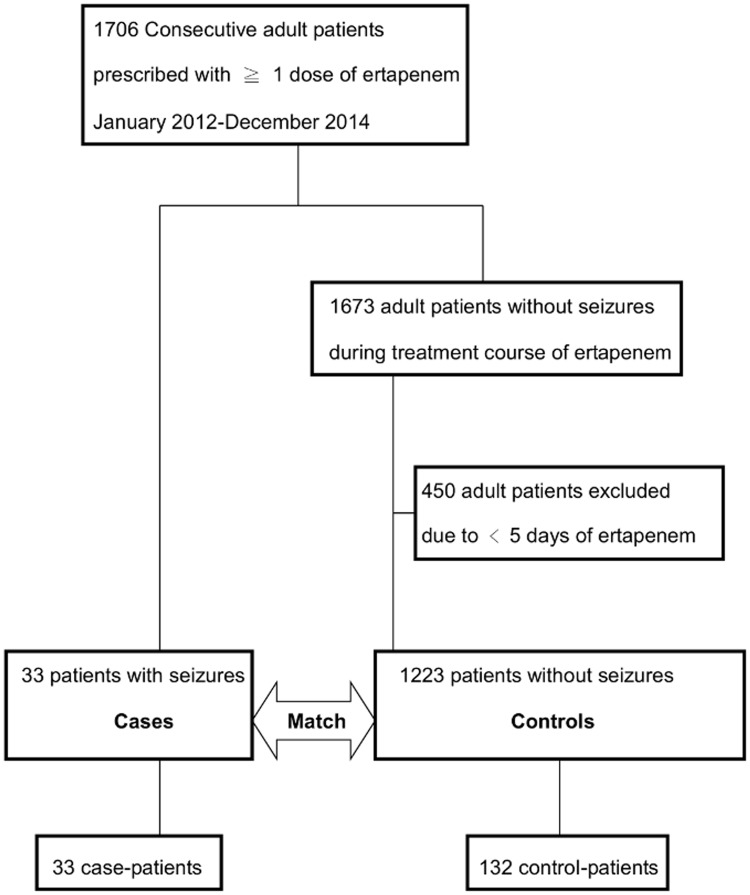
During the 3-year study period, a total of 1706 adult patients were prescribed with at least one dose of ertapenem therapy at CYCH, and seizures developed in 33 patients (1.9%, case patients) following the administration of ertapenem. Among the 1673 patients without seizures (designated as the pool of controls), 450 patients were excluded because they received less than five days of ertapenem. Out of the remaining 1223 potential controls, 132 were matched by sex, age, and the date of prescription of ertapenem to the 33 cases patients to survey the associated factors of attacks of seizures (Fig 1). The baseline characteristics of the 165 enrolled patients are summarized in Tables 1 and 2 (S1 File). Compared with control patients, case patients were more likely to have a history of old stroke (either hemorrhage or infarct), epilepsy, and receiving brain image studies within one year prior to this admission, and a higher Charlson comorbidity score. Concerning prior exposure to various medications, fewer case patients were prescribed with sedative-hypnotics than with the controls (p = 0.044), but no significant difference existed within other categories of drugs. Additionally, a lower proportion of case patients received concomitant use of steroids with ertapenem, but no other categories of medications concomitantly used with ertapenem differed significantly. Lower blood glucose, and more patients with anemia (hemoglobin < 11 g/dl) or thrombocytopenia (platelets < 150k /mm3) were observed in the case patients.
Fig 1. Flow chart of participants with or without seizures included in the analysis.
Table 1. Clinical characteristics in matched pairs (1:4) of those ertapenem-treated patients with or without development of seizures.
| Variables | Patients with seizures | Patients without siezures | p-value |
|---|---|---|---|
| No. of patients | 33 | 132 | - |
| Gender (male) | 20 (60.6) | 80 (60.6) | - |
| Age | 79.3±7.5 | 78.8±7.7 | 0.7116† |
| Hypertension | 23 (69.7) | 78 (59.1) | 0.2634¥ |
| Diabetes mellitus | 13 (39.4) | 58 (43.9) | 0.6371¥ |
| Old stroke | 22 (66.7) | 32 (24.2) | <.0001¥ |
| Cerebrovascular disease type | <.0001¥ | ||
| Intracerebral hemorrhage | 10 (30.3) | 9 (6.8) | |
| Ischemic infarction | 12 (36.4) | 23 (17.4) | |
| Parkinsonism | 5 (15.2) | 15 (11.4) | 0.5556¥ |
| Dementia | 8 (24.2) | 24 (18.2) | 0.4309¥ |
| Epilepsy | 4 (12.1) | 3 (2.3) | 0.0302¥ |
| Heart failure | 1 (3.0) | 18 (13.6) | 0.1264¥ |
| Coronary artery disease | 2 (6.1) | 23 (17.4) | 0.1034¥ |
| Peripheral arterial occlusive disease | 3 (9.1) | 4 (3.0) | 0.1437¥ |
| Cancer | 12 (36.4) | 30 (22.7) | 0.1077¥ |
| Gout | 3 (9.1) | 14 (10.6) | - |
| Liver cirrhosis | 6 (18.2) | 14 (10.6) | 0.2402¥ |
| Chronic obstructive pulmonary disease | 1 (3.0) | 17 (12.9) | 0.1277¥ |
| Peptic ulcer disease | 11 (33.3) | 45 (34.1) | 0.9345¥ |
| Chronic kidney disease | 9 (27.3) | 31 (23.5) | 0.6497¥ |
| End stage renal disease | 2 (6.1) | 11 (8.3) | - |
| Charlson score | 4.5±2.4 | 3.4±2.5 | 0.0244† |
| Brain image within one year prior to this admission | 16 (48.5) | 28 (21.2) | 0.0015¥ |
| Intracranial hemorrhage | 2 | 1 | 0.5920¥ |
| Infarction | 14 | 23 | 0.9919¥ |
| Hydrocephalus | 9 | 12 | 0.3977¥ |
| Chronic subdural effusion | 0 | 1 | - |
| Meningioma | 0 | 1 | - |
| Initial vital signs before use of ertapenem | |||
| Conscious change | 8 (24.2) | 20 (15.2) | 0.2134¥ |
| Systolic blood pressure | 136.8±28.3 | 132.6±31.9 | 0.4886† |
| Diastolic blood pressure | 76.4±18.4 | 72.7±16.8 | 0.2653† |
| Heart rate | 99.0±17.4 | 94.6±19.5 | 0.2410† |
| Body temperature | 37.3±1.3 | 37.4±1.1 | 0.7166† |
| Respiratory rate | 23.2±4.5 | 21.7±7.4 | 0.1433† |
| Initial lab data before use of ertapenem | |||
| Blood sugar | 147.7±58.7 | 184.5±107.9 | 0.0184† |
| White blood count | 8520.1±6007.1 | 10818.8±5986.3 | 0.0503† |
| White blood count < 4000 or > 11000 | 18 (54.6) | 66 (50.0) | 0.6404¥ |
| Hemoglobin | 10.5±4.0 | 10.8±2.2 | 0.7137† |
| Hemoglobin < 11 g/dl | 26 (78.8) | 67 (50.8) | 0.0037¥ |
| Platelet | 178.1±102.1 | 205.0±103.3 | 0.1873† |
| Platelet < 150k/mm3 | 16 (50.0) | 40 (30.3) | 0.0350¥ |
| C-reactive protein | 10.5±8.3 | 9.4±8.7 | 0.6071† |
| Aspartate aminotransferase | 79.1±106.5 | 46.7±53.3 | 0.1794† |
| Alanine aminotransferase | 79.0±147.1 | 50.5±130.5 | 0.3374† |
| Blood urea nitrogen | 39.0±33.0 | 35.4±31.3 | 0.5767† |
| Creatinine | 1.9±2.1 | 1.9±1.8 | 0.9885† |
| Creatinine > 1.4 | 10 (32.3) | 53 (41.7) | 0.3341¥ |
| Sodium | 135.9±12.1 | 136.1±7.4 | 0.9035† |
| Potassium | 3.7±0.8 | 3.8±0.6 | 0.4548† |
| Baseline creatinine | 1.6±2.3 | 1.2±1.0 | 0.2864† |
| Intensive care unit admission | 10 (30.3) | 37 (28.0) | 0.7958¥ |
| Recommended dose | 20 (60.6) | 98 (74.2) | 0.1206¥ |
| Duration of hospitalization, days | 24.2±17.1 | 19.3±12.4 | 0.1250† |
| In-hospital mortality | 11 (33.3) | 14 (10.6) | 0.0011¥ |
| The mean time-to-onset of seizures, days | 3.3±2.6 | - | |
| Computed tomography scan of brain | 17 (51.5) | - | |
| Electroencephalography | 24 (72.7) | - | |
| Medications for control of seizures (31) | |||
| Benzodiazepines | 23 (74.2) | - | |
| Antiepileptic drugs | 30 (96.8) | - |
† Student t;
¥ Chi-Square test.
Table 2. Prior and concomitant medications used in those ertapenem-treated patients with or without development of seizures.
| Variables | Patients with seizures (33) | Patients without siezures(132) | p-value |
|---|---|---|---|
| Previous drug history within one month | 29 (87.9) | 98 (74.2) | 0.0961¥ |
| Calcium channel blocker | 15 (45.5) | 43 (32.6) | 0.1658¥ |
| ACEI or ARB | 4 (12.1) | 22 (16.7) | 0.5215¥ |
| Diuretics | 6 (18.2) | 26 (19.7) | 0.8439¥ |
| Beta blocker | 2 (6.1) | 15 (11.4) | 0.5286¥ |
| Alpha blocker | 1 (3.0) | 10 (7.6) | 0.6954¥ |
| Harnalidge | 4 (12.1) | 12 (9.1) | 0.5293¥ |
| Nitrate | 1 (3.0) | 10 (7.6) | 0.6954¥ |
| Statin | 3 (9.1) | 9 (6.8) | 0.7081¥ |
| Antibiotics | 7 (21.2) | 19 (14.4) | 0.3363¥ |
| H2-blocker | 9 (27.3) | 33 (25.0) | 0.7886¥ |
| Proton pump inhibitors | 4 (12.1) | 23 (17.4) | 0.4614¥ |
| Steroid | 3 (9.1) | 17 (12.9) | 0.7673¥ |
| Antiplatelet agents | 5 (15.2) | 31 (23.5) | 0.2999¥ |
| Prokinetic agents | 9 (27.3) | 29 (22.0) | 0.5175¥ |
| Drugs for peripheral vascular disorder | 3 (9.1) | 16 (12.1) | 0.7677¥ |
| Sedative/hypnotics | 2 (6.1) | 28 (21.2) | 0.0435¥ |
| Concomitant drugs with ertapenem | 33 (100.0) | 130 (98.5) | - |
| Proton pump inhibitors | 17 (51.5) | 52 (39.4) | 0.2067¥ |
| H2-blockers | 11 (33.3) | 49 (37.1) | 0.6858¥ |
| Prokinetic agents | 12 (36.4) | 41 (31.1) | 0.5595¥ |
| Harnalidge | 2 (6.1) | 15 (11.4) | 0.5286¥ |
| Albumin | 6 (18.2) | 27 (20.5) | 0.7703¥ |
| Opioids | 6 (18.2) | 32 (24.2) | 0.4595¥ |
| Non-steroid anti-inflammatory drug | 0 (0.0) | 9 (6.8) | 0.2067¥ |
| Steroid | 4 (12.1) | 41 (31.1) | 0.0289¥ |
| Inhaled bronchodilators | 14 (42.4) | 47 (35.6) | 0.4680¥ |
| Beta-blockers | 2 (6.1) | 18 (13.6) | 0.3711¥ |
| Diuretics | 10 (30.3) | 46 (34.9) | 0.6218¥ |
| Calcium channel blockers | 10 (30.3) | 51 (38.6) | 0.3751¥ |
| Statin | 2 (6.1) | 6 (4.6) | 0.6609¥ |
| Nitrate | 3 (9.1) | 10 (7.6) | 0.7249¥ |
| Cordarone | 1 (3.0) | 10 (7.6) | 0.6954¥ |
| Knowful | 4 (12.1) | 10 (7.6) | 0.4826¥ |
| Antiplatelet agents | 3 (9.1) | 28 (21.2) | 0.1108¥ |
| Drugs for peripheral vascular disorder | 2 (6.1) | 8 (6.1) | - |
| Transamine | 7 (21.2) | 18 (13.6) | 0.2776¥ |
| Sedative/hypnotics | 5 (15.2) | 25 (18.9) | 0.6138¥ |
| Antibiotics | 6 (18.2) | 32 (24.2) | 0.4595¥ |
| Contrast medium | 2 (6.1) | 20 (15.2) | 0.2529¥ |
¥ Chi-Square test.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Ertapenem was mainly used to treat patients with a diagnosis of UTI, followed by pneumonia, and application of this antimicrobial agent showed no significant difference between case and control patients. Around 60% (20/33) of case patients and approximately three-fourths of control patients received the recommended doses, and the proportion of ICU admission initially was similar (30.3% vs. 28.0%). In this study, 11 case subjects and 29 control patients received normal dose based on their renal function. Otherwise, lower dose than recommended was prescribed in 2 cases and 5 controls, respectively. No association with subtherapeutic dose and the development of seizure existed (p = 0.85). No patients in the case or control group received supratherapeutic dose. Concerning treatment outcome, longer length of hospitalization and higher crude in-hospital mortality were noted in the case group (p = 0.0011). The median duration of ertapenem therapy was 4 days (IQR, 2–6), and 8 days (IQR, 7–11) in case subjects and control patients, respectively.
The first episode of seizure among case patients occurred within 3.3 ± 2.6 days after initiation of ertapenem treatment. Of these 33 patients with the development of seizures, 23 individuals presented as generalized tonic clonic seizure, 9 as focal seizure, and one as absence seizure. CT scans of the brain and EEGs were arranged for 17 (51.5%) and 24 (72.7%) patients, respectively. The CT findings included infarction in 15, hydrocephalus in 9, normal results in 2, and hemorrhage in one; and the EEGs showed diffuse cortical dysfunction in 22, epileptogenic discharge in 5, normal results in one, and generalized sharp waves in one. Only two patients did not take medications used to control the seizures. Of 31 patients receiving medical therapy for their ertapenem-associated seizure, 23 (74.2%) cases took benzodiazepines, including diazepam, midazolam and lorazepam, and 30 (96.8%) individuals were prescribed with at least one of the following antiepileptic drugs: phenytoin (21 patients, 67.7%), valproic acid (11, 35.5%), levetiracetam (9, 29%), and topiramate (1, 3.2%). Ten (30.3%) patients were admitted to the ICU initially, and the mean of hospital stay and the crude in-hospital mortality rate were 24.2 ± 17.1 days and 33.3%, respectively.
Factors associated with the development of seizures among patients treated with ertapenem
In univariate analysis using a conditional logistic regression model, old stroke, epilepsy, undergoing brain images within one year prior to this admission, a higher Charlson co-morbidity score, lower hemoglobin level, lower platelet count, and no concomitant use of steroids with ertapenem were associated with the development of seizures during ertapenem treatment (Table 3). Multivariate analysis identified old stroke (OR, 14.36; 95% CI, 4.38–47.02; p < 0.0001), undergoing brain images within one year prior to this admission (OR, 5.73; 95% CI, 1.78–18.43; p = 0.0034), lower hemoglobin level (OR, 3.88; 95% CI, 1.28–12.75; p = 0.0165), and lower platelet count (OR, 4,94; 95% CI, 1.56–15.68; p = 0.0067) as significant predictors for the development of seizure. However, heart failure (OR, 0.04; 95% CI, 0.00–0.63; p = 0.0222), use of steroids (OR, 0.19; 95% CI, 0.05–0.77; p = 0.0201) or antiplatelet agents concurrentwith ertapenem (OR, 0.12; 95% CI, 0.02–0.63, p = 0.0123) were independently protective factors.
Table 3. Multivariate analysis for factors associated with the development of seizures in patients treated with ertapenem.
| Covariate | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Old stroke | 6.17 | (2.71–14.04) | <.0001 | 14.36 | (4.38–47.02) | <.0001 |
| Epilepsy | 5.84 | (1.25–27.30) | 0.0249 | |||
| Heart failure | 0.20 | (0.03–1.55) | 0.1228 | 0.04 | (0.00–0.63) | 0.0222 |
| Coronary artery disease | 0.31 | (0.07–1.37) | 0.1225 | |||
| peripheral arterial occlusive disease | 3.17 | (0.68–14.81) | 0.1423 | |||
| Malignancy | 1.94 | (0.86–4.37) | 0.1124 | |||
| chronic obstructive pulmonary disease | 0.21 | (0.03–1.66) | 0.1393 | |||
| Charlson score | 1.18 | (1.02–1.37) | 0.0280 | |||
| Brain image within one year before this admission | 3.46 | (1.56–7.69) | 0.0022 | 5.73 | (1.78–18.43) | 0.0034 |
| Prior use of calcium channel blocker within 1 month | 1.72 | (0.79–3.73) | 0.1698 | |||
| Prior use of sedative or hypnotics within 1 month | 0.24 | (0.05–1.07) | 0.0609 | |||
| Initial hemoglobin < 11 g/dl | 3.58 | (1.46–8.79) | 0.0055 | 3.88 | (1.28–11.75) | 0.0165 |
| Initial platelet < 150k/mm3 | 2.29 | (1.05–5.01) | 0.0384 | 4.94 | (1.56–15.68) | 0.0067 |
| Recommended dose | 0.54 | (0.24–1.19) | 0.1250 | |||
| Concomitant use of steroid | 0.31 | (0.10.0.93) | 0.0369 | 0.19 | (0.05–0.77) | 0.0201 |
| Concomitant use of antiplatelet agents | 0.37 | (0.11–1.31) | 0.1240 | 0.12 | (0.02–0.63) | 0.0123 |
| Concomitant use of contrast medium | 0.36 | (0.08–1.63) | 0.1868 | |||
Discussion
To our knowledge, this study was the first matched case-control study to compare the clinical characteristics of patients with or without development of seizures during the treatment course of ertapenem, and investigate the predictors for the development of seizures among these patients. The proportion of patients developing seizures among those individuals treated with ertapenem was about 1.9% in the present study, and the interval from the initiation of ertapenem to the onset of seizure was 3.3 ± 2.6 days. The independent predictors associated with the development of seizures were old stroke, undergoing brain images within one year previous to this admission, lower hemoglobin level, and lower platelet count; and heart failure, concomitant use of steroid or antiplatelet agent with ertapenem were protective factors from seizures.
Several preceding clinical trials have reported that the proportion developing seizure among those patients treated with ertapenem was around 0.1% [24] to 0.5% [25], much lower than that in our study (1.9%). This might be because patients enrolled in our study were older (mean age, 71 vs. <60 years), and old patients at higher risk for ertapenem-associated seizures has been illustrated previously [19,24,26]. In addition, the interval from the administration of ertapenem to the onset of seizures was shorter than that noted in prior clinical trials [16–18]. As we understand it, multiple complicated co-morbidities were excluded in previous clinical trials, such as chronic kidney disease [16], thrombocytopenia [16,17], or malignancy [17]; all of these were noted among our studied patients. That might be the reason why earlier development of seizures occurred in our ertapenem-treated patients, but the actual mechanism for latency from symptom onset to diagnosis of seizures should be further surveyed.
Several prior studies have demonstrated that pre-existing central nervous system (CNS) disorders, including old cerebrovascular accidents (either thrombotic or embolic events), were risk factors for the development of seizures among ertapenem-treated patients [20–23]. One possible hypothesis was that ertapenem might act as a mediator lowering the seizure threshold in patients with previous neurological comorbidities [22]. Another deduction has been that damage to the blood-brain barrier with a subsequently higher concentration of ertapenem in the brain tissue, observed in patients with a history of cerebrovascular events, would result in development of antibiotic-related seizures [27]. Our study echoed this finding, and the requirement of neuroimages for neurologic manifestations within one year prior to this hospitalization, which might imply pre-existing CNS disorders, also increased the risk of seizures.
If, clinically, some surrogate markers or laboratory parameters could be recognized to predict the potential of developing antibiotic-associated seizures, it would be helpful for physicians to be alert to, detect, and then manage them as soon as possible. Among pediatric patients, iron deficiency anemia (hemoglobin value < 11 g/dl), as a significant risk factor for febrile seizures has been clarified in some case-control studies [28–30]. Low hemoglobin might impair oxygen delivery to brain tissue, which in turn might contribute to the development of brain dysfunction, including the development of seizures. However, the detailed mechanisms need further investigation.
Berggren et al. observed that thrombocytopenia was more common among those alcohol-dependent individuals with development of seizures compared to those without alcohol-related seizures: Numminen et al. showed thrombocytopenia correlated with the onset of brain infarction in alcoholics; and Kim et al reported that 62.5% of patients had thrombocytopenia when they developed seizures [31–33]. All these findings supported that thrombocytopenia is possibly one of the underlying mechanisms contributing to the development of seizures. In addition, thrombocytopenia might be a surrogate marker of poor general condition. And patients with poorer general condition might thus more easily develop seizures while they receive ertapenem.
The immunomodulatory or anti-inflammatory properties of corticosteroids made them effective for treatment of seizures or epilepsy, with the first report published in 1942 [34–36]. Corticosteroids can suppress the corticotropin-releasing hormone levels in the CNS and then lower the neuronal excitability; also, they can affect GABAA receptors or enhance the action of neurosteroids [37]. Thus, it is reasonable that concomitant use of steroids with ertapenem would protect those patients from developing seizures, as shown in our study.
Several recent clinical reviews have emphasized activation of the inflammatory process that is probably involved with the occurrence of epilepsy [38–40]. Among atherothrombotic diseases, such as myocardial infarction or ischemic stroke, platelets contribute to vascular inflammation, so use of antiplatelet agents would manifest an anti-inflammatory effect by attenuating the release of inflammatory mediators [41]. Furthermore, acetylsalicylic acid (ASA) in high concentrations has had direct neuroprotective effects in animal models [42], and even ASA itself or in combination with anti-epileptic drugs lowers the incidence of seizures [43–45]. Therefore, the protective effects of antiplatelet agents occurring concurrently with the use of ertapenem from the development of seizures as demonstrated in our results is reasonable, but the actual platelet-mediated inflammatory pathways in the CNS correlated with seizures caused by ertapenem should be surveyed in the future.
Heart failure has been well recognized as a complex clinical syndrome resulting from structural or functional impairment of ventricular filling or ejection of blood. As it occurs, subsequent compensatory mechanisms that activate the renin-angiotensin-aldosterone system would start to restore adequate cardiac output [46,47]. Xu B et al. have demonstrated that angiotensin II in the central nervous system potentiates GABA release, which in turn increases the seizure threshold [48]. This might partially explain why patients with heart failure would be less likely to develop seizure while receiving ertapenem.
There were limitations in our study. First, it was retrospective and conducted at a single center, so unavoidable bias, confounding, and missing data, and generalization cautiously would be anticipated. In particular, history of old stroke or epilepsy could be only documented by medical records. It was difficult to verify the information about if these patients with a history of old stroke or epilepsy had acute symptomatic seizures in their life previously or had a lesion due to a stroke visible on brain image studies, which might lead to imprecise estimation of our statistics. Additionally, the history of febrile or afebrile seizures in patients or families might be a risk factor for such drug induced seizures, but acquiring the information was not feasible in our retrospective study. Second, drug concentrations of ertapenem in the serum and cerebral tissues were not calculated, so it was difficult to judge the impact of drug levels on the development of seizures. Finally, some patients receiving brain image studies within one year prior to this admission, but we cannot clarify whether these lesions were epileptogenic and would therefore directly cause subsequent episodes of seizure or not. Nonetheless, our study results remain useful for clinical physicians in dealing with ertapenem-treated patients because we have identified several factors associated with the development of seizure among these patients. This should alert clinicians to detect and manage seizure disorders as soon as possible.
In conclusion, with the widespread use of ertapenem due to the increasing rate of resistant pathogens, ertapenem-associated seizures, in the elderly particularly, would be expected to occur more frequently, and even much earlier. Our study results may help clinicians to estimate the risk of developing seizures when ertapenem is prescribed to treat bacterial infections in those patients, and to prescribe ertapenem more safely.
Supporting information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Doi Y, Chambers HF. Bennett JE, Dolin R, Blaser MJ, editors. Other β-Lactam Antibiotics. Chapter 22. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. The Elsevier Saunders, 8th ed Philadelphia, PA:2015. [Google Scholar]
- 2.Balfour JA, Bryson HM, Brogden RN. Imipenem/cilastatin: an update of its antibacterial activity, pharmacokinetics and therapeutic efficacy in the treatment of serious infections. Drugs 1996; 51(1):99–136. [DOI] [PubMed] [Google Scholar]
- 3.Mohr JF 3rd. Update on the efficacy and tolerability of meropenem in the treatment of serious bacterial infections. Clin Infect Dis 2008; 47 Suppl 1:S41–51. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM, Perry CM. Ertapenem: a review of its use in the treatment of bacterial infections. Drugs 2005; 65(15):2151–78. [DOI] [PubMed] [Google Scholar]
- 5.Mandell L. Doripenem: a new carbapenem in the treatment of nosocomial infection. Clin Infect Dis 2009; 49 Suppl 1:S1–3. [DOI] [PubMed] [Google Scholar]
- 6.Merrem [package insert]. Wilmington, DE: Astra Zeneca, 2010. [Google Scholar]
- 7.Invanz [package insert]. Whitehouse Station, NJ: Merck & Co., Inc., 2012. [Google Scholar]
- 8.Primaxin [package insert]. Whitehouse Station, NJ: Merck & Co., Inc., 2012. [Google Scholar]
- 9.Doribax [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc., 2007. [Google Scholar]
- 10.Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 2005; 24(10):649–53. doi: 10.1007/s10096-005-0021-y [DOI] [PubMed] [Google Scholar]
- 11.De Sarro A, De Sarro GB, Ascioti C, Nisticó G. Epileptogenic activity of some beta-lactam derivatives: structure-activity relationship. Neuropharmacology 1989; 28(4):359–65. [DOI] [PubMed] [Google Scholar]
- 12.Lowenstein DH. Harrison TR, Kasper DL, Hauser SL. Seizures and Epilepsy Chapter 445. Harrison's Principles of Internal Medicine. The McGraw-Hill Education, 19th ed the United States:2012. [Google Scholar]
- 13.Redman R, File TM Jr. Safety of intravenous infusion of doripenem. Clin Infect Dis 2009; 49 Suppl 1:S28–35. [DOI] [PubMed] [Google Scholar]
- 14.Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother 2014; 69(8):2043–55. doi: 10.1093/jac/dku111 [DOI] [PubMed] [Google Scholar]
- 15.Calandra GB, Ricci FM, Wang C, Brown KR. The efficacy results and safety profile of imipenem/cilastatin from the clinical research trials. J Clin Pharmacol 1988; 28(2):120–7. [DOI] [PubMed] [Google Scholar]
- 16.Solomkin JS, Yellin AE, Rotstein OD, Christou NV, Dellinger EP, Tellado JM et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg 2003; 237(2):235–45. doi: 10.1097/01.SLA.0000048551.32606.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz-Ruiz G, Caballero-Lopez J, Friedland IR, Woods GL, Carides A; Protocol 018 Ertapenem Community-Acquired Pneumonia Study Group. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin Infect Dis 2002; 34(8):1076–83. doi: 10.1086/339543 [DOI] [PubMed] [Google Scholar]
- 18.Vetter N, Cambronero-Hernandez E, Rohlf J, Simon S, Carides A, Oliveria T et al. A prospective, randomized, double-blind multicenter comparison of parenteral ertapenem and ceftriaxone for the treatment of hospitalized adults with community-acquired pneumonia. Clin Ther 2002; 24(11):1770–85. [DOI] [PubMed] [Google Scholar]
- 19.Seto AH, Song JC, Guest SS. Ertapenem-associated seizures in a peritoneal dialysis patient. Ann Pharmacother 2005; 39(2):352–6. doi: 10.1345/aph.1E421 [DOI] [PubMed] [Google Scholar]
- 20.Saidel-Odes L, Borer A, Riesenberg K, Smolyakov R, Schlaeffer F. History of cerebrovascular events: a relative contraindication to ertapenem treatment. Clin Infect Dis 2006; 43(2):262–3. doi: 10.1086/505304 [DOI] [PubMed] [Google Scholar]
- 21.Lunde JL, Nelson RE, Storandt HF. Acute seizures in a patient receiving divalproex sodium after starting ertapenem therapy. Pharmacotherapy 2007; 27(8):1202–5. doi: 10.1592/phco.27.8.1202 [DOI] [PubMed] [Google Scholar]
- 22.Fica AE, Abusada NJ. Seizures associated with ertapenem use in patients with CNS disorders and renal insufficiency. Scand J Infect Dis 2008; 40(11–12):983–5. doi: 10.1080/00365540802375570 [DOI] [PubMed] [Google Scholar]
- 23.Ong C, Chua AC, Tambyah PA, Fei YS. Seizures associated with ertapenem. Int J Antimicrob Agents 2008; 31(3):290 doi: 10.1016/j.ijantimicag.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 24.Miller AD, Ball AM, Bookstaver PB, Dornblaser EK, Bennett CL. Epileptogenic potential of carbapenem agents: mechanism of action, seizure rates, and clinical considerations. Pharmacotherapy 2011; 31(4):408–23. doi: 10.1592/phco.31.4.408 [DOI] [PubMed] [Google Scholar]
- 25.Teppler H, Gesser RM, Friedland IR, Woods GL, Meibohm A, Herman G et al. Safety and tolerability of ertapenem. J Antimicrob Chemother 2004; 53 Suppl 2:ii75–81. [DOI] [PubMed] [Google Scholar]
- 26.Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central Nervous System Toxicity Associated with Ertapenem Use. Ann Pharmacother 2011; 45:e6 doi: 10.1345/aph.1P528 [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Chew ST. Status Epilepticus and Delirium Associated with Ertapenem in a Very Elderly Patient with Chronic Kidney Disease and Silent Ischaemic Cerebrovascular Disease. Drug Saf Case Rep. 2015; 2(1): 19 doi: 10.1007/s40800-015-0021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartfield DS, Tan J, Yager JY, Rosychuk RJ, Spady D, Haines C et al. The association between iron deficiency and febrile seizures in childhood. Clin Pediatr 2009; 48(4):420–6. [DOI] [PubMed] [Google Scholar]
- 29.Kumari PL, Nair MK, Nair SM, Kailas L, Geetha S. Iron deficiency as a risk factor for simple febrile seizures—a case control study. Indian Pediatr 2012; 49(1):17–9. [DOI] [PubMed] [Google Scholar]
- 30.Fallah R, Tirandazi B, Akhavan Karbasi S, Golestan M. Iron deficiency and iron deficiency anemia in children with febrile seizure. Iran J Ped Hematol Oncol 2013; 3(1):200–3. [PMC free article] [PubMed] [Google Scholar]
- 31.Berggren U, Fahlke C, Berglund KJ, Blennow K, Zetterberg H, Balldin J. Thrombocytopenia in early alcohol withdrawal is associated with development of delirium tremens or seizures. Alcohol Alcohol 2009; 44(4):382–6. doi: 10.1093/alcalc/agp012 [DOI] [PubMed] [Google Scholar]
- 32.Numminen H, Hillbom M, Juvela S. Platelets, alcohol consumption, and onset of brain infarction. J Neurol Neurosurg Psychiatry 1996; 61(4):376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim W, Kwon SY, Cho AH, Lim SC, Kim YI, Shon YM. Effectiveness of topiramate in medically complicated patients with status epilepticus or acute refractory seizures. J Epilepsy Res 2011; 1(2):52–6. doi: 10.14581/jer.11010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQuarrie I, Anderson JA, Ziegler MR. Observations on the Antagonistic Effects of Posterior Pituitary and Cortico-Adrenal Hormones in the Epileptic Subject. J Clin Endocrinol 1942; 2:406–410. [Google Scholar]
- 35.Laswell EM, Chambers KD, Whitsel DR, Poudel K. New-onset refractory status epilepticus in an adult with an atypical presentation of cat-scratch disease: successful treatment with high-dose corticosteroids. Pharmacotherapy 2015; 35(6):e106–10. doi: 10.1002/phar.1595 [DOI] [PubMed] [Google Scholar]
- 36.Pera MC, Randazzo G, Masnada S, Dontin SD, De Giorgis V, Balottin U et al. Intravenous methylprednisolone pulse therapy for children with epileptic encephalopathy. Funct Neurol 2015; 30(3):173–9. doi: 10.11138/FNeur/2015.30.3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Özkara Ç, Vigevano F. Immuno- and antiinflammatory therapies in epileptic disorders. Epilepsia 2011; 52 Suppl 3:45–51. [DOI] [PubMed] [Google Scholar]
- 38.Aarli JA. Epilepsy and the immune system. Arch Neurol 2000; 57(12):1689–92. [DOI] [PubMed] [Google Scholar]
- 39.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005; 46(11):1724–43. doi: 10.1111/j.1528-1167.2005.00298.x [DOI] [PubMed] [Google Scholar]
- 40.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol 2011; 7(1):31–40. doi: 10.1038/nrneurol.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Lüscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med 2007; 12(2):113–22. doi: 10.1177/1358863X07077462 [DOI] [PubMed] [Google Scholar]
- 42.Moro MA, De Alba J, Cárdenas A, De Cristóbal J, Leza JC, Lizasoain I et al. Mechanisms of the neuroprotective effect of aspirin after oxygen and glucose deprivation in rat forebrain slices. Neuropharmacology 2000; 39(7):1309–18. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava AK, Gupta YK. Aspirin modulates the anticonvulsant effect of diazepam and sodium valproate in pentylenetetrazole and maximal electroshock induced seizures in mice. Indian J Physiol Pharmacol 2001; 45(4):475–80. [PubMed] [Google Scholar]
- 44.Tandon M, Anuradha K, Pandhi P. Evaluation of antiepileptic activity of aspirin in combination with newer antiepileptic lamotrigine in mice. Methods Find Exp Clin Pharmacol 2003; 25(8):607–10. [DOI] [PubMed] [Google Scholar]
- 45.Dhir A, Naidu PS, Kulkarni SK. Effect of cyclooxygenase inhibitors on pentylenetetrazol (PTZ)-induced convulsions: Possible mechanism of action. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30(8):1478–85. doi: 10.1016/j.pnpbp.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 46.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009; 54(19):1747–62. doi: 10.1016/j.jacc.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 47.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol 2012; 59(2):117–22. doi: 10.1016/j.jjcc.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Li H. Brain mechanisms of sympathetic activation in heart failure: Roles of the renin-angiotensin system, nitric oxide and pro-inflammatory cytokines (Review). Mol Med Rep 2015; 12(6):7823–9. doi: 10.3892/mmr.2015.4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.