Abstract
Objectives: Tenofovir alafenamide, a prodrug of tenofovir, produces higher PBMC concentrations of tenofovir diphosphate (tenofovir-dp) than tenofovir disoproxil fumarate. To understand tenofovir alafenamide’s mucosal tissue distribution and its implications for pre-exposure prophylaxis, we characterized tenofovir-dp in female genital tract (FGT) and lower gastrointestinal (GI) tissues.
Methods: Healthy seronegative women were given 5, 10 or 25 mg of tenofovir alafenamide (n = 8/group). Each participant provided plasma, PBMC and cervical, vaginal and rectal tissue samples over 14 days. Plasma, cell lysate and tissue homogenate concentrations were analysed by LC-MS/MS. Dose proportionality was declared in plasma and PBMCs if the natural log AUC versus natural log dose regression line 90% CI was within 0.57–1.43. In vitro tenofovir-dp formation was assessed in PBMCs and ectocervical (Ect1/E6E7) and vaginal (VK2/E6E7) cells incubated in 0.5 and 10 μM tenofovir alafenamide or tenofovir. clinicaltrials.gov: NCT02357602.
Results: Following single doses of 5, 10 and 25 mg, median (IQR) tenofovir plasma AUC0–14 days was 52.8 (49.5–59.6), 78.1 (68.2–86.9) and 169.7 (131.2–211.4) ng·h/mL and tenofovir-dp PBMC AUC0–14 days was 2268 (1519–4090), 4584 (3113–5734) and 9306 (6891–10785) fmol·h/106 cells, respectively. Tenofovir was quantifiable in 52% and 92% of FGT and GI tissues, whereas tenofovir-dp was quantifiable in only 5% and 19% of FGT and GI tissues, respectively. Plasma tenofovir and PBMC tenofovir-dp were dose proportional (90% CI = 0.87–1.15 and 0.62–1.02, respectively). In vitro tenofovir-dp was 1.7–17-fold higher in epithelial cells than PBMCs.
Conclusions: After tenofovir alafenamide dosing in vivo, tenofovir-dp was unquantifiable in most tissues (91%) although cervical and vaginal epithelial cells efficiently formed tenofovir-dp from tenofovir alafenamide in vitro. These findings warrant further investigation of tenofovir alafenamide’s pharmacology.
Introduction
Globally, 50% of HIV-infected individuals are women.1 This high prevalence may be partly due to an inability for women to negotiate HIV prevention methods such as condoms.2 Developing preventative agents that women control is essential in lowering the global HIV burden. Recently, the US FDA approved tenofovir disoproxil fumarate with emtricitabine as once-daily pre-exposure prophylaxis (PrEP).3 The efficacy of daily oral tenofovir disoproxil fumarate with or without emtricitabine for PrEP, has been investigated in several large clinical trials with mixed results in women.4–9 Low adherence has been noted as the reason for PrEP futility in some female study populations where <30% of women exhibited pharmacokinetic (PK) evidence of recent product use.8,9 However, tenofovir disoproxil fumarate with emtricitabine demonstrated a 44% reduction in HIV incidence among MSM and transgender women, despite similarly poor rates of detectable drug concentrations.6
Recent PK modelling of tenofovir disoproxil fumarate and emtricitabine in mucosal tissues suggests at least 86% adherence (≥6 doses/week) is required to achieve protective drug exposure in the female genital tract (FGT) and 29% adherence (≥2 doses/week) in the lower gastrointestinal (GI) tract.10 Similar to tenofovir disoproxil fumarate, tenofovir alafenamide is a prodrug of tenofovir currently approved for HIV treatment.11 Compared with 300 mg of tenofovir disoproxil fumarate, 25 mg of tenofovir alafenamide results in ∼7-fold higher concentrations of tenofovir’s active metabolite [tenofovir diphosphate (tenofovir-dp)] in PBMCs and ∼7-fold lower tenofovir exposure in plasma.12 As data on drug penetration in mucosal tissues remained unavailable, this study sought to investigate the dose proportionality of tenofovir and tenofovir-dp in mucosal tissues to evaluate the utility of tenofovir alafenamide for PrEP.
Methods
Ethics
This study was registered (clinicaltrials.gov NCT02357602) and conducted in accordance with ICH Good Clinical Practice standards and the Declaration of Helsinki. Study procedures were approved by the University of North Carolina’s Biomedical Institutional Review Board (protocol no. 14–2797). All participants provided written informed consent prior to enrolment.
Clinical trial design
This single-centre, open-label, dose-ranging PK study investigated a single tenofovir alafenamide dose in healthy, premenopausal HIV seronegative female volunteers 18–49 years old with intact FGTs and GI tracts and regular menstrual cycles. Participants were excluded due to: medication allergies; clinically significant medical conditions or abnormal laboratory tests, including abnormal Pap smears; symptomatic bacterial vaginosis; sexually transmitted infections, HIV or hepatitis B or C; pregnancy or breastfeeding; positive tests for drugs of abuse; and not using an approved method of contraception. Adverse events (AEs) were assessed at all visits using a standard questionnaire and graded according to the NIAID Division of AIDS AE grading table.13
Participants were assigned to one of three dosing groups: 5, 10 or 25 mg (n = 8 evaluable participants per group). Within 42 days of screening and 7 days after the end of their menstrual period, participants were admitted to UNC HealthCare’s Clinical Trials Research Center for an overnight visit and received a single oral dose of tenofovir alafenamide, provided by Gilead Sciences, Inc. (Foster City, CA, USA). Participants fasted for 8 h prior to and 2 h after witnessed medication administration. Serial blood samples were collected for plasma (baseline and 1, 3, 6, 12 and 24 h) and PBMCs (baseline and 3, 6, 12 and 24 h). Each participant provided cervical (two tissue pieces), vaginal (two tissue pieces) and colorectal (10 tissue pieces) samples during the overnight visit collected at 3, 6, 12 or 24 h post-dose (n = 2 per timepoint per dosing group). Participants were on a low fibre diet for 3 days then clear liquids for 12 h prior to colorectal biopsies. Each participant returned for four sampling visits at 3, 7, 10 and 14 days following their dose, with blood collected for plasma and PBMCs. Each participant provided a second cervical (two tissue pieces), vaginal (two tissue pieces) and colorectal (10 tissue pieces) sample at one of these outpatient visits (n = 2 per day per dosing group). Follow-up was within 14 days of the last visit. Women were screened for pregnancy at all visits and safety labs were obtained at follow-up.
Sample collection and processing
Cervical and vaginal tissues were collected with Baby Tischler biopsy forceps (Cooper Surgical, CT, USA). Colorectal tissues were collected using single-use RJ4 large-capacity forceps (Boston Scientific, MA, USA). All tissues were immediately placed in a cryovial and snap-frozen in liquid nitrogen. Whole blood, collected in 3 mL EDTA tubes and 8 mL BD Vacutainer® CPT™ tubes (Becton Dickinson and Company, NJ, USA), was processed for plasma and PBMCs, respectively, as previously described.10 Isolated PBMCs were counted on a Muse™ Cell Analyzer (EMD Millipore Cooperation, Billerica, MA, USA) and lysed in 70:30 methanol/water. All samples were stored at −80°C.
Cell culture methods
Ectocervical (Ect1/E6E7) ATCC® CRL-2614™ and vaginal (VK2/E6E7) ATCC® CRL-2616™ epithelial cell lines were obtained and sub-cultured for >4 passes after the initial thaw. Ect1/E6E7 and VK2/E6E7, 2×105 cells/well, were incubated overnight in 6-well culture plates with serum-free keratinocyte medium containing calcium, bovine pituitary extract, epidermal growth factor and antibiotics (Gibco™, Waltham, MA, USA) according to the cell lines’ product information.14,15 Freshly isolated PBMCs, collected from a single healthy volunteer, were incubated overnight at 2×106 cells/well in 12-well culture plates with RPMI 1640 medium containing FBS, antibiotics and IL-2 (Roche Diagnostics, Indianapolis, IN, USA). 1 mg/mL drug stocks were prepared by diluting tenofovir alafenamide (MedChem Express, Monmouth Junction, NJ, USA) in 100% DMSO and tenofovir (Toronto Research Chemicals, Toronto, ON, Canada) in purified water. Cells were incubated with 0.5 and 10 μM tenofovir alafenamide (i.e. 238.2 and 4764 ng/mL, respectively) or tenofovir (i.e. 143.6 and 2872 ng/mL, respectively) for 3, 12, 24, 48 and 72 h. Differences in drug stock diluents were controlled by matching concentrations of DMSO and purified water for the tenofovir- and tenofovir alafenamide-treated media, respectively. To harvest, epithelial cells were trypsinized with 0.25% Trypsin-EDTA (Gibco™, Waltham, MA, USA). Detached epithelial cells and PBMCs were centrifuged at 4°C, washed with cold PBS, counted, pelleted and lysed with cold 70:30 methanol/water for tenofovir-dp quantification. Experiments were performed in three independent replicates.
Analytical methods
Drug concentrations were measured in all matrices using LC-MS/MS methods on an AB Sciex API-5000 triple quadrupole mass spectrometer with ±15% [20% at the lower limit of quantification (LLOQ)] precision and accuracy. Plasma samples were analysed for tenofovir and tenofovir alafenamide following protein precipitation extractions with internal standards 13C5-tenofovir (for tenofovir) and raltegravir-d6 (for tenofovir alafenamide) by reverse phase chromatography (calibrated ranges = 0.25–250 and 0.05–100 ng/mL, respectively). Cell lysates (PBMCs and epithelial cells) were analysed for tenofovir-dp using a stable isotopically labelled internal standard (13C5-tenofovir-dp) by anion exchange chromatography (calibrated range = 0.02–20 ng/mL). Frozen tissue biopsies were weighed then homogenized in Precellys® hard tissue grinding kit tubes (Cayman Chemical, MI, USA) with cold 70:30 acetonitrile/1 mM ammonium phosphate buffer (pH 7.4). Following protein precipitation extraction with dolutegravir-13C, d5 (tenofovir alafenamide), 13C5-tenofovir [tenofovir monophosphate (tenofovir-mp) and tenofovir] and 13C5-tenofovir-dp (tenofovir-dp), samples were analysed by LC-MS/MS coupled with reverse phase (for tenofovir alafenamide and tenofovir) and anion exchange (for tenofovir-mp and tenofovir-dp) chromatographic conditions. The calibrated range was 0.005–100 ng/mL homogenate for tenofovir alafenamide, 0.04–1 ng/mL homogenate for tenofovir-mp and 0.02–20 ng/mL homogenate for tenofovir and tenofovir-dp.
PK methods
Non-compartmental analysis (NCA) was performed using Phoenix WinNonlin® version 6.4 (Pharsight Cooperation, Cary, NC, USA). Plasma and PBMC AUCs were calculated using the linear up–log down trapezoidal rule and reported as median (IQR). Since no significant differences (P = 0.98) were observed between cervical and vaginal tissue concentrations, these datasets were pooled for analysis of overall FGT concentrations. AUCs for GI and FGT tissues were calculated using the sparse sampling function and linear trapezoidal rule. Values below the limits of quantification (BLQ) were handled according to the Beal M5 method (BLQ = LLOQ ÷ 2).16 Terminal elimination rates were determined by including only the first imputed BLQ concentration. For cell culture analyses, AUC0–72 h was calculated using the sparse sampling function and linear trapezoidal rule. In vitro tenofovir-dp accumulation rate constants (Kobs) were determined by fitting a one-phase association curve with concentrations from 0 to 48 h for 0.5 μM tenofovir alafenamide and tenofovir using GraphPad Prism7 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical methods
Dose proportionality was assessed for tenofovir in plasma and tenofovir-dp in PBMCs by fitting a linear regression model to natural log (Ln) AUC and Ln dose. Perfect dose proportionality was defined as regression line slope (β1) = 1. We specified a priori that dose proportionality would be declared if the 90% CI of β1 fell within 0.57–1.43 based on the published equation where r = 5 (the highest to lowest dose ratio), θL = 0.5 and θU = 2.17 Assuming a percentage coefficient of variation ≤45%, eight women per dosing group provided ≥80% power to declare dose proportionality. Pearson correlation was used to quantify the relationship among cell types for dose-normalized, log10-transformed, time- and dose-matched tenofovir-dp concentrations. These analyses were performed in SAS version 9.3 (SAS institute, Cary, NC, USA).
Results
Demographics and AEs
Twenty-four women received a single 5, 10 or 25 mg dose of tenofovir alafenamide from May to October 2015. Briefly, median age was 27.5 years, 83% were white, 88% were non-Hispanic and median BMI was 24.4 kg/m2 (Table 1). AEs were reported most frequently by the 25 mg cohort (55% of reported AEs; Table 1). Eighty-nine percent of all AEs were grade 1, with intravenous site pain, pelvic pain, headache and nausea each contributing to 11% of reported AEs. Three AEs (two episodes of nausea and one of loose stool) were deemed possibly related to the study medication. No AEs required study interruption or discontinuation, and all resolved by follow-up without sequela.
Table 1.
Subject demographics and AEs
| 5 mg (n=8) | 10 mg (n=8) | 25 mg (n=8) | |
|---|---|---|---|
| Demographics | |||
| age, median (min–max) | 23.5 (19–46) | 24.5 (21–41) | 27.5 (23–41) |
| race, n (%) | |||
| white | 8 (100) | 6 (75) | 6 (75) |
| black | 0 | 2 (25) | 2 (25) |
| ethnicity, n (%) | |||
| Hispanic | 1 (12.5) | 1 (12.5) | 1 (12.5) |
| non-Hispanic | 7 (87.5) | 7 (87.5) | 7 (87.5) |
| BMI, median (min–max) | 22.7 (19.3–33.5) | 28.4 (19–34.9) | 24.4 (18.8–34.6) |
| AEs, n | |||
| all grade 1 AEsa | 7 | 1 | 8 |
| intravenous site pain | 2 | 0 | 0 |
| pelvic pain | 1 | 0 | 1 |
| headache | 0 | 1 | 1 |
| nausea | 0 | 0 | 2 |
| grade 2 AEsb | 0 | 0 | 2 |
Grade 1 AEs reported by fewer than two subjects: sampling discomfort, dry lips, abnormal menstrual cycle, blood in stool, diarrhoea, change in affect, tinnitus and leucocytosis.
Grade 2 AEs reported by fewer than two subjects: chest wall pain and loose stool.
Drug concentrations and dose proportionality in blood plasma and PBMCs
Plasma tenofovir and PBMC tenofovir-dp concentrations are plotted over time in Figure 1 and NCA estimates are reported in Table 2. For all doses, the time to maximal tenofovir concentration (Tmax) in plasma was 1–3 h with a median terminal half-life (t1/2) of 40 h. Plasma tenofovir was quantifiable over 24 h in the 5 mg cohort and 3 and 7 days in the 10 and 25 mg cohorts, respectively. Tenofovir alafenamide was only quantifiable in 25% of plasma samples at 6 h. Median (IQR) tenofovir alafenamide concentrations at 1 h [40.4 (28.45–66.45) ng/mL] were the highest observed and the AUC0–6 h was 38 ng·h/mL. In PBMCs, tenofovir-dp Tmax was achieved by 9–12 h with a median t1/2 of 54 h. By 14 days, 0%, 12.5% and 37.5% were quantifiable in the 5, 10 and 25 mg groups, respectively. Dose proportionality was declared for plasma tenofovir AUC0–24 h as well as PBMC tenofovir-dp AUC0–7 days and AUC0–14days [β1 (90% CI) = 1.01 (0.87–1.15), 0.83 (0.62–1.04) and 0.82 (0.62–1.02), respectively].
Figure 1.
Median (IQR) tenofovir and tenofovir-dp in blood plasma and PBMCs following a single dose of tenofovir alafenamide. Tenofovir over 14 days in plasma (a) and tenofovir-dp in PBMCs (b). Plot inserts depict concentrations over 24 h for each matrix. Concentration values BLQ were imputed at half the LLOQ for graphing purposes. Broken horizontal lines represent the LLOQ. At least 32% of plasma samples had unquantifiable tenofovir and at least 8% of PBMC samples had unquantifiable tenofovir-dp. Percentage of samples that were BLQ at each sampling time is tabulated below each graph. TAF, tenofovir alafenamide.
Table 2.
Tenofovir and tenofovir-dp NCA
| PK parameter | 5 mg (n = 8) | 10 mg (n = 8) | 25 mg (n = 8) |
|---|---|---|---|
| Tenofovir in plasmaa, median (IQR) | |||
| Cmax (ng/mL) | 0.8 (0.6–1) | 1.5 (1.4–1.7) | 4.7 (3.7–5) |
| AUC0–24 h (ng·h/mL) | 10.5 (8.8–14.5) | 21.5 (19.5–23.2) | 57.7 (42.5–67) |
| AUC0–14 days (ng·h/mL) | 52.8 (49.5–59.6) | 78.1 (68.2–86.9) | 169.7 (131.2–211.4) |
| Tenofovir-dp in PBMCsb, median (IQR) | |||
| Cmax (fmol/106 cells) | 33.7 (26.4–50.6) | 68 (56.9–88.8) | 113.6 (80.9–150.2) |
| AUC0–24 h (fmol·h/106 cells) | 657 (400–1027) | 1086 (888–1460) | 2094 (1528–2816) |
| AUC0–14 days (fmol·h/106 cells) | 2268 (1519–4090) | 4584 (3113–5734) | 9306 (6891–10785) |
| Tenofovir in female genital tissuec | |||
| Cmax (ng/g) | 5.9 | 7.2 | 15.8 |
| AUC0–24 h (ng·h/g) | 96.6 | 102.1 | 313.8 |
| AUC0–14 days (ng·h/g) | 604.7 | 730.6 | 1394 |
| Tenofovir in rectal tissued | |||
| Cmax (ng/g) | 121.7 | 1402.3 | 255.7 |
| AUC0–24 h (ng·h/g) | 963.5 | 8602 | 876.8 |
| AUC0–14 days (ng·h/g) | 4752 | 44545 | 22403 |
| Tenofovir-dp in female genital tissuee | |||
| Cmax (fmol/g) | BLQ | 4499 | 9552 |
| AUC0–24 h (fmol·h/g) | BLQ | 63703 | 104086 |
| AUC0–14 days (fmol·h/g) | BLQ | 986421 | 1309305 |
| Tenofovir-dp in rectal tissuef | |||
| Cmax (fmol/g) | BLQ | 3456 | 11163 |
| AUC0–24 h (fmol·h/g) | BLQ | 45103 | 39162 |
| AUC0–14 days (fmol·h/g) | BLQ | 315145 | 1041352 |
%BLQ in the 5, 10 and 25 mg dosing arms = 51%, 38% and 32%, respectively.
%BLQ in the 5, 10 and 25 mg dosing arms = 30%, 17% and 8%, respectively.
%BLQ in the 5, 10 and 25 mg dosing arms = 66%, 47% and 31%, respectively.
%BLQ in the 5, 10 and 25 mg dosing arms = 25%, 0% and 0%, respectively.
%BLQ in the 5, 10 and 25 mg dosing arms = 100%, 97% and 87.5%, respectively.
%BLQ in the 5, 10 and 25 mg dosing arms = 100%, 69% and 75%, respectively.
Drug concentrations in mucosal tissues
Rectal concentrations are plotted over time in Figure 2. Rectal tenofovir was quantifiable in 0%, 100% and 100% of samples at 14 days following a 5, 10 and 25 mg dose, respectively. The average sample-specific LLOQ (based on tissue sample mass) was 0.46 ng/g. Following a 25 mg dose, Tmax was 72 h and Cmax was 256 ng/g. For all dosing groups, AUC0–14days ranged from 4751 to 44545 ng·h/g. In contrast, tenofovir alafenamide was not quantifiable in rectal tissues. Tenofovir-mp was only quantifiable in two rectal tissue samples collected at 24 and 72 h both from the 25 mg cohort. The maximum concentration was 18317 fmol/g at 72 h (15% lower than tenofovir-dp in that sample). Rectal tenofovir-dp was quantifiable in 0%, 31% and 25% of samples from the 5, 10 and 25 mg cohorts, respectively. The average sample-specific LLOQ was 1032 fmol/g. Following a 25 mg dose, Tmax was 72 h and Cmax was 11163 fmol/g. For all dosing groups, AUC0–14days ranged from 315145 to 1041351 fmol·h/g (see Table 2 for percentage imputed BLQ values included in estimates).
Figure 2.
Median (IQR) tenofovir and tenofovir-dp in rectal tissues following a single dose of tenofovir alafenamide. Tenofovir (continuous lines) and tenofovir-dp (broken lines) over 14 days in rectal tissue. Concentration values BLQ were imputed at half the sample-specific LLOQ for graphing purposes. Representative LLOQs are depicted for tenofovir (continuous horizontal line) and tenofovir-dp (broken horizontal line). At least 25% and 69% of samples had unquantifiable tenofovir and tenofovir-dp, respectively. Percentage of samples that were BLQ at each sampling time is tabulated below the graph. TAF, tenofovir alafenamide; TFV, tenofovir; TFVdp, tenofovir-dp.
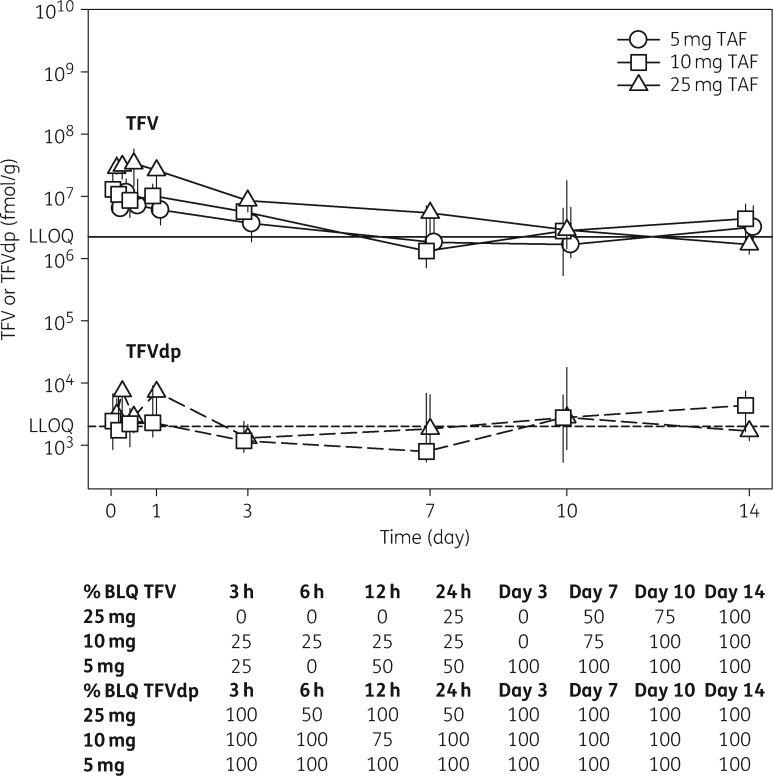
FGT concentrations are plotted over time in Figure 3. FGT tenofovir was quantified in 0%, 0% and 25% of samples at 14 days following a 5, 10 and 25 mg dose, respectively. The average sample-specific LLOQ was 2.96 ng/g. Following a 25 mg dose, Tmax was 12 h and Cmax was 16 ng/g. For all dosing groups, AUC0–14days ranged from 605 to 1394 ng·h/g. FGT tenofovir-dp was quantifiable in 0%, 3% and 12.5% of samples from the 5, 10 and 25 mg cohorts. The average sample-specific LLOQ was 6617 fmol/g. Following a 25 mg dose, Tmax was 6 h and Cmax was 9552 fmol/g. For all dosing groups, AUC0–14days ranged from 986421 to 1309305 fmol·h/g (see Table 2 for percentage imputed BLQ values included in estimates). Neither tenofovir alafenamide nor tenofovir-mp was quantifiable in FGT tissues. Owing to the low number of detectable concentrations, tissue dose proportionality was not assessed.
Figure 3.
Median (IQR) tenofovir and tenofovir-dp in female genital tissues following a single dose of tenofovir alafenamide. Tenofovir (continuous lines) and tenofovir-dp (broken lines) over 14 days in FGT tissue. Concentration values BLQ were imputed at half the sample-specific LLOQ for graphing purposes. Representative LLOQs are depicted for tenofovir (continuous horizontal line) and tenofovir-dp (broken horizontal line). At least 31% and 87.5% of samples had unquantifiable tenofovir and tenofovir-dp, respectively. Percentage of samples that were BLQ at each sampling time is tabulated below the graph. TAF, tenofovir alafenamide; TFV, tenofovir; TFVdp, tenofovir-dp.
In vitro tenofovir-dp concentrations in PBMCs and epithelial cells
To explore whether low tenofovir-dp concentrations within tissue epithelial cells might explain low mucosal tissue tenofovir-dp concentrations in homogenates, we measured tenofovir-dp in epithelial cell lines (Ect1/E6E7 and VK2/E6E7) and primary PBMCs incubated over 72 h in tenofovir alafenamide or tenofovir. Tenofovir disoproxil fumarate is rapidly hydrolysed in human plasma and intestinal tissue (t1/2 <5 min).18 Therefore, we used tenofovir, the primary molecule available for in vivo cellular uptake, for this experiment. Tenofovir-dp exposure was 1.7–17-fold higher in epithelial cells versus PBMCs and 192–1309-fold higher in cells dosed with tenofovir alafenamide versus tenofovir (Table 3). Tenofovir-dp concentrations plateaued by 48 h in PBMCs and Ect1/E6E7 cells and by 24 h in VK2/E6E7 cells (Figure 4). For 0.5 μM tenofovir alafenamide, tenofovir-dp’s Kobs was 0.06, 0.13 and 0.19 h−1 and time to half-maximal accumulation (half-time) was 11.7, 7.6 and 3.7 h in PBMCs, Ect1/E6E7 cells and VK2/E6E7 cells, respectively. For 0.5 μM tenofovir, Kobs was 0.03, 0.09 and 0.25 h−1 and half-time was 21.7, 11.2 and 2.8 h in PBMCs, Ect1/E6E7 cells and VK2/E6E7 cells, respectively. We observed significant correlations in tenofovir-dp concentrations between all cell types for both tenofovir alafenamide and tenofovir (Pearson’s r = 0.42–0.9, P < 0.05).
Table 3.
In vitro tenofovir-dp formation in PBMCs and epithelial cells
| Cell type | Tenofovir-dp AUC0–72 ha |
|||
|---|---|---|---|---|
| 0.5 μM tenofovir alafenamide | 10 μM tenofovir alafenamide | 0.5 μM tenofovir | 10 μM tenofovir | |
| PBMCs | 2122 | 12646 | 1.626b | 33.51 |
| VK2/E6E7 | 3587 | 60604 | 18.62c | 256.2 |
| Ect1/E6E7 | 10036 | 210382 | 26.66 | 468.8 |
AUC0–72 h expressed in pmol·h/million cells units.
Two concentrations at 3 h, one concentration at 12 h and one concentration at 24 h were BLQ.
One concentration at 3 h was BLQ.
Figure 4.
Median (range) tenofovir-dp in PBMCs and epithelial cells incubated in tenofovir and tenofovir alafenamide. Tenofovir-dp concentration over time in human PBMCs (triangles), Ect1/E6E7 ectocervical epithelial cells (circles) and VK2/E6E7 vaginal epithelial cells (squares) incubated in 0.5 μM (a) or 10 μM (b) tenofovir alafenamide (filled symbols and continuous lines) or tenofovir (open symbols and broken lines). Tenofovir-dp accumulation plateaued by 24 h in vaginal epithelial cells and 48 h in cervical epithelial cells and PBMCs for both tenofovir alafenamide and tenofovir.
Discussion
Oral tenofovir disoproxil fumarate, with or without emtricitabine, PrEP has been evaluated in several Phase III trials with varying success among women.4,5,8,9 While the Partners PrEP Study Team demonstrated 66%–71% efficacy among women,4 FEM-PrEP and VOICE8,9 demonstrated futility. Since tenofovir was detected in <30% of plasma samples from FEM-PrEP and VOICE, these results have largely been attributed to varying adherence rates among study populations with differing risk perception and demonstrate the need to design PrEP options with improved forgiveness for missed doses and/or reduced frequency dosing. While other technologies in varying stages of clinical development are being studied for this purpose (e.g. long-acting injectables, implants and vaginal rings19–21), oral agents continue to be of interest. This study is the first to describe the distribution of tenofovir alafenamide, tenofovir and tenofovir-dp in mucosal tissues after tenofovir alafenamide dosing in women and was designed to support PK modelling to explore whether less frequent dosing of PrEP might be feasible with tenofovir alafenamide.
The PK profiles of tenofovir alafenamide and tenofovir in blood plasma and tenofovir-dp in PBMCs observed in this study after a 25 mg dose were consistent with previous reports.12 Both plasma tenofovir AUC0–24 h and PBMC tenofovir-dp AUC0–14days demonstrated dose proportionality. AUC0–24 h was used for the plasma analysis because tenofovir was unquantifiable after 24 h in the 5 mg dosing arm.
Given tenofovir alafenamide’s favourable phosphorylation in PBMCs, we expected tenofovir and tenofovir-dp concentrations in mucosal tissues to be higher and more sustained following a single dose of tenofovir alafenamide versus tenofovir disoproxil fumarate. Conversely, the majority of tissue tenofovir-dp concentrations were unquantifiable (95% and 81% in FGT and rectal tissue, respectively) and could not be quantified in any samples collected after 72 h. Because of this, we were unable to assess dose proportionality within mucosal tissues. However, the median tenofovir-dp concentration in tissue samples was 2.6 times higher for the 25 versus 10 mg dosing arm, which may suggest a predictable relationship between tissue concentrations and dose.
In comparing FGT and rectal tissues, tenofovir Tmax was delayed by 60 h in the rectum. Tenofovir-dp peaked at 3 days in the rectum versus 6 h in the FGT and was quantifiable out to 1 day in the FGT versus 3 days in the rectum. While tenofovir and tenofovir-dp were quantified more frequently in the rectum compared with the FGT, the tenofovir-dp Cmax (11163 versus 9552 fmol/g, respectively) was comparable.
We previously conducted a similar PK evaluation of tenofovir-dp in mucosal tissues after a single dose of tenofovir disoproxil fumarate.10 Tissue collection and bioanalytical techniques were identical between these studies except that tenofovir alafenamide study samples were stored for ∼6 months before analysis versus ∼18 months for tenofovir disoproxil fumarate study samples. In comparing these data, tenofovir exposures (AUC0–48 h) following a single 25 mg dose of tenofovir alafenamide were 2- and 10-fold lower in the FGT and rectal tissue compared with 300 mg of tenofovir disoproxil fumarate, respectively.10 Tenofovir-dp exposures were 1.3–13-fold lower in FGT and rectal tissue compared with tenofovir disoproxil fumarate. Additionally, tenofovir-dp was unquantifiable in more samples with tenofovir alafenamide dosing in FGT (35% more) and rectal tissue (75% more).10
To understand better the pharmacology of tenofovir alafenamide in mucosal tissues, we conducted additional investigations to characterize parent (tenofovir alafenamide) and intermediary metabolite (tenofovir-mp) concentrations in mucosal tissues. Tenofovir alafenamide was analysed in plasma and tissue homogenates collected from participants in the 25 mg dosing arm (n = 8). Despite quantifiable tenofovir alafenamide concentrations in all plasma samples collected prior to 6 h, tenofovir alafenamide was unquantifiable in 100% of tissue samples across all sampling times. Analyte degradation is an unlikely explanation of these findings. To minimize opportunity for degradation, our biopsies were snap-frozen within 5 min of collection and remained frozen until homogenization. Our quality control tissue homogenate samples demonstrated analyte stability in homogenates with ≥75% within 20% accuracy. Lastly, all samples were analysed within 230 days of collection and tenofovir alafenamide was stable in frozen plasma for 360 days (data not shown). Thus, tenofovir alafenamide itself does not appear to distribute to mucosal tissues. Interestingly, tenofovir alafenamide is a substrate of multidrug resistance protein 1 and breast cancer resistance protein, whereas tenofovir is not.22 These efflux transporters are expressed within FGT and GI tissues, and may provide a clearance mechanism that excludes tenofovir alafenamide but not tenofovir from mucosal tissues.23,24 Therefore, following tenofovir alafenamide dosing, mucosal tissue tenofovir-dp may result from circulating tenofovir rather than local conversion of tenofovir alafenamide.
Tenofovir-mp is an intermediary metabolite, which is rapidly phosphorylated to tenofovir-dp by intracellular kinases.25In vivo concentrations of this analyte are proportionally small compared with tenofovir-dp; however, degradation of tenofovir-dp or altered intracellular kinetics could result in higher tenofovir-mp concentrations.26 Tenofovir-mp was only quantifiable in two tissue samples. Thus, ex vivo tenofovir-dp degradation or altered cellular kinetics does not explain our results.
Cathepsin A is the enzyme responsible for catalysing tenofovir alafenamide’s intracellular conversion into tenofovir. Variable expression in cathepsin A among different cell and tissue types has been noted27,28 and may result in reduced conversion of tenofovir alafenamide. Since tissue homogenates provide an averaged drug concentration across multiple cell types, including epithelial cells, tenofovir-dp concentrations could be diluted in mucosal tissues if epithelial cells have reduced ability to convert tenofovir alafenamide. Therefore, we characterized tenofovir-dp formation over time in two human epithelial cell lines versus PBMCs. We found ectocervical and vaginal cells efficiently metabolize tenofovir alafenamide to tenofovir-dp compared with PBMCs. Assuming dose proportionality, previous work to characterize tenofovir-dp in primary epithelial cells exposed to tenofovir in vitro suggests that the tenofovir-dp in our ectocervical cell line would be ∼1.7-fold higher than in primary ectocervical cells.29 Thus these cell lines provide a good surrogate for understanding tenofovir-dp exposure in epithelial cells and do not suggest a dilution of concentrations by the use of homogenates.
This study had some limitations, including the single-dose study design, which was intended to support PK modelling to predict steady-state mucosal tenofovir-dp concentrations. This analysis also did not account for any contribution of cellular trafficking to tissue tenofovir-dp concentrations. No doses >25 mg were used. The doses selected for this investigation were chosen to generate PK data for tenofovir alafenamide’s HIV treatment doses, which are 10 mg when dosed with strong CYP3A4 inhibitors and 25 mg otherwise.11 Since tenofovir alafenamide does not exhibit Michaelis–Menten PK, studying single doses >25 mg may have increased the likelihood of detecting tenofovir-dp mucosal concentrations. While additional PK studies exploring higher single or multiple doses are needed, it is important to note these findings are consistent with recent animal data showing lower tenofovir-dp concentrations in rectal tissue when rhesus macaques were dosed with tenofovir alafenamide and emtricitabine compared with those dosed with tenofovir disoproxil fumarate and emtricitabine.30 These investigators also noted no protection against simian/human immunodeficiency virus (SHIV) following repeated rectal challenges when tenofovir alafenamide monotherapy was administered 3 days prior to challenge31 but significant protection when tenofovir alafenamide + emtricitabine was dosed 24 h before and 2 h after challenge.30
We did not perform matrix effects tests for tenofovir alafenamide or tenofovir-mp. These analyses were prompted by our unexpected results and intended for discovery purposes only. However, a post-hoc infusion test indicated enhancement or suppression of tenofovir alafenamide signal by human plasma is unlikely. Furthermore, the extreme magnitude of suppression for tissue tenofovir alafenamide or tenofovir-mp signal to be below limits of detection is unlikely. Thus, we do not anticipate that matrix effects confounded our conclusions.
Conclusions
In conclusion, our results demonstrate tenofovir alafenamide is well-tolerated in healthy volunteers and phosphorylated to a greater extent in PBMCs compared with tenofovir disoproxil fumarate. Unexpectedly, we noted limited phosphorylated metabolite within mucosal tissues. Further investigation of mucosal tissue pharmacology is needed to understand tenofovir alafenamide’s role in HIV prevention.
Acknowledgements
These data were presented in part at the Twenty-third Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016 (Abstract no. 102LB) and at the 2016 International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy (Abstract O_10).
We wish to thank the participants of this study as well as the research staff at the UNC Clinical Trials Research Center (NCATS UL1TR001111). We thank all members of the Clinical Pharmacology and Analytical Core laboratory along with the Center for AIDS Research.
Funding
This work was supported by: Gilead Sciences, Inc. (Investigator Sponsored Research Grant Number IN-US-120-D001); the National Institute of General Medical Sciences (Grant Number T32GM086330); the National Institute of Allergy and Infectious Diseases (Grant Number P30AI50410); and the National Center for Advancing Translational Sciences (Grant Number UL1TR001111).
Transparency declarations
Gilead Sciences, Inc. developed and owns tenofovir alafenamide. This study was funded by Gilead Sciences, Inc. with a grant to the University of North Carolina. Both J. F. R. and S. M. are employees and stockholders of Gilead Sciences, Inc. All other authors: none to declare.
Author contributions
M. L. C., K. L. G. and A. D. M. K.: wrote the article. C. S., H. M. A. P., A. S., C. W. E., A. P., J. F. R., S. M. and C. G.: critical review of manuscript. H. M. A. P., C. G. and A. P.: oversight of sample collection and clinical study protocols. M. L. C., K. L. G., C. S., J. F. R., S. M. and A. D. M. K.: designed research. M. L. C., K. L. G., C. S., A. S., C. W. E., H. M. A. P. and A. P.: performed research. M. L. C., K. L. G. and A. D. M. K.: analysed data.
References
- 1. WHO. HIV/AIDS: Data and Statistics. Global Summary of HIV/AIDS Epidemic, December 2015 http://www.who.int/hiv/data/epi_core_2016.png?ua=1.
- 2. Langen TT. Gender power imbalance on women's capacity to negotiate self-protection against HIV/AIDS in Botswana and South Africa. Afr Health Sci 2005; 5: 188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Truvada Full Prescribing Information. Foster City, CA: Gilead Sciences, Inc, 2016. http://www.gilead.com/∼/media/Files/pdfs/medicines/hiv/truvada/truvada_pi.pdf. [Google Scholar]
- 4. Baeten JM, Donnell D, Ndase P. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thigpen MC, Kebaabetswe PM, Paxton LA. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. [DOI] [PubMed] [Google Scholar]
- 6. Grant RM, Lama JR, Anderson PL. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choopanya K, Martin M, Suntharasamai P. et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381: 2083–90. [DOI] [PubMed] [Google Scholar]
- 8. Marrazzo JM, Ramjee G, Richardson BA. et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372: 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Damme L, Corneli A, Ahmed K. et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cottrell ML, Yang KH, Prince HM. et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Descovy Full Prescribing Information. Foster City, CA: Gilead Sciences, Inc, 2016. http://www.gilead.com/∼/media/files/pdfs/medicines/hiv/descovy/descovy_pi.pdf?la=en. [Google Scholar]
- 12. Ruane PJ, DeJesus E, Berger D. et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63: 449–55. [DOI] [PubMed] [Google Scholar]
- 13. Microbicide Trials Network (MTN). The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (“DAIDS AE Grading Table”) http://www.mtnstopshiv.org/sites/default/files/attachments/DAIDS_AE_GradingTable_ClarificationAug2009_Final_%5B1%5D.pdf.
- 14. Ect1/E6E7 (ATCC® CRL 2614™) Product Sheet. Manassas, VA: ATCC, 2014. [Google Scholar]
- 15. VK2/E6E7 (ATCC® CRL 2616™) Product Sheet. Manassas, VA: ATCC, 2014. [Google Scholar]
- 16. Beal S. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 17. Hummel J, McKendrick S, Brindley C. et al. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat 2009; 8: 38–49. [DOI] [PubMed] [Google Scholar]
- 18. US FDA Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Application Number 21-356. Drug Approval Package VIREAD (Tenofovir Disoproxil Fumarate) Tablets Web Site http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-356_Viread.cfm.
- 19. Baeten JM, Palanee-Phillips T, Brown ER. et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375: 2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markowitz M, Frank I, Grant R. et al. ÉCLAIR: Phase 2A safety and PK study of cabotegravir LA in HIV uninfected men. In: Abstracts of the Twenty-third Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016. Abstract 106. Foundation for Retrovirology and Human Health, Alexandria, VA, USA.
- 21. Gunawardana M, Remedios-Chan M, Miller C. et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 2015; 59: 3913.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lepist EI, Phan TK, Roy A. et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother 2012; 56: 5409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou T, Hu M, Pearlman A. et al. Expression and localization of P-glycoprotein, multidrug resistance protein 4, and breast cancer resistance protein in the female lower genital tract of human and pigtailed macaque. AIDS Res Hum Retroviruses 2014; 30: 1106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nicol MR, Fedoriw Y, Mathews M. et al. Expression of six drug transporters in vaginal, cervical, and colorectal tissues: Implications for drug disposition in HIV prevention. J Clin Pharmacol 2014; 54: 574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birkus G, Bam RA, Willkom M. et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother 2015; 60: 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tenofovir Disoproxil Fumarate (PMPA Prodrug) GS-4331-05 Complete Investigator Brochure. Foster City, CA: Gilead Sciences, Inc, 2001. [Google Scholar]
- 27.The Broad Institute of MIT and Harvard. GTEx Portal: Gene Expression for CTSA (Cathepsin A) http://www.gtexportal.org/home/gene/CTSA.
- 28. Satake A, Itoh K, Shimmoto M. et al. Distribution of lysosomal protective protein in human tissues. Biochem Biophys Res Commun 1994; 205: 38–43. [DOI] [PubMed] [Google Scholar]
- 29. Shen Z, Fahey JV, Bodwell JE. et al. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One 2014; 9: e100863.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Massud I, Mitchell J, Babusis D. et al. Chemoprophylaxis with oral emtricitabine and tenofovir alafenamide combination protects macaques from rectal simian/human immunodeficiency virus infection. J Infect Dis 2016; 214: 1058–62. [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Lerma JG, Aung W, Cong ME. et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011; 85: 6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]