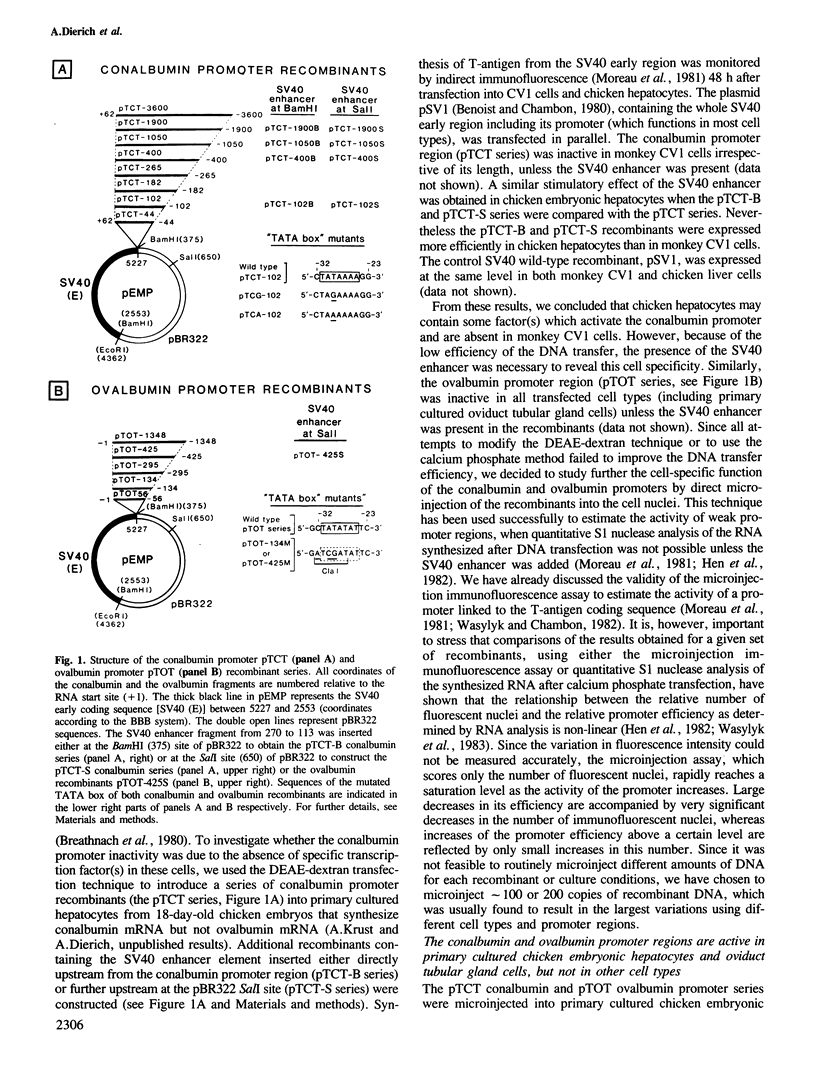
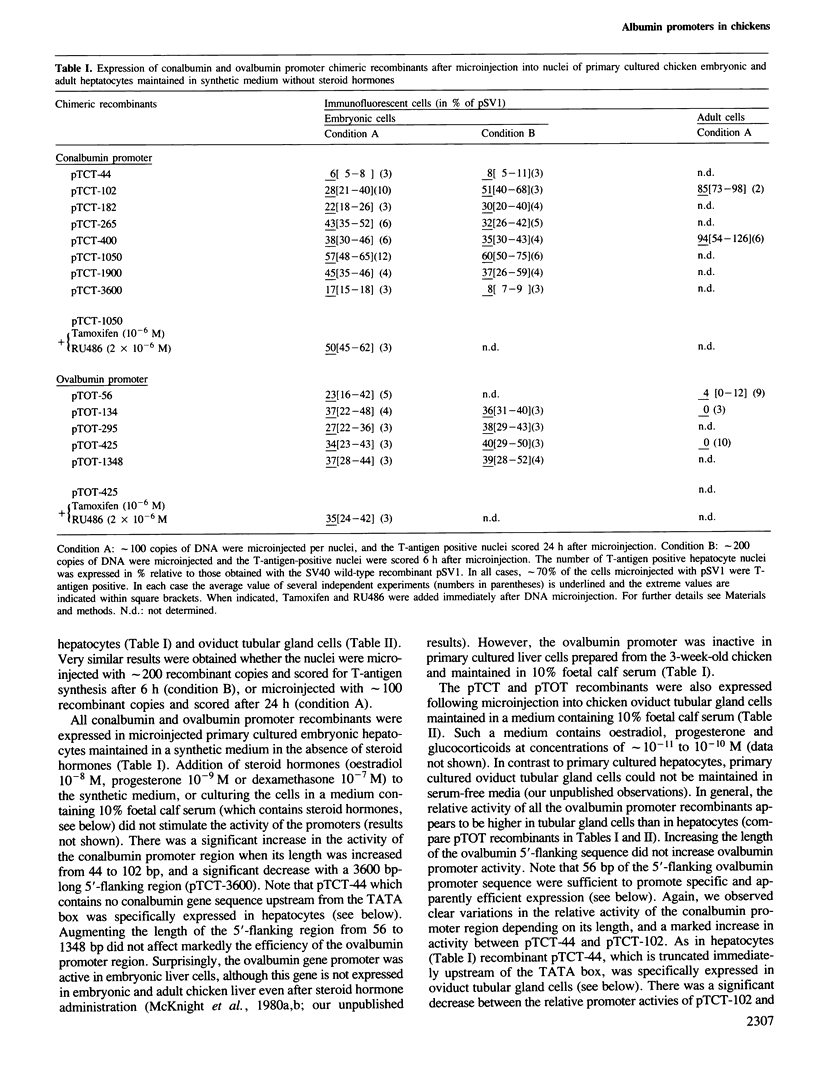
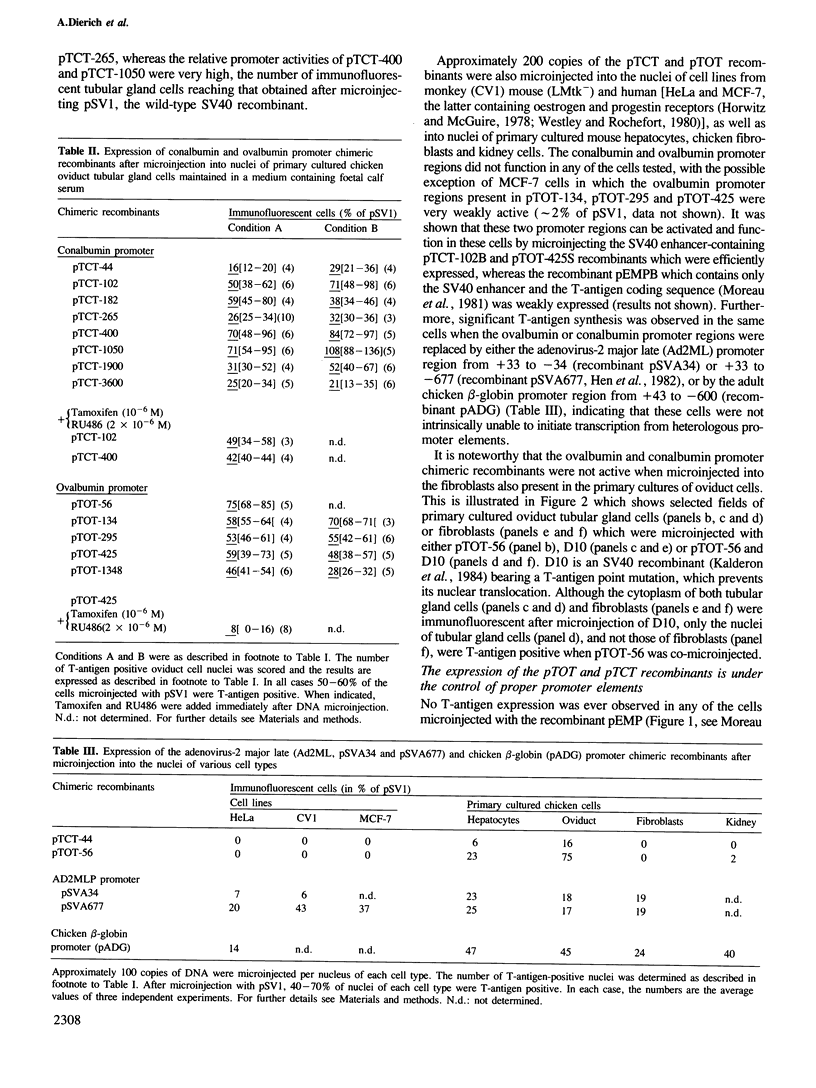
Abstract
A series of recombinant plasmids containing increasing lengths of the 5'-flanking promoter sequences of the chicken conalbumin and ovalbumin genes fused to the sequences coding for the SV40 T-antigen have been constructed. These recombinants were introduced into a variety of established cell lines and primary cultured cells by nuclear microinjection. Promoter activity was estimated by monitoring T-antigen synthesis by indirect immunofluorescence. We show that the microinjected ovalbumin and conalbumin promoter regions do not function in chicken fibroblasts, kidney cells and in a variety of non-chicken cells, irrespective of the presence of steroid hormone receptors. In contrast, these promoter regions are active in primary cultured chicken embryonic hepatocytes and oviduct tubular gland cells, suggesting the presence of cell-specific transcription factors in these cells. Unexpectedly, promoter sequences close to the TATA boxes of both the ovalbumin and conalbumin genes are sufficient to confer cell-specific expression. Most of the controls exerted on the ovalbumin and conalbumin promoters in the whole animal appear to be reproduced in vitro by nuclear microinjection of the chimeric genes into the primary cultured cells. However, the microinjected ovalbumin promoter is active in embryonic hepatocytes and thus escapes the regulation imposed on the corresponding inactive endogenous gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W. Improved system for capillary microinjection into living cells. Exp Cell Res. 1982 Jul;140(1):31–37. doi: 10.1016/0014-4827(82)90152-5. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mantei N., Chambon P. Corrected splicing of a chicken ovalbumin gene transcript in mouse L cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):740–744. doi: 10.1073/pnas.77.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Dodgson J. B., Stadt S. J., Choi O. R., Dolan M., Fischer H. D., Engel J. D. The nucleotide sequence of the embryonic chicken beta-type globin genes. J Biol Chem. 1983 Oct 25;258(20):12685–12692. [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods Enzymol. 1980;65(1):816–825. doi: 10.1016/s0076-6879(80)65076-9. [DOI] [PubMed] [Google Scholar]
- Groyer A., Le Bouc Y., Joab I., Radanyi C., Renoir J. M., Robel P., Baulieu E. E. Chick oviduct glucocorticosteroid receptor. Specific binding of the synthetic steroid RU 486 and immunological studies with antibodies to chick oviduct progesterone receptor. Eur J Biochem. 1985 Jun 3;149(2):445–451. doi: 10.1111/j.1432-1033.1985.tb08945.x. [DOI] [PubMed] [Google Scholar]
- Grundström T., Zenke W. M., Wintzerith M., Matthes H. W., Staub A., Chambon P. Oligonucleotide-directed mutagenesis by microscale 'shot-gun' gene synthesis. Nucleic Acids Res. 1985 May 10;13(9):3305–3316. doi: 10.1093/nar/13.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., McKnight G. S., Palmiter R. D. Glucocorticoid induction of egg white mRNAs in chick oviduct. J Biol Chem. 1980 Aug 25;255(16):7796–7800. [PubMed] [Google Scholar]
- Hen R., Sassone-Corsi P., Corden J., Gaub M. P., Chambon P. Sequences upstream from the T-A-T-A box are required in vivo and in vitro for efficient transcription from the adenovirus serotype 2 major late promoter. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7132–7136. doi: 10.1073/pnas.79.23.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kaye J. S., Pratt-Kaye S., Bellard M., Dretzen G., Bellard F., Chambon P. Steroid hormone dependence of four DNase I-hypersensitive regions located within the 7000-bp 5'-flanking segment of the ovalbumin gene. EMBO J. 1986 Feb;5(2):277–285. doi: 10.1002/j.1460-2075.1986.tb04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Riser M. E., O'Malley B. W. Regulated expression of the chicken ovalbumin gene in a human estrogen-responsive cell line. J Biol Chem. 1983 Oct 25;258(20):12693–12701. [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Lee D. C., McKnight G. S., Palmiter R. D. The action of estrogen and progesterone on the expression of the transferrin gene. A comparison of the response in chick liver and oviduct. J Biol Chem. 1978 May 25;253(10):3494–3503. [PubMed] [Google Scholar]
- Matthias P. D., Renkawitz R., Grez M., Schütz G. Transient expression of the chicken lysozyme gene after transfer into human cells. EMBO J. 1982;1(10):1207–1212. doi: 10.1002/j.1460-2075.1982.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Hemmaplardh D., Finch C. A., Palmiter R. D. Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980 Jan 10;255(1):144–147. [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Palmiter R. D. Transferrin gene expression. Regulation of mRNA transcription in chick liver by steroid hormones and iron deficiency. J Biol Chem. 1980 Jan 10;255(1):148–153. [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Roop D. R., Lai E. C., Nordstrom J. L., Catterall J. F., Swaneck G. E., Colbert D. A., Tsai M. J., Dugaiczyk A., Woo S. L. The ovalbumin gene: organization, structure, transcription, and regulation. Recent Prog Horm Res. 1979;35:1–46. doi: 10.1016/b978-0-12-571135-7.50005-9. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Pastorcic M., Wang H., Elbrecht A., Tsai S. Y., Tsai M. J., O'Malley B. W. Control of transcription initiation in vitro requires binding of a transcription factor to the distal promoter of the ovalbumin gene. Mol Cell Biol. 1986 Aug;6(8):2784–2791. doi: 10.1128/mcb.6.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz R., Beug H., Graf T., Matthias P., Grez M., Schütz G. Expression of a chicken lysozyme recombinant gene is regulated by progesterone and dexamethasone after microinjection into oviduct cells. Cell. 1982 Nov;31(1):167–176. doi: 10.1016/0092-8674(82)90416-0. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Mester J., Baulieu E. E. Tamoxifen is a potent "pure" anti-oestrogen in chick oviduct. Nature. 1977 Jun 2;267(5610):434–435. doi: 10.1038/267434a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. A T to A base substitution and small deletions in the conalbumin TATA box drastically decrease specific in vitro transcription. Nucleic Acids Res. 1981 Apr 24;9(8):1813–1824. doi: 10.1093/nar/9.8.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Potentiator effect of the SV40 72-bp repeat on initiation of transcription from heterologous promoter elements. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):921–934. doi: 10.1101/sqb.1983.047.01.106. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Cozens P. J., Mattaj I. W., Jost J. P. Estrogen induces a demethylation at the 5' end region of the chicken vitellogenin gene. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4252–4255. doi: 10.1073/pnas.79.14.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A., Cato A. C., Cozens P. J., Mattaj I. W., Jost J. P. Isolation and fine structure organisation of an avian vitellogenin gene coding for the major estrogen-inducible mRNA. Gene. 1981 Dec;16(1-3):249–259. doi: 10.1016/0378-1119(81)90081-0. [DOI] [PubMed] [Google Scholar]