Abstract
Head and neck squamous cell carcinoma (HNSCC) is one of many cancers that are strongly associated with tobacco use. Whereas HNSCC is often seen in tobacco users, many tobacco users do not develop carcinoma; and the differences between smokers with and without HNSCC are poorly studied to date. Some smokers may be inherently more susceptible to developing carcinoma due to patterns of tobacco use, innate metabolism of carcinogens, or altered excretion. Identifying those smokers at greatest risk for HNSCC would have great benefit through targeted smoking cessation efforts and enhanced surveillance. One approach to better understand the extent of exposure to, and metabolism of, tobacco carcinogens is through the use of tobacco-specific metabolites. Tobacco-specific metabolites can identify patterns of dose, exposure, and metabolism, and perhaps ultimately characterize the important differences between smokers who develop HNSCC and smokers who do not.
Keywords: carcinogenesis, tobacco metabolites, head and neck cancer, DNA adducts, biomarkers
Tobacco product use is an important etiologic factor in head and neck squamous cell carcinoma (HNSCC), a disease that affects 40,000 Americans and 500,000 people worldwide each year.1–3
Tobacco use via cigarette smoking began in the first half of the 20th century. Cigarette use initially climbed steadily starting in the early 1900s with only short pauses at the time of the great depression and after publication of the first large cohorts demonstrating the dangers of cigarette use.4–6 Increasing public awareness of the dangers of tobacco use after the publication of the 1964 Surgeon General’s report extended over the second half of the 20th century, resulting in decreased cigarette consumption. In the United States, this trend has been helped by aggressive anti-tobacco advertising, legislation banning smoking in public areas, increasing taxes on cigarettes, provisions for tobacco cessation treatment, restrictions in youth access, and prevention education (IOM 2007).7 Still, tobacco use remains a major problem with an estimated 46 million Americans being classified as current smokers in 2008.8,9 Worldwide, there are about 1200 million smokers and hundreds of millions of smokeless tobacco users.10,11
Although there has been progress in reducing tobacco exposure in some parts of the world, HNSCC related to tobacco use remains a significant problem. Tobacco smoking causes cancer of the oral cavity, nasopharynx, oropharynx, hypopharynx, nasal cavity and paranasal sinuses, larynx, and esophagus.11 The risk for HNSCC in smokers is approximately 10 times higher than that of never-smokers.12 Although tobacco cessation does reduce the risk of carcinoma, there is conflicting data as to whether a past smoker’s risk ever decreases to the level of a never-smoker.13 Alcohol enhances the risk of HNSCC in tobacco users but also acts as an individual risk factor.14 When considering all new presentations of HNSCC, 80% to 90% are associated with tobacco and alcohol use.14 Although squamous cell carcinoma of some head and neck subsites is decreasing in incidence, other subsites have seen an increase. This includes oropharyngeal and oral cavity carcinoma. This may be due in part to the use of smokeless tobacco products, which is significant in many countries. For example, the popularity of these products in Southeast Asia has led to HNSCC becoming the most common cancer in men in that part of the world.15
Within the group of tumors that are tobacco/alcohol associated, experienced head and neck surgeons and oncologists would agree that there is a spectrum of HNSCC behavior from less-aggressive to highly aggressive tumors with the latter demonstrating explosive growth over short periods of time. Indeed, it is not uncommon for 2 similar tumors treated with identical therapy to have dramatically different outcomes. This presents a quandary for head and neck oncologists, as it is difficult to know which treatment is the best option for each individual patient. In recent years, treatment options have broadened for patients with HNSCC. Whereas surgery has historically been first-line treatment for these tumors, chemotherapy and radiation are now commonly used as adjuvant or definitive therapy. We are currently not able to definitively discern which treatment modality is best for each tumor. As a result, recent work has been focused on biomarkers to further characterize head and neck tumors in the hopes that these biomarkers will guide treatment selection for individual head and neck tumors.
Although a detailed discussion of HNSCC biomarkers is outside the scope of this review, biomarker data highlights the potential of tobacco metabolites. At present, some of the biomarkers that have been studied relating to head and neck cancer include vascular endothelial growth factor, epidermal growth factor receptor, human papillomavirus (HPV) p16, P53, and bcl-xL, to name only a few.16 Studies of these markers have shown that elevated p16 is associated with a response to induction chemotherapy, whereas production of epidermal growth factor receptor is inversely associated with response. These data suggest that markers can be predictive of tumor behavior and likelihood of treatment response.16
Much of the work on biomarkers in HNSCC to date has focused on the treatment-related and prognostic implications of biomarker data. HPV is an excellent example of this approach. Tumors associated with HPV have demonstrated an improved response to treatment when compared to HPV-negative tumors.17,18 As such, HPV status is often obtained before the start of treatment to provide prognostic information. In the near future, given recent data, patients with HPV-positive tumors may qualify for a “de-intensified” treatment regimen. HPV-negative HNSCC is believed to be associated with tobacco and/or alcohol use. Within the group of tumors that are HPV-positive, patients with a history of tobacco use seem to have worse outcomes compared to nonsmokers with HPV-positive tumors.18 Therefore, the presence of HPV in tobacco-associated tumors results in behavior that is intermediate between those that are HPV positive in nonusers and those that are HPV negative in tobacco users.
Despite acceptance of the link between tobacco use and carcinoma, and emerging evidence that HPV-positive tumors in non-users respond better to treatment, metabolites and biomarkers associated with tobacco have not been studied in HNSCC.
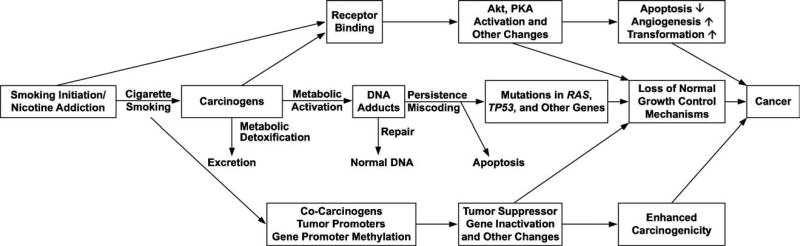
A great deal of work has been done in the field of tobacco carcinogenesis aimed at understanding mechanisms by which tobacco products induce carcinoma.19 It is widely accepted that tobacco carcinogens and their metabolites can bind covalently to DNA resulting in the formation of DNA adducts. DNA adducts, if unrepaired, can cause miscoding and permanent mutations which can activate oncogenes such as K-ras, or inactivate tumor suppressor genes such as p5320–22 (Figure 1). This has led to the use of tobacco carcinogen and toxicant metabolites and DNA adducts as biomarkers of exposure to, and metabolism of, compounds associated with tobacco use.23 Although the role of tobacco products as etiologic agents in HNSCC is not disputed, little work has been done thus far to explore the potential of these tobacco carcinogens and toxicant biomarkers in characterizing HNSCC. There are not currently any identified tobacco carcinogen or toxicant biomarkers that can distinguish between the opposing ends of the HNSCC spectrum of behavior. Furthermore, tobacco carcinogen and toxicant biomarkers have not been studied as screening tools for HNSCC.
FIGURE 1.
The development of carcinoma after tobacco-use associated carcinogenic damage.
The purpose of this review was to provide an overview of some tobacco carcinogen and toxicant biomarkers which may be associated with HNSCC. We will also discuss potential opportunities for the use of these biomarkers as screening tools and prognostic biomarkers in HNSCC.
TOBACCO METABOLITES: BACKGROUND
TOBACCO CARCINOGEN AND TOXICANT METABOLITES AND DNA ADDUCTS AS BIOMARKERS OF EXPOSURE TO TOBACCO PRODUCTS
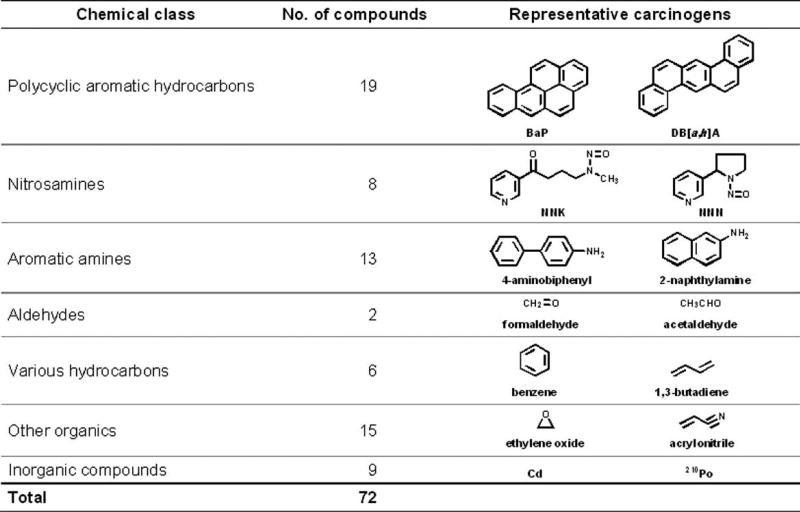
Multiple carcinogens and toxicants have been identified in tobacco products11,24–29 (Figure 2).30,31 Humans are exposed to these compounds upon use of both burned and unburned tobacco products. The common denominator in all tobacco products, and the main reason for their continued use, is the addictive compound nicotine. Nicotine is not an overt carcinogen but has been shown to act as a promoter of proliferation and survival in non-small cell lung cancer cells.32 In addition to nicotine, each puff of a cigarette or pinch of smokeless tobacco delivers a mixture of carcinogens. Humans metabolize nicotine to cotinine, 3′-hydroxycotinine, and their glucuronides. These metabolites along with several others are excreted in urine.
FIGURE 2.
Compounds in tobacco smoke that have been evaluated as carcinogens in either laboratory animals or humans by the International Agency for Research on Cancer. BaP, benzo[a]pyrene; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N’-nitrosonornicotine.
One group of carcinogens commonly associated with tobacco use is the tobacco-specific nitrosamines. These nitrosamines are formed from tobacco alkaloids during the curing and processing of tobacco.33–35 The most carcinogenic of these compounds are 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN). Two others, N′-nitrosoanabasine and N′-nitrosoanatabine are less active or inactive.34 NNK is felt to be related to the development of lung and pancreatic cancer whereas NNN is thought be a cause of esophageal cancer.20,36 Both NNK and NNN induce nasal tumors in laboratory animals.37
Another group of carcinogens which is likely to play a significant role in the induction of tobacco-related cancers is the polycyclic aromatic hydrocarbons (PAHs).38,39 In contrast to nicotine and tobacco-specific nitrosamines, these compounds are not tobacco-specific, but rather are found in all situations in which incomplete combustion of organic matter has occurred. Thus, PAHs are commonly detected in polluted air and water, engine exhaust, broiled foods, and in tobacco products. PAHs always occur as mixtures and 1 common component of these mixtures, benzo[a]pyrene, a strong carcinogen, has often been used as a surrogate for the multiple PAHs in these mixtures.
Acetaldehyde and formaldehyde are tobacco smoke constituents that are associated with head and neck cancer in both laboratory animals and humans.40,41 Acetaldehyde is also the primary metabolite of ethanol and is likely to be involved as a causative factor in alcohol-related cancers.42
Tobacco-specific biomarkers, such as those derived from nicotine or tobacco-specific nitrosamines, are felt to be a superior gauge of tobacco product exposure when compared, for example, to user-reported “cigarettes per day” estimates. This is because each tobacco user consumes his/her product of choice in a unique fashion resulting in wide variability of actual exposure for a given amount of tobacco “used.” For example, some smokers inhale more deeply, smoke more puffs, and smoke cigarettes more completely than other smokers, thereby getting a higher dose of carcinogen or toxicant per cigarette. As a result, tobacco-use quantification is best done by determination of tobacco-specific metabolites. Techniques to quantify uptake of nicotine and NNK metabolites have been refined and described.35,43–47 In addition, further work has demonstrated the feasibility and value of studying total NNN in urine.48
Biomarkers of non-tobacco specific compounds include 1-hydroxypyrene in urine, a metabolite of pyrene which occurs in all PAH mixtures, and acetaldehyde-DNA and formaldehyde-DNA adducts in leukocytes.20,36,49 These biomarkers can, under the right circumstances, distinguish between those who are exposed and unexposed to tobacco products, and may be related to risk.
Some Examples of Tobacco Carcinogen and Toxicant Biomarkers
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides
NNK is tobacco-specific and a highly effective carcinogen. It is extensively metabolized in humans, leading to a variety of compounds in urine, among which are 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (NNAL-Glucs).50 The sum of NNAL and NNAL-Glucs represents total NNAL. Although these compounds can be quantified separately, measurement of their sum is a good indicator of NNK exposure. Advantages of measuring urinary total NNAL include tobacco specificity, linkage to carcinogen intake, and consistent detection in those exposed to tobacco.
Total NNAL has proven to be highly useful in determining actual carcinogen exposure. Smokers have been reliably shown to have higher levels of NNAL when compared to nonsmokers.19 Studies of smokers’ total level of NNAL demonstrated decreased levels upon reduction or cessation of smoking.51,52
Total NNAL in the urine has been studied in nonsmoking hospitality workers.53 Participants in the study were nonsmokers who worked in restaurants or bars that permitted smoking. This study showed a statistically significant increase in urinary levels of total NNAL, nicotine, and cotinine on working days compared to nonworking days. Thus, NNAL is a reliable indicator of tobacco exposure, even when the exposure is environmental (or “second-hand”) in nature. Elevated urinary NNAL has been observed in multiple studies of second-hand cigarette smoke exposure.54
NNK uptake was shown to be similar in users of regular, light, and ultralight cigarettes. This finding is consistent with epidemiologic studies that demonstrate no protection against lung cancer in smokers of light cigarettes when compared to those using regular cigarettes.55
Recent data have shown a linear relationship between NNAL levels in smokers and risk of the development of lung cancer.56,57 In a prospective analysis of Chinese smokers, urinary levels of total NNAL were significantly associated with risk of lung cancer in a dose-dependent manner. Relative to the lowest tertile, risks associated with the second and third tertiles of total NNAL were 1.43 and 2.11, respectively, after adjustment for self-reported smoking history and urinary total cotinine. Perhaps most notably, smokers in the highest tertiles of urinary total NNAL and total cotinine exhibited an 8.5-fold increased risk for lung cancer relative to smokers with comparable smoking history but possessing the lowest tertiles of urinary total NNAL and total cotinine. These data are promising in that they offer the possibility of NNAL as a screening tool for those smokers who are at highest risk of developing lung cancer.
N′-Nitrosonornicotine
NNN is thought to play a role in both esophageal and oral cancer.58 It can be readily quantified in human urine by assaying for free, unchanged NNN plus its metabolite NNN-N-Gluc, the sum being referred to as total NNN.59 NNN and its metabolites are present in the urine of both smokers and smokeless tobacco users.48 The level of urinary total NNN was significantly higher in smokeless tobacco users when compared to smokers.59
1-Hydroxypyrene
Various markers of PAH exposure, including PAH-DNA adducts, have been used to assess exposure to carcinogens. Probably the most extensively applied and reliable biomarker of PAH exposure is 1-hydroxypyrene (1-HOP). It is a urinary metabolite of pyrene, which is always present in PAH mixtures. Since its introduction by Jongeneelen et al,60 many studies have quantified 1-HOP in the urine of people exposed to PAH.61 Sensitive, accurate, and precise high throughput assays for quantifying 1-HOP are available.62 Although 1-HOP is not tobacco specific, urinary levels are generally 2 to 3 times higher in smokers when compared to nonsmokers, although there can be considerable variation due to environmental and occupational exposure.23,52,58
Acetaldehyde DNA Adducts
Acetaldehyde is found widely in the environment and is known to be genotoxic and carcinogenic.57 It causes mutations, micronuclei, and aneuploidy in mammalian cells and gene mutations in bacteria.63,64 Levels of acetaldehyde in cigarette smoke typically range from 500 to 1000 micrograms/cigarette.31,65 Acetaldehyde exerts its carcinogenic effects by its reaction with DNA via adduct formation. A method of quantifying acetaldehyde-DNA adduct formation was developed and validated.66 This method quantifies the major DNA adduct of acetaldehyde (N2-ethylidene-dGuo) by assaying N2-ethyl-dGuo, the product of N2-ethylidene-dGuo reduction in the presence of NaBH3CN.
The study by Chen et al66 also investigated the effect of smoking cessation on levels of this acetaldehyde DNA adduct. Study subjects who had quit smoking for 4 weeks showed a 28% decrease in adduct level. Of interest, there was a race-related difference in that African Americans demonstrated a much greater average decrease in adduct levels when compared to white people upon cessation. This difference may be related to racial predilections for the development of carcinoma and thus holds promise for study in this area.
Acetaldehyde is also a product of alcohol metabolism. A typical drink contains 10 to 15 g of ethanol, much of which may be metabolized to acetaldehyde. In comparison, exposure to acetaldehyde from smoking would be about 20 mg per day, although the route of administration is different. Alcohol may act synergistically with tobacco in the development of HNSCC and is also an independent risk factor for HNSCC. As a result, acetaldehyde adduct levels may reflect the impact of both tobacco and alcohol use in patients with HNSCC. Individuals who are deficient in their ability to metabolize acetaldehyde due to polymorphisms in the aldehyde dehydrogenase gene leading to acetaldehyde accumulation and flushing, but who nevertheless consume significant amounts of alcohol, are at higher risk for head and neck cancer. The International Agency for Research on Cancer, therefore, concluded that acetaldehyde associated with alcoholic beverages is carcinogenic to humans.42
Formaldehyde DNA Adducts
Formaldehyde is considered “carcinogenic to humans” by the International Agency for Research on Cancer.24 It is genotoxic and cytotoxic, and both properties are a result of its ability to form DNA adducts, and are believed to play important roles in its carcinogenicity.24 It is well established that formaldehyde forms DNA adducts and cross-links in vitro.67–70 The most abundant of these is N6-hydroxymethyldeoxyadenosine. Until recently, there were no data in the literature on specific formaldehyde DNA adducts in humans.
In a recent investigation, we refined and extended our mass spectrometric methodology for quantitation of N6-hydroxymethyldeoxyadenosine, applying it to the analysis of human leukocyte DNA from smokers and nonsmokers.49,71 The results demonstrated clear differences in its levels between smokers and nonsmokers, indicating that this biomarker may be important for further studies on HNSCC.
Exposure versus Risk
An important distinction in the discussion of tobacco metabolites must be made between carcinogen exposure and risk of carcinoma. Metabolites such as NNAL and NNN are useful in measuring carcinogen exposure as they correlate with levels of nicotine and cotinine. However, when the levels are corrected for cotinine, cigarettes per day, and pack years of smoking, NNAL and NNN levels are elevated in those patients at greater risk for lung cancer. Therefore, these latter 2 metabolites are likely markers of exposure and risk whereas cotinine level indicates exposure alone. Further research in patients with lung cancer or HNSCC has the potential to further elucidate the nature of the metabolites discussed in this review as markers of exposure and/or markers of risk.
TOBACCO METABOLITES: POTENTIAL RESEARCH DIRECTIONS APPLIED TO HEAD AND NECK ONCOLOGY
As head and neck surgical oncologists, some of the fundamental questions we wish to address are: (1) Although many patients presenting with HNSCC are current or former tobacco users, why do only some smokers develop HNSCC whereas the majority do not? (2) Is there any way to identify these “highest risk” smokers before they develop HNSCC? (3) If so, can these patients be targeted for additional smoking cessation efforts and regular screening examinations for HNSCC?
Question #1 above is generally felt to be a didactic issue rather than a question that directly impacts patient treatment and outcome. When a patient with HNSCC and a history of tobacco use presents for treatment, the treating physician generally ascribes the HNSCC to tobacco use and carries on with the treatment. Because tobacco and alcohol consumption are implicated in 75% to 90% of HNSCC,72–74 this is a reasonable approach for treating these patients. When viewed from a disease prevention standpoint, however, it is reasonable to question what makes these patients different from those smokers who do not develop HNSCC. Why did patient “A” who has smoked 1 pack of cigarettes per day for 20 years develop HNSCC whereas several others who smoke similarly do not?
This leads to question #2: what biochemical or genetic factors make the smoker with HNSCC different from the smoker who does not develop HNSCC? The answer to this question may partially lie in variable metabolism of tobacco smoke carcinogens. That is, if some smokers are able to more efficiently break down or excrete carcinogens and their metabolites, their carcinogenic impact may be partially mitigated. This hypothesis could help to explain why those smokers may be less likely to develop a carcinoma. As discussed above, tobacco exerts carcinogenic effects via the formation of DNA adducts. Although acetaldehyde-DNA and formaldehyde-DNA adducts are described here, notable adduct formation also occurs with NNK/NNN and PAH. Individual differences in the ability to perform DNA repair would lead to variability in the significance of adduct formation among smokers. In addition to variation in repair rates, differences in formation of DNA adducts may cause some smokers to generate greater DNA damage and mutations.
A better understanding of tobacco carcinogen dose, metabolism, and DNA adduct formation provides an opportunity to identify those smokers who are at greatest risk for HNSCC. This approach focuses on the use of metabolites and DNA adducts for prevention and early detection of HNSCC. This approach is unique in that it seeks to identify those most at risk for HNSCC before it actually occurs so that these patients may be specifically targeted for smoking cessation efforts. As our healthcare system moves toward an emphasis on prevention rather than treatment, the identification of smokers who are most likely to develop HNSCC will allow for a greater focus on cancer prevention. Smoking cessation is a critical cancer prevention initiative that has the potential to prevent new cancers at multiple anatomic sites. However, an understanding of which smokers are at highest risk provides the opportunity to focus even greater attention on these smokers for cessation efforts. The NNAL data derived from a Chinese cohort of smokers discussed above suggests the possibility of screening smokers for total NNAL to inform them of their relative risk of developing a carcinoma.
When applied practically, some smokers who are informed that they are “highest risk” may quit outright, although other smokers who are identified as “highest risk” by NNAL levels may still fail smoking cessation efforts. In this subgroup of smokers, the information provided by tobacco metabolite and adduct analysis would still be very useful. Using intense clinical surveillance of these “at-risk” smokers could allow carcinomas in these patients to be detected at an earlier stage. In this way, diagnosis and treatment would occur when the disease is stage 1 or 2 rather than stage 3 or 4. A decrease in cost associated with treatment is another potential benefit of a “prevention-oriented” approach. That is, disease diagnosed earlier would require less intense treatment, less hospital time, and fewer procedures.
A final area of potential for tobacco metabolites is as prognostic markers. Just as HPV status provides information regarding the likelihood of disease control after therapy, profiling of the tobacco carcinogen and toxicant biomarkers described in this review may demonstrate patterns that correlate with prognosis. As the number of well-understood prognostic biomarkers grows larger, our ability to predict tumor behavior and response to a specific treatment will gradually be refined. This can result in improved patient outcomes with minimized morbidity via tailored treatment.
In summary, tobacco carcinogen and toxicant biomarkers represent an excellent opportunity for head and neck oncologists as potential tools for prevention of, and screening for, HNSCC. The early data using these metabolites in the arena of lung cancer are very promising and suggest that urinary total NNAL, possibly together with other biomarkers, may be used to screen for smokers at highest risk for developing carcinoma. Furthermore, the as yet unstudied potential role of tobacco carcinogen and toxicant metabolites and DNA adducts as biomarkers of tumor behavior and prognosis warrants investigation to further characterize HNSCC in the tobacco user.
Acknowledgments
Dorothy Hatsukami recieves fund from Nabi Biopharmaceuticals for his clinical trial examining nicotine immunotherapy.
References
- 1.WHO. Tobacco or health: a global status report. Geneva: World Health Organization; 1997. [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Hill A. Lung cancer and other causes of death in relation to smoking: a second report on the mortality British doctors. Br Med J. 1956;2:1071–1081. doi: 10.1136/bmj.2.5001.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond EC, Horn D. The relationship between human smoking habits and death rates: follow-up study of 187,766 men. JAMA. 1954;155:1316–1328. doi: 10.1001/jama.1954.03690330020006. [DOI] [PubMed] [Google Scholar]
- 6.Hammond E, Horn D. Smoking and death rates — report on forty-four months of follow-up on 187,783 men. JAMA. 1958;166:1159–1172. doi: 10.1001/jama.1958.02990100047009. [DOI] [PubMed] [Google Scholar]
- 7.IOM (Institute of Medicine) Ending the tobacco problem: A blueprint for the nation. Washington, DC: The National Academic Press; 2007. [Google Scholar]
- 8.MMWR. Cigarette smoking among adults and trends in smoking cessation — United States, 2008. MMWR: Centers for Disease Control; 2009. pp. 1227–1232. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148. [PubMed] [Google Scholar]
- 10.Mackay J, Eriksen M. The Tobacco Atlas. Geneva: World Health Organization; 2002. [Google Scholar]
- 11.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon (France): 2004. Tobacco smoke and involuntary smoking. [PMC free article] [PubMed] [Google Scholar]
- 12.Schlecht NF, Franco EL, Pintos J, Kowalski LP. Effect of smoking cessation and tobacco type on the risk of cancers in the upper aero-digestive tract in Brazil. Epidemiology. 1999;10:412–418. doi: 10.1097/00001648-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 14.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004;31:726–733. doi: 10.1053/j.seminoncol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Sturgis EM. A review of social and behavioral efforts at oral cancer preventions in India. Head Neck. 2004;26:937–944. doi: 10.1002/hed.20075. [DOI] [PubMed] [Google Scholar]
- 16.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in orpharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 18.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 21.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 23.Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IARC. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Lyon (France): 1986. Tobacco smoking. [Google Scholar]
- 25.Hoffmann D, Adams JD, Lisk D, Fisenne I, Brunnemann KD. Toxic and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst. 1987;79:1281–1286. [PubMed] [Google Scholar]
- 26.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 27.Swauger JE, Steichen TJ, Murphy PA, Kinsler S. An analysis of the mainstream smoke chemistry of samples of the U.S. cigarette market acquired between 1995 and 2000. Regul Toxicol Pharmacol. 2002;35(2 Pt 1):142–156. doi: 10.1006/rtph.2001.1521. [DOI] [PubMed] [Google Scholar]
- 28.IARC, editor. IARC. Smokeless Tobacco and Tobacco-Specific Nitrosamines. Lyon: 2007. pp. 57–86. [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS. Etiology of cancer: tobacco. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Wolters Kluwer/ Lippincott Williams & Wilkins; 2008. pp. 147–155. [Google Scholar]
- 30.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 31.IARC. Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2004:35–102. [PMC free article] [PubMed] [Google Scholar]
- 32.Tsurutani J, Castillo SS, Brognard J, et al. Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–1995. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 33.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 34.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 35.Spiegelhalder B, Bartsch H. Tobacco-specific nitrosamines. Eur J Cancer Prev. 1996;5(Suppl 1):33–38. [PubMed] [Google Scholar]
- 36.Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–294. [PubMed] [Google Scholar]
- 37.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 38.Luch A. In: Polycyclic aromatic hydrocarbon-induced carcinogenesisan introduction. Luch A, editor. London: Imperial College Press; 2005. pp. 1–18. [Google Scholar]
- 39.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 40.IARC. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-Ol. Lyon, FR: 2006. [PMC free article] [PubMed] [Google Scholar]
- 41.IARC. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide (part two) 1999 [PMC free article] [PubMed] [Google Scholar]
- 42.Secretan B, Straif K, Baan R, et al. A review of human carcinogens–Part E: tobacco, areca, nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 43.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers’ urine. Cancer Res. 1993;53:721–724. [PubMed] [Google Scholar]
- 44.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1257–1261. [PubMed] [Google Scholar]
- 45.Carmella SG, Han S, Villalta PW, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers’ blood. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2669– 2672. doi: 10.1158/1055-9965.EPI-05-0129. [DOI] [PubMed] [Google Scholar]
- 46.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 47.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 48.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Cheng G, Balbo S, Carmella SG, Villalta PW, Hecht SS. Clear differences in levels of a formaldehyde-DNA adduct in leukocytes of smokers and nonsmokers. Cancer Res. 2009;69:7170–7174. doi: 10.1158/0008-5472.CAN-09-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1257–1261. [PubMed] [Google Scholar]
- 51.Hecht SS, Murphy SE, Carmella SG, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–115. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- 52.Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tulunay OE, Hecht SS, Carmella SG, et al. Urinary metabolites of a tobacco-specific carcinogen in nonsmoking hospitality workers. Cancer Epidemiol Biomarkers Prev. 2005;14:1283–1286. doi: 10.1158/1055-9965.EPI-04-0570. [DOI] [PubMed] [Google Scholar]
- 54.Hecht SS, Carmella SG, Le KA, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2006;15:988–992. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- 55.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693–698. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 56.Yuan JM, Koh WP, Murphy SE, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Dept. of Health and Human Services. Report on carcinogens. 11. Research Triangle Park, NC: 2004. pp. III-1–III-3. [Google Scholar]
- 58.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 59.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 60.Jongeneelen FJ, Anzion RB, Leijdekkers CM, Bos RP, Henderson PT. 1-hydroxypyrene in human urine after exposure to coal tar and a coal tar derived product. Int Arch Occup Environ Health. 1985;57:47–55. doi: 10.1007/BF00383545. [DOI] [PubMed] [Google Scholar]
- 61.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies–a review. Int J Hyg Environ Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Carmella SG, Le KA, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol Biomarkers Prev. 2004;13:1261–1264. [PubMed] [Google Scholar]
- 63.IARC. Allyl compounds, aldehydes, epoxides and peroxides. iarc monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol. 36. Lyon, France: 1985. pp. 101–132. [PubMed] [Google Scholar]
- 64.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: 1999. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide (part two) pp. 318–335. [PMC free article] [PubMed] [Google Scholar]
- 65.Chepiga TA, Morton MJ, Murphy PA, et al. A comparison of the mainstream smoke chemistry and mutagenicity of a representative sample of the US cigarette market with two Kentucky reference cigarettes (K1R4F and K1R5F) Food Chem Toxicol. 2000;38:949–962. doi: 10.1016/s0278-6915(00)00086-7. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Wang M, Villalta PW, et al. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chem Res Toxicol. 2007;20:108–113. doi: 10.1021/tx060232x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaw YF, Crane LE, Lange P, Shapiro R. Isolation and identification of cross-links from formaldehyde-treated nucleic acids. Biochemistry. 1980;19:5525–5531. doi: 10.1021/bi00565a010. [DOI] [PubMed] [Google Scholar]
- 68.Beland FA, Fullerton NF, Heflich RH. Rapid isolation, hydrolysis and chromatography of formaldehyde-modified DNA. J Chromatogr. 1984;308:121–131. doi: 10.1016/s0021-9673(01)87539-7. [DOI] [PubMed] [Google Scholar]
- 69.Huang H, Hopkins PB. DNA interstrand cross-linking by formaldehyde: nucleotide sequence preference and covalent structure of the predominant cross-link formed in synthetic oligonucleotides. J Am Chem Soc. 1993;115:9402–9408. [Google Scholar]
- 70.Cheng G, Shi Y, Shana SJ, et al. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem Res Toxicol. 2003;16:145–152. doi: 10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 71.Wang M, Cheng G, Villalta PW, Hecht SS. Development of liquid chromatography electrospray ionization tandem mass spectrometry methods for analysis of DNA adducts of formaldehyde and their application to rats treated with N-nitrosodimethylamine or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem Res Toxicol. 2007;20:1141–1148. doi: 10.1021/tx700189c. [DOI] [PubMed] [Google Scholar]
- 72.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 73.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 74.Tuyns AJ, Estève J, Raymond L, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France) Int J Cancer. 1988;41:483–491. doi: 10.1002/ijc.2910410403. [DOI] [PubMed] [Google Scholar]