Abstract
Putative mechanisms leading to the development of alcoholic cardiomyopathy (ACM) include the interrelated cellular processes of mitochondria metabolism, oxidative stress and apoptosis. As mitochondria fuel the constant energy demands of this continually contracting tissue, it is not surprising that alcohol-induced molecular changes in this organelle contribute to cardiac dysfunction and ACM. As the causal relationship of these processes with ACM has already been established, the primary objective of this review is to provide an update of the experimental findings to more completely understand the aforementioned mechanisms. Accordingly, recent data indicate that alcohol impairs mitochondria function assessed by membrane potential and respiratory chain activity. Indictors of oxidative stress including superoxide dismutase, glutathione metabolites and malondialdehyde are also adversely affected by alcohol oftentimes in a sex-dependent manner. Additionally, myocardial apoptosis is increased based on assessment of TUNEL staining and caspase activity. Recent work has also emerged linking alcohol-induced oxidative stress with apoptosis providing new insight on the codependence of these interrelated mechanisms in ACM. Attention is also given to methodological differences including the dose of alcohol, experimental model system and the use of males versus females to highlight inconsistencies and areas that would benefit from establishment of a consistent model.
Keywords: reactive oxygen species, respiratory chain, ethanol, heart, caspase
Introduction
Alcoholic cardiomyopathy (ACM) is a consequence of excessive alcohol intake for a prolonged period of time and is oftentimes accompanied by impaired cardiac contractility and function (e.g., blood pressure, cardiac output, etc.) (1). As no definitive diagnostic criteria have been established, ACM is typically diagnosed based on exclusion of other contributing factors in patients with dilated cardiomyopathy and a history of excessive alcohol use. In developed countries where per capita alcohol consumption is high, ACM represents a leading cause of non-ischemic dilated cardiomyopathy (2). The prevalence of ACM has been reported to represent between 4 – 47% of all cases of dilated cardiomyopathy and this high variability appears largely dependent on the specific inclusion criteria (i.e., cumulative alcohol intake, sex, geographic location) used for its classification (3–7). While not all those classified with alcohol use disorder (AUD) develop the disease, its contribution to mortality associated with alcohol intake is unequivocal. Therefore, determining the cellular and molecular factors leading to the onset and progression of ACM is central to developing effective treatment strategies.
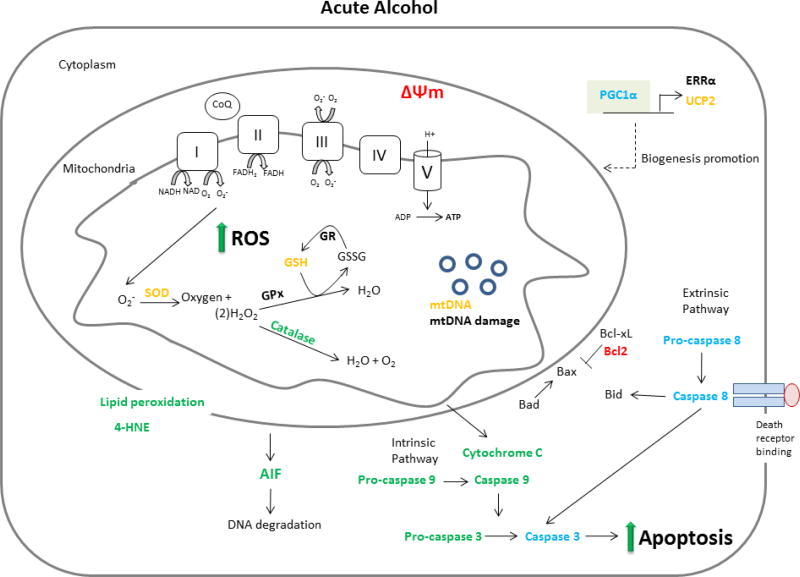
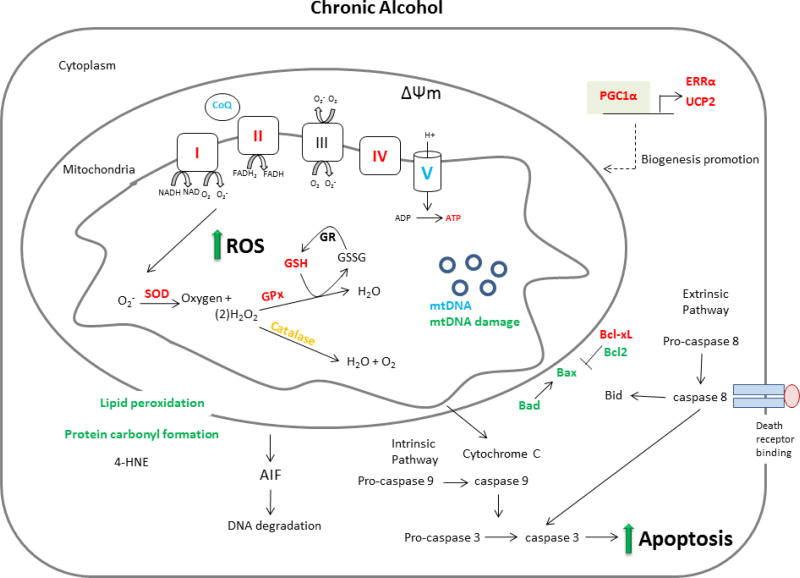
ACM is a multifactorial disease and defects in mitochondrial function, oxidative stress and apoptosis appear fundamental to its etiology. The aim of this article is to provide an unbiased review of the new and important discoveries related to the role and association between mitochondria, oxidative stress and apoptosis in the development of ACM. To achieve this objective and provide a concise presentation, only research published between 2010 and the first half of 2017 (when this review was written) will be discussed aside from the limited inclusion of seminal articles that provide the context necessary for the understanding of the current findings. As such, the reader is directed to other high quality reviews for further historical perspective (8–11). The scope of this review is therefore limited to work published since 2010 that included the measurement of mitochondria function, oxidative stress and/or apoptosis subsequent to either acute or chronic alcohol treatment in vitro, in animal models or in samples from human. Additionally, effort was made to identify methodological differences, including those related to alcohol dose and duration as well as the sex of the subject as these represent an important source of variability that likely contributed to observed differences in experimental outcomes. Finally, to ensure an unbiased review of the literature, we have included all well-designed studies whether or not their findings support the prevailing hypothesis in an area. Figures 1 and 2 which summarize the response of heart and cardiomyocytes to acute and chronic exposure to alcohol, respectively, are presented to provide a visual framework for which the subsequent literature review.
Figure 1.
Overview of the impact of acute alcohol intoxication on mitochondrial mediated aspects of ACM. Those factors listed in red were decreased by alcohol, those in green were increased and those in blue were unchanged. Orange print indicates that measured changes were inconsistent between studies and black signifies that no measurement was made in the included literature between 2010–2017.
Figure 2.
Overview of the impact of chronic alcohol intoxication on mitochondrial mediated aspects of ACM. Those factors listed in red were decreased by alcohol, those in green were increased and those in blue were unchanged. Orange print indicates that measured changes were inconsistent between studies and black signifies that no measurement was made in the included literature between 2010–2017.
1. Mitochondria
1.1 Structure and function
The impact of alcohol on heart mitochondria structure was established by early work that demonstrated alcohol produced mitochondria enlargement and degeneration of inner mitochondria membrane folds (12, 13). These findings were recently extended to show that chronic alcohol consumption decreased mitochondrial number, but not size (14), and increased mitochondria fragmentation (15).
1.1.1 Membrane potential
Consistent with the reported structural alterations, alcohol also impaired mitochondrial function. Mitochondrial membrane potential or more specifically early membrane depolarization has been used as an indicator of mitochondrial dysfunction (16). When cardiomyocytes were cultured with alcohol (100 mM, 6 days or 50–200 mM, 24 hr) the membrane potential was decreased and the percentage of depolarized mitochondria was increased (17–19). Acute alcohol intoxication (1 or 3 day, 3g/kg) in male mice led to a similar decrement in membrane potential (20, 21). The generation of alcohol metabolites may have contributed to the alcohol mediated mitochondrial changes as the decrease in membrane potential was greater in mice lacking aldehyde dehydrogenase (ALDH) or alcohol dehydrogenase (ADH) than in wild-type littermates (20, 21). Dysregulation of the SHC (SRC homology 2 containing) (p66Shc)/Pin1 pathway was also implicated in the alcohol-induced change in mitochondrial membrane potential. Specifically, Pin1 overexpression exacerbated the alcohol-induced decrease in mitochondrial membrane potential, whereas mitochondrial dysfunction was prevented by the knockdown of Pin1 or blockade of either p66Shc or protein kinase C (PKC)-β (18, 19).
1.1.2 Citric acid cycle and respiratory chain complexes
Reduced enzyme activity and the content of proteins in the tricarboxylic acid (TCA) cycle and electron transport chain (ETC) are also indicative of the detrimental effects of alcohol on mitochondria function. For example, past (pre-2010) work reported that alcohol decreased the mitochondrial respiratory control index/ratio (RCI) (the ratio of state III/IV respiration) (22, 23), state III respiration, and mitochondrial oxygen consumption suggesting uncoupling of oxidation phosphorylation (8, 22–27). Further, alcohol decreased the activity of several enzymes within the TCA cycle providing additional evidence of its negative impact on mitochondrial function (8, 28).
This work has recently been extended and alcohol-induced changes in respiratory chain components have now been quantified. First, complex I which is responsible for NADH oxidation may be dose-dependently decreased by alcohol. No change in complex I activity was detected in male rats provided beer (5.4% alcohol) for 21 days or male mice fed alcohol (5% v/v liquid diet) for 10 days (29, 30). However, when more severe alcohol feeding paradigms were used, including 10 days of alcohol in combination with a single binge episode, and 20 or 40 days of alcohol intake supplemented with and without binges every 10 days (male mice) complex I activity was decreased (30).
While complex I transfers protons across the inner mitochondrial membrane to create an energy gradient, complex II transfers electrons via flavin adenine dinucleotide (FAD+) and is the only complex that is part of both the TCA cycle and ETC. As electrons are gained in the conversion of succinate to fumarate, succinate dehydrogenase activity is often assessed as a marker of complex II activity. Succinate dehydrogenase activity was decreased in cardiomyocytes after both long-term (6 days; 10, 50, 100 mM) and short-term (24 h; 100, 200 mM) alcohol exposure (17, 31). This decrease in complex II activity was also evident in vivo in male mice after 20 days of alcohol (including 2 binge episodes) or 40 days of alcohol (with or without 4 binge episodes) (30). Conversely, when the alcohol dose or duration was lowered the effect on complex II activity was also lost (30, 31).
Alcohol treatment of cardiomyocytes (50, 100 and 200 mM for 24 h) also dose-dependently decreased complex IV. Additionally, chronic alcohol intake in male mice (20 or 40 days plus binge episodes every 10 days) or male rats (4 months of alcohol feeding) reduced cytochrome oxidase activity (17, 30). Acute alcohol intoxication (3 days, 3 g/kg/day) also decreased myocardial cytochrome c oxidase (COX IV) protein content in male mice (32). This decrease may be mediated by AMP-activated protein kinase (AMPK) as it was partially ameliorated by whole-body AMPK knockout (32). Coenzyme Q (CoQ) is an essential component of the respiratory chain as it carries electrons from complex I and II to complex III. Limited data in this regard indicated that CoQ activity in heart did not differ between control and alcohol-fed (21 days of beer 5.4% alcohol) rats (29). Overall, dose- and/or time- dependent effects of alcohol on respiratory complex activity were observed with higher alcohol doses and treatment durations leading to decreased enzyme activity.
1.1.3 ATP synthesis
Finally, protein expression of the α- and β-subunits of mitochondrial ATP synthase has been measured in cardiomyocytes following acute alcohol. While no change was detected 30 min post alcohol, higher concentrations alcohol (50 mM and 100 mM but not 10 mM) for 24 hr increased both α- and β-subunit (33). There were several noteworthy aspects of these findings. First, decreased, not increased subunit abundance would be expected based on prior reports of the negative effects of alcohol on respiratory chain activity (17, 30, 31). Further, no concurrent change in cellular ATP content was observed under these experimental conditions. These data are in contrast to the findings from several other studies that showed alcohol decreased ATP levels. For instance, decreased ATP content was observed in cardiomyocytes after 6 d of alcohol exposure (50 mM) (17) and in whole heart from alcohol-fed (18% in drinking water, 3 months) male mice (34). Similarly, ATPase activity, ATP content as well as adenine nucleotide translocator 1 (ANT1) mRNA, protein and activity were all reduced after 2, 4 or 6 months of alcohol feeding in male rats (14). Second, alcohol only altered protein expression (not mRNA levels) suggesting the time point of the measurement was not optimal for detecting changes in mRNA, the alcohol effect was primarily at the translational level, or ANT1 protein degradation was reduced as a compensatory mechanism (33). Lastly, the increased expression of ATP synthase subunits may represent a compensatory response to the increased mitochondrial size discussed above. These findings are in contrast to the reported decrease in respiratory chain activity and indicate that further verification of the effects of alcohol on ATP production and its associated machinery is required especially using in vivo systems.
1.2 Regulators of mitochondrial biogenesis
1.2.1 PGC1α, UCP2 and ERRα
Alcohol may also impact mitochondrial function via regulation of proteins implicated in mitochondria biogenesis including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (15, 30, 35). Specifically, chronic alcohol intake (4% v/v liquid diet, 16 wk) in male mice decreased PGC-1α protein in heart (15) and 10 to 40 days of chronic alcohol feeding paired with a binge episode every 10 days decreased PGC-1α mRNA (30). In contrast, myocardial PGC-1α protein or mRNA content were not altered by acute alcohol (1 g/kg, 3 days) (35) possibly suggesting a time- or dose-dependent effect on this endpoint. PGC-1α enhances mitochondrial biogenesis via up-regulation of uncoupling protein-2 (UCP2) and estrogen related receptor alpha (ERRα) (36). Consistent with the effect observed for PGC-1α, chronic alcohol feeding (4% v/v liquid diet, 16 wk) decreased UCP2 protein in male mice (15), whereas in contrast, acute intoxication (3 days, 1 g/kg) did not alter UCP2 protein content (35). Lastly, 10–40 days (± binge episodes at 20 and 40 days) of alcohol intake in male mice decreased ERRα mRNA which was in agreement with its regulation by PGC-1α (30). Therefore, it was concluded that chronic alcohol intake suppressed PGC-1α, UCP2 and ERRα, whereas acute doses of alcohol lasting less than 3 days were not sufficient to impair the expression of these regulatory factors.
1.2.2 mtDNA
As mitochondria contain their own DNA, the abundance of mitochondrial DNA (mtDNA) provides a measure of biogenesis or damage to the organelle. Results from early studies were equivocal as mtDNA content was reported to be either increased (37) or unchanged (38) after chronic alcohol consumption. Acute consumption led to time-dependent changes characterized by an initial transient decrease in mtDNA followed by an increase at 24 and 48 hr (39). The most recent work by Laurent et al. (2014) showed that mtDNA copy number was decreased and mtDNA damage was increased in cardiomyocytes exposed to alcohol [6 days, 100 mM (but not 50 mM)] (17). Further, chronic alcohol feeding in male rats (10% v/v in water, 4 mo) selectively increased mtDNA damage assessed by increased mitochondrial topoisomerase-dependent DNA strand breakage without altering copy number (17). Therefore, alcohol may damage the replicative potential of mitochondria in addition to the functional aspects for which mtDNA primarily encodes. The mtDNA damage may have resulted from an alcohol-mediated induction of oxidative stress as mitochondria are a primary site of reactive oxygen species (ROS) production. With this in mind, the following section discusses the literature related to oxidative stress produced by alcohol in heart in relation to ACM.
2 Oxidative stress
Mitochondria produce reactive oxygen species which, depending on the context and concentration, have either adaptive or damaging effects. For example, high levels of ROS production may eventually lead to apoptosis by promoting oxidation of lipids, proteins and DNA, as is seen in the development of ACM (40, 41). For the purpose of this review when ROS was experimentally reported (and not specified), it was assumed to include the generation of oxygen free radicals, such as superoxide anion radical (O2·−) and hydroxyl radical (˙OH), and non-radical oxidants, such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) among other free radical molecules. In general, past work indicates that alcohol (acute and chronic) increased or did not change myocardial oxidative stress, although the specific markers used to reach this conclusion varied by study and model making definitive conclusions elusive. For example, the activities of catalase that accelerates H2O2 breakdown and superoxide dismutase (SOD) which has anti-oxidant properties were increased (42–45) or unchanged (46, 47) by chronic alcohol (8, 45–47). Further, the myocardial content of the anti-oxidant glutathione (GSH) (48) and glutathione peroxidase (47) was decreased in response to chronic alcohol feeding. The potential importance of increased oxidative stress in ACM was also evidenced by data indicating that various anti-oxidant agents including vitamin E, cyanidanol-3 (48) and N-acetylcysteine (47) ameliorated oxidative stress markers relative to ACM. Despite the general consensus that oxidative stress contributes to ACM development current results have been somewhat contradictory meriting further study to better define the reasons for the apparent discrepancy. As the role of oxidative stress in ACM has been well-defined in several prior reviews (8, 11, 49, 50) it is our intent to focus solely on recent data and provide a more detailed look at the influence of alcohol on each oxidative product while accounting for differences in alcohol dose and treatment schedule.
2.1 Alcohol and ROS production
Levels of ROS assessed in vitro, ex vivo and in vivo are generally increased following alcohol exposure; however, time- and dose-dependent differences exist. For example, in isolated cardiomyocytes, alcohol (50, 100 and 200 mM; 24 h) increased ROS production (18, 19). Likewise, a high (100 mM) concentration of alcohol (for 30 min) increased ROS in cardiomyocytes harvested following ex vivo perfusion of male rat hearts, although a lower alcohol concentration (5 mM) decreased hydrogen peroxide (51). Further, a low alcohol concentration (20 mM) for a short period of time (5 min-1 hr) also did not change ROS production in human aortic cells (52).
In vivo studies examining ROS production after acute alcohol intoxication have also provided inconsistent results as ROS was increased at 10, 15, 20, 25 and 30 min after an acute bolus of alcohol (1 g/kg) in male rats (53), but not in a separate investigation that used similar doses and exposure times (0.5, 1, and 1.5 g/kg; 0–60 min) (54). The later study, however, established a role for sex hormones as estrogen (E2) administration to male rats prior to the alcohol bolus (1 g/kg and 1.5 g/kg) increased ROS (54) to match the increase normally observed in female rats (0.5 or 1.5 g/kg; 1 g/kg, intragastric) (55, 56). Moreover, ovariectomy prevented the alcohol-induced increase, while exogenous administration of E2 restored the normal response and strongly supported a sexual dimorphic effect.
Similar to acute alcohol, chronic alcohol feeding (either 6 wks or 16 wks) increased superoxide production, a marker of ROS, in male mice (15, 57). The selective overexpression of insulin-like growth factor – I (IGF-I) in heart antagonized this increase (15), as did the administration of a cytochrome P450 2E1 (CYP2E1) inhibitor that blocked the metabolism of alcohol (57). Overall, the consensus supports an alcohol-induced increase in ROS. Over the long-term the tendency for alcohol to increase ROS may contribute to the development of ACM, although acutely the impact of alcohol on ROS is less clear. Further work is needed to clarify the sex-specific effects as well as other mechanisms and targets for ameliorating ROS production following alcohol intake.
2.2 Superoxide dismutase and byproducts
The enzyme superoxide dismutase (SOD) degrades the superoxide anion (O2·−) radical into oxygen and hydrogen peroxide (less harmful than O2·−) and thereby plays an antioxidant role in the control of alcohol-related oxidative damage. There are three primary SOD enzymes specifically, copper/zinc-containing SOD (Cu/ZnSOD, SOD1), manganese SOD (MnSOD, SOD2) and extracellular superoxide dismutase (ECSOD, SOD3). As much of the work presented below reported SOD levels without specifying which enzyme was measured, those findings will be discussed first before the specific enzymes are highlighted in relation to acute and chronic alcohol intake.
2.2.1 Superoxide dismutase
The preponderance of data indicate that chronic alcohol consumption decreased SOD activity in cardiac tissue of mice and rats (58–62). Daily feeding regimes as short as 10 days (1.7 g/kg/day, mice) (61) or 15 days (3 g/kg/day, rats) (62) decreased cardiac SOD activity. Baring one exception (63), longer durations of alcohol consumption (4–16 wks) of varying doses in mice or rats reduced SOD activity (58–60). In contrast, acute alcohol intoxication yielded more variable results as SOD activity has been reported to be either increased (64) or decreased (65, 66). However, methodological differences between those studies precluded direct comparison and identification of a single contributing factor (64–66). For instance, a single dose of alcohol (12 mL/kg; 50% alcohol solution) administered to female (Sprague Dawley) rats 12 h prior to tissue collection decreased SOD activity (65) as did 5 doses (2 g/kg) in male rats (66). In contrast, 3 doses of alcohol (5 g/kg/dose) given every 12 h to female Sprague Dawley rats increased SOD activity in heart isolated 6 h after the last dose of alcohol (64). The increase in SOD activity in this later study was hypothesized to be an adaptive response to protect the myocardium from alcohol damage similar to the cardio-protective effects commonly reported at low doses; however, the doses used were relatively high and this hypothesis was not experimentally addressed (64). Therefore, excluding this last study, acute and chronic alcohol decreased SOD activity leading to oxidative damage.
2.2.2 Antioxidants
The alcohol-induced decrease in SOD activity has been successfully ameliorated by several interventions administered prior to acute alcohol intoxication or during long-term alcohol feeding. For example, pretreatment (30 days) with the anti-oxidants selenium or vitamin E partially offset the acute alcohol-induced decrease in SOD activity (65). Caloric restriction was also protective as 5 wks of a restricted diet (30–40% of normal intake) prevented the decrease in SOD activity caused by 5 doses of alcohol (2 g/kg/dose, every 12 hr) given over the last 3 days of the dietary restriction period (66). In contrast, a more severe caloric restriction (i.e., 40–50% of normal intake) exaggerated the alcohol-induced decrease in SOD (66). In the setting of chronic alcohol intake, an anti-oxidant extract from the thespesia populnea plant prevented the alcohol-induced decrease in SOD activity (59) as did daily supplementation with sodium hydrosulfide (NaHS), a donor of hydrogen sulfide (H2S) (60).
2.2.3 Specific SOD enzymes
Similar to SOD, chronic alcohol feeding to rats or mice decreased Cu/ZnSOD activity (15, 66), while activity was either decreased (4 g/kg/d, 3 days) (66) or unchanged after acute alcohol (1 g/kg, 3 days) (35). The chronic alcohol-related decrease was ameliorated by NaHS treatment (60) or cardiac-specific overexpression of IGF-I (15), suggesting plausible mechanisms for the alcohol-mediated increase in oxidative stress.
Manganese superoxide dismutase (MnSOD) metabolizes the superoxide free radical produced during mitochondrial respiration and maintains homeostasis in response to cellular oxidative stress. Both acute alcohol intoxication (5 doses in 3 days, 2 g/kg) (66) and chronic feeding in male rats (18 wks, 30% alcohol in water + 12 mL/kg, 3x/day, via intragastric administration) (60) decreased MnSOD. Analogous to SOD and Cu/ZnSOD, treatment with NaHS also offset the chronic alcohol-induced decrease in MnSOD (60).
The last isoform of the SOD enzyme is the anti-oxidant SOD3 that is secreted into the extracellular fluid and binds to the surface of endothelial cells and the extracellular matrix. Reduced levels of SOD3 are observed in heart failure and are associated with an increased risk of hypertension and ischemic heart disease, while elimination of SOD3 leads to cardiomyocyte hypertrophy and fibrosis (67–70). Despite its association with heart disease, only a single study has measured the effect of alcohol on its activity. Ex vivo perfusion of the heart with low levels of alcohol (5 mM) decreased cardiac SOD3, whereas moderate levels of alcohol (25 mM) did not alter SOD3 and high levels (100 mM) increased SOD3 content (51). Hence, the regulation of myocardial SOD3 by alcohol was dose-dependent, at least in response to acute alcohol exposure. However, these results contradicted the inverse relationship between alcohol dose and SOD activity observed for the other SOD isoforms. Additional work is needed to confirm these observations and clarify this relationship.
2.2.4 Hydrogen peroxide, metabolites and related enzymes
Hydrogen peroxide (H2O2), typically produced from the reaction catalyzed by SOD, is degraded to a molecule of water and oxygen by catalase thereby reducing the oxidant burden. The effect of chronic alcohol on catalase may be duration-dependent as enzyme activity was increased after 7–8 wks of alcohol intake (58, 63) while a shorter duration of alcohol ingestion (15, 30 or 37 days) decreased catalase (59, 62, 71). The relative importance of sex hormones in regulating catalase activity has also been highlighted by data that showed that estrogen pretreatment increased catalase activity in male rats in a binge drinking model (54, 56) and after a single acute dose of alcohol (0.5, 1.0, 1.5 g/kg) (54). Conversely, increased catalase activity was not seen in ovariectomized female rats given an acute dose of alcohol (1.5 g/kg) but was observed after estrogen replacement (56). Therefore, these data suggest a potential mechanism by which females maybe more susceptible to ACM.
Hydrogen peroxide can also be metabolized to water by glutathione peroxidase (GPx) which in the process converts glutathione (GSH) to glutathione disulfide (oxidized glutathione; GSSG). Alcohol has been reported to downregulate both GPx and GSH (59, 60, 63, 71, 72) in all but one study (58). GPx may also be influenced by sex as no change was observed following acute or chronic alcohol intake in female rats (64, 72). The anti-oxidant GSH was also decreased by chronic alcohol in mice and rats after shorter periods of alcohol (10–30 days) (59, 61, 62, 71), while it was increased in response to alcohol consumption of longer duration (50 days) (58). In agreement, acute alcohol intoxication (1 dose (65) or 3 doses (64)) decreased (65) or did not change (64) GSH in myocardial tissue from female rats. Similar to SOD, pretreatment with Vitamin E or selenium partially ameliorated the acute alcohol-induced decrease in GSH (65). Likewise, treatment with either NaHS (60) or a processed fish protein hydrolysate with a high anti-oxidant capacity (62) attenuated the chronic alcohol-induced decrease in GPx.
Another important enzyme in ROS detoxification is glutathione S-transferase (GST), a phase II enzyme that allows endogenous water soluble substrates like GSH to activate and remove xenobiotics. The effect of alcohol on this important anti-oxidant is inconsistent both in past (pre 2010) and more current work as chronic alcohol increased (46, 58) or decreased (59, 73) GST in male rats and GST content was unchanged in female rats (74). The length of alcohol treatment and dose differed in each study and these variables along with sex differences could explain the discrepant findings. Similar to chronic alcohol fed female rats, acute alcohol (1.5 days) did not alter GST levels in female rats (64). Overall, the consensus indicates that alcohol downregulates the myocardial content of various anti-oxidants, including those important in the metabolism of hydrogen peroxide. The highlighted work also exemplifies the sex-dependent nature of several of the anti-oxidants potentially meriting different sex based prevention and treatment strategies.
2.3 Additional markers of oxidative stress
2.3.1 Protein carbonylation
Protein carbonyl status is also used as an indicator of oxidative stress due to the protein oxidation caused by ROS (including superoxide, hydrogen peroxide and other radical species). Protein carbonyl is generally found to be increased in response to chronic alcohol ingestion (57, 59, 62, 65), although one report noted no significant change after 8 wks of alcohol feeding (male Wistar rats, 30% v/v in drinking water) (63). The alcohol-induced increase in protein carbonyl was prevented by pretreatment with the anti-oxidants selenium or vitamin E (65), administration of sardinelle heads (62), or inhibition of alcohol metabolism by blocking CYP2E1 (57).
2.3.2 Malondialdehyde (MDA)
Similar to protein carbonyl production, lipids can also be modified and indicative of oxidative stress. Malondialdehyde (MDA) is commonly used as a marker of lipid peroxidation and is typically assessed via the thiobarbituric acid reactive substances (TBARS) assay, although this assay can be somewhat nonspecific as it can react with other aldehydes in addition to MDA (75). The majority of results obtained from studies using acute alcohol models showed myocardial MDA to be increased in both male and female rats (56, 64–66); however, exceptions were noted (54, 55) and as with other variables discussed it was not possible to discern whether such differences are due to sex, time post-alcohol and/or dose. In regards to sex, or more accurately the presence of estrogen, MDA was increased in male rats following 5 doses of alcohol (1 dose every 12 hr) (66), while in another study estrogen was required for the induction of MDA by alcohol (54). Similarly, ovariectomy in female mice prevented the acute alcohol-induced increase in MDA until estrogen was replaced (56). Dose and time effects were also noted as myocardial MDA content was not altered by low (0.5 g/kg) (54, 56) or moderate (1 g/kg) doses of alcohol at time points earlier than 60 – 90 minutes (55).
Although consistent increases in MDA were seen in rats after chronic alcohol feeding ranging from 15 days −18 wks (59, 60, 62, 71), one study showed no change following 50 days of alcohol (58), while another reported MDA was decreased after 8 wks of alcohol consumption (63). As the duration and dose of alcohol differed in each of these studies, the consensus of evidence favored an alcohol-induced increase in lipid peroxidation. Lastly, as with other oxidative markers discussed, treatment with NaHS or anti-oxidants either reduced or prevented the alcohol-induced increase in MDA (60, 62).
2.3.4 4-hydroxynonenal (4-HNE)
Another marker of oxidative stress and lipid peroxidation is 4-hydroxynonenal (4-HNE). As with MDA, the effects of alcohol on 4-HNE appear dose, time and sex-dependent. At alcohol concentrations greater than 1 g/kg, acute intoxication increased 4-HNE, although not before 60 minutes (55, 56). Similar to MDA, ovariectomy prevented the alcohol-induced increase in 4-HNE in female mice, while estrogen replacement restored the response (56). Future work should assess cardiac 4-HNE levels in response to chronic alcohol feeding as well as directly validate the sex-dependent effect.
2.3.5 Heat shock proteins
Heat shock proteins (Hsp) play an important role in cellular protection from oxidative stress; however, from the limited available data they do not appear to have a prominent role in mediating the development of ACM. For example, alcohol did not significantly alter heat shock factor 1 (HSF1) (chronic alcohol) (61), Hsp25 (chronic alcohol) (58), Hsp 70 (chronic alcohol) (74), Hsp72 (chronic alcohol) (61), or Hsp90 (acute alcohol) (32). However, lower levels of alcohol intake in male mice increased total and phosphorylated Hsp27 (76). In contrast, work published before 2010 indicated that Hsp 60 and 70 were decreased in hearts from chronic alcohol-fed rats (77, 78). Differences between in vitro and in vivo findings were also evident in previous reports as H9c2 cells incubated with alcohol for 1 hr increased Hsp70 (79). Taken together, alterations in the heat shock proteins do not appear essential for the development of ACM.
In summary, a relatively large number of studies have measured markers of oxidative stress following acute and chronic alcohol intake during the last 6 years. In general, alcohol negatively altered the oxidative stress markers SOD activity, protein carbonyl and MDA levels while other markers were either modulated in a sex-dependent manner (catalase, ROS, GSH, GPx) or the response so variable that unequivocal conclusions could not be reached. Further research should focus on clarifying the contribution of oxidative stress to ACM, especially in humans, but at the very least should use more consistent animal models to improve rigor and reproducibility.
2.4 Oxidative stress and contractility
Assessing a direct, causal link between oxidative stress and contractile dysfunction is difficult as a multitude of factors potentially contribute precluding specific mechanistic manipulations. With that in mind, antioxidant agents or superoxide mimetics have instead been employed to investigate the potential relationship between cardiac function and oxidative stress in the context of ACM. For example, the SOD mimetic, tempol, prevented the acute alcohol-induced increase in ROS production and normalized the alcohol-induced decrease in the maximal rise of ventricular pressure over time (dP/dt max) measured in conscious animals (55). In contrast, induction of the protein heme oxygenase-1 (HO-1) (via cobalt protoporphyrin treatment) to protect against oxidative stress, did not abrogate the negative effects of alcohol on contractile endpoints including peak shortening, the maximal velocity of shortening/relengthening (±dL/dt), and time to 90% of relengthening (TR90) in cardiomyocytes (57). Overall, definitive causal evidence is lacking to conclusively link increased oxidative stress specifically to the development of functional deficits following acute alcohol intake.
3 Apoptosis
There is a well-established link between apoptosis and cardiovascular disease in general, and the evidence of a relationship between apoptosis and the development of ACM in particular has continued to grow. Early work noted the number of myocyte nuclei in the left ventricle was decreased in rats after chronic (8 months) alcohol consumption (80), and altered myocyte morphology was also detected in samples of human hearts obtained at necropsy that was consistent with increased apoptosis (13). Subsequently, alcohol was shown to increase apoptosis in rat H9c2 cardiomyocytes (81) as well as in cardiomyocytes from animals and humans (82). Specifically, alcohol dose-dependently (50–100 mM or 100–400 mM) increased apoptosis in H9c2 cells or freshly isolated cardiomyocytes (83, 84). Further, alcohol exacerbated apoptosis produced by serum withdrawal in primary cardiomyocytes and such an effect was prevented by IGF-I (81). Similarly, the number of TUNEL-positive cells (suggesting DNA fragmentation) and the expression of Bax and Bcl-2 (markers of pro-apoptotic actions) were increased in hearts of alcoholic patients (82). Caspase 3 activity, one of the most commonly measured surrogate markers for apoptosis, was increased following acute alcohol intoxication in mice, in part due to enhanced ROS production caused by acetaldehyde accumulation (85, 86). Despite the well documented enhancement of apoptosis by alcohol (13, 80–87), the precise mechanism remains elusive although several contributing factors have been posited including: angiotensin II type I receptors (88, 89), metabolism of alcohol to acetaldehyde via CYP2E1 (57), and signaling of growth factors including myostatin and IGF-I (81, 90, 91). These factors likely contribute to apoptosis through production of oxidative stress and mitochondrial damage (83, 89). Significant work has been done subsequent to these early reports to further clarify this relationship and these findings are described in the following section.
3.1 Alcohol and markers of apoptosis
3.1.1 TUNEL staining
Alcohol increased several indices of apoptosis including the number of TUNEL-stained cells, expression of pro-apoptotic proteins caspase 3 and Bax, and the abundance of the anti-apoptotic protein Bcl-2. For instance, chronic alcohol intake increased apoptotic bodies in hearts from humans (90), male and female mice (15, 57, 89, 92), and male rats (88, 93). Based on methodological variations it appears that apoptosis can be increased within as little as 6 wks (4% v/v liquid diet) in mice (57) and 4 months (5% v/v alcohol in drinking water) in rats (93). The use of Annexin V staining with fluorescence-activated cell sorting has confirmed an alcohol-mediated increase in apoptosis in cardiomyocytes (94, 95). Similarly, the number of apoptotic nuclei detected via TUNEL was increased after acute alcohol in male mice (3 day, 3 g/kg alcohol/day) (20) and male rats (8 g/kg alcohol) (96), as well as in H9c2 cardiomyocytes (100–400 mg/dL, 24 hr), neonatal rat cardiac myocytes (200 mM, 4 hr) and primary cardiomyocytes from adult rats (0.3–0.5% alcohol in media, 4 days). This effect appears specific to cardiomyocytes as there was no detectable increase in apoptosis in alcohol-treated cardiac fibroblasts (97). Overall, apoptosis detected via TUNEL was increased by alcohol in both animal and cell models at varying concentrations. However, some caution should be exercised when using TUNEL staining as the extent of apoptosis may be over-estimated because stained nuclei can represent either apoptotic cell death or DNA repair due to the reversible nature of DNA fragmentation (98).
3.1.2 Caspase 3
Caspase 3 is a pro-apoptotic protein that lies at the intersection of several apoptotic pathways, has been employed as a general apoptotic marker, and has been reported to be consistently increased by alcohol. Specifically, alcohol increased caspase 3 staining (4–5 months liquid diet) in male Wistar rats (99) and caspase 3 protein and activity in alcohol-fed male mice (15, 57). Acutely, cleaved caspase-3 was also increased by alcohol (3g/kg/day, 3 days) in male mice (95). Under in vitro conditions, pro-caspase 3/active caspase 3 protein expression was increased in endothelial progenitor cells (25 mM, 48 hr) (100), while cleaved caspase 3 was increased in H9c2 cells (100–400 mM, 24 hr) and neonatal rat cardiac myocytes (200 mM, 4 hr) (89, 95). In contrast, caspase 3 activity was unchanged in rat hearts in response to acute alcohol intoxication, despite increased caspase 3 staining in isolated cardiomyocytes (96). Therefore, the majority of recent data indicate that caspase 3 is increased in response to acute and chronic alcohol indicating apoptotic pathway activation.
3.2 Regulation of apoptotic pathways by alcohol
3.2.1 Intrinsic pathway
The extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway are the primary apoptotic pathways that have been evaluated. The mitochondrial pathway can be activated by several stimuli including growth factors, oxidative stress, DNA damage and hypoxia (101). Following activation of apoptosis, proteins in the Bcl-2 protein family regulate mitochondria membrane integrity and permeability. Bcl-2 is an anti-apoptotic protein that was unexpectedly shown to be increased in human alcoholic hearts (90), while it was decreased in male mice by acute alcohol intoxication (3g/kg/d, 3 days) (20, 95), possibly suggesting divergent effects of acute versus chronic alcohol. Staining for the anti-apoptotic protein Bcl-xL was also decreased in hearts from chronic alcohol-fed dogs (6 months) (88). Pro-apoptotic proteins from the Bcl-2 family, including Bax and Bad, were consistently increased by alcohol (20, 57, 88, 90, 95). For example, Bax was increased by acute (20, 95) and chronic alcohol (4% v/v, 16 wk) (57) in male mice and chronic alcohol in human donor hearts (90), with enhanced Bad staining seen in the hearts of mongrel dogs following chronic alcohol consumption (88). When the actions of Bcl-2 do not counter the enhanced pro-apoptotic signaling, increased mitochondrial membrane permeability occurs and cytochrome c is released into the cytoplasm. Acute alcohol (3 g/kg, 3 days) in male mice increased cytosolic cytochrome c as well as apoptosis inducing factor (AIF) (20) in agreement with the decreased mitochondrial cytochrome c (i.e., increased cytosolic) observed in vitro. Following cytochrome c release, caspase 9 and 3 are activated and apoptosis occurs. Accordingly, acute alcohol increased cytosolic pro-caspase 9 and caspase 3 (as discussed above) (20).
3.2.2 Extrinsic pathway
The death receptor pathway is mediated via activation of the death receptor by the ligands tumor necrosis factor alpha (TNF-α) and Fas ligand (FasL). Ligand binding to the death receptor recruits a death domain (FADD) that activates downstream caspases, including caspase 8 and ultimately caspase 3. However, protein levels of caspase 8, cytosolic pro-caspase 8, TNF-α, Fas receptor and FasL were unchanged in mice after acute alcohol (20), and although an increase in cardiac TNFα mRNA was detected in alcohol-fed female adult and older Fischer 344 rats, this change was apparently insufficient to activate the extrinsic apoptotic pathway (102, 103). These data suggest that activation of the intrinsic pathway represents the predominant mechanism by which alcohol induces apoptosis.
3.3 Alcohol metabolism and apoptosis
The byproducts of alcohol metabolism contribute meaningfully to the induction of apoptosis by alcohol and this has been shown through the manipulation of the enzymes responsible for catalyzing the oxidative metabolism of alcohol. For example, while the activity of CYP2E1 in the heart low, compared to the liver, alcohol can modulate apoptosis by regulating the conversion of alcohol to acetaldehyde (57). Specifically, inhibition of CYP2E1 activity with diallyl sulfide attenuated the alcohol-induced increase in caspase 3 protein expression and activity, the expression of pro-apoptotic BAX and the number of TUNEL-stained nuclei (57). Consistent with these observations, cardiac-specific overexpression of ADH increased apoptosis in male mice following acute alcohol intoxication (3g/kg/d, 3 days), as evidenced by an increased number of TUNEL-stained nuclei, caspase 3 protein expression, Bax expression and cytosolic pro-caspase 9 (20). ALDH2 is responsible for the breakdown of the toxic metabolite acetaldehyde and has also been implicated in the alcohol-mediated regulation of apoptosis. In this regard, ALDH2 was repressed and apoptosis was increased in cardiomyocytes incubated with alcohol (0.3% and 0.5%, 4 days), and these changes were associated with enhanced expression of micro(mi)RNA-378a-5p (94). Moreover, the over-expression of miR-378a-5p decreased ALDH2 mRNA content. These data provide convincing evidence that alcohol-induced apoptosis can be mediated directly via ALDH2 gene suppression. Therefore, targeted regulation of these metabolizing enzymes may prove beneficial in patients to reduce acetaldehyde load and apoptosis until abstinence can be achieved.
4. Interaction between oxidative stress and apoptosis
The precise mechanism for alcohol-induced cardiac apoptosis is unknown although the protein Pin1 has been identified as a contributing factor (18). Pin1 is a peptidyl-prolyl cis-trans isomerase of the parvulin family of PPIase enzymes with a role in the regulation of apoptosis (104). In cardiomyocytes from neonatal mouse hearts, apoptosis was correlated with the alcohol-mediated upregulation of Pin1 mRNA and protein expression in a dose-dependent manner (18). Depletion of Pin1 by siRNA reduced the detrimental effects of alcohol on cell viability and attenuated alcohol-induced apoptosis as assessed by reduced caspase 9, caspase 3 activity and TUNEL staining. Conversely, overexpression of Pin1 augmented alcohol-induced apoptosis and cell viability (18). Pin1 may also be involved in the ROS-mediated induction of apoptosis similar to other signaling molecules including p66Shc and PKC-β. Briefly, mechanisms through which p66Shc led to ROS production included enhanced RAC1, suppression of ROS scavenging enzymes following its interaction with FOXO (Forkhead box) transcription factors, and induction of H2O2 production after its phosphorylation by PKC-β and subsequent prolyl-isomerization by Pin1 (105, 106). Further, siRNA downregulation of p66Shc or inhibition of PKC-β decreased the alcohol (200 mM, 24 hr) mediated increase in apoptosis and ROS production in primary cardiomyocytes (19). Finally, blocking PKC-β decreased binding between Pin1 and phosphorylated p66Shc that was enhanced by alcohol (19). It is quite evident then that Pin1 and related proteins have a central role in linking apoptosis with oxidative stress-induced by alcohol.
In a related mechanism, Tan et al. (89) reported that PKC-β1 activation via the angiotensin II type I (AT1) receptor increased nitrative stress (i.e., nitrogen oxides) and produced cardiac cell death following acute alcohol (89). Antagonizing the AT1 receptor prevented the alcohol-induced increase in oxidative stress and cellular apoptosis (89) providing additional evidence for this mechanism in regulating apoptosis. As these studies provide a putative link between alcohol-induced ROS and apoptosis in cardiomyocytes, future in vivo work will need to confirm the importance of this signaling pathway and its role in apoptosis and oxidative stress during ACM.
Another novel factor recently identified to play a causative role in ACM in relation to apoptosis is the regulator of G protein signaling 6 gene (RGS6) that is implicated in extracellular signaling responses via G-protein coupled receptors. Whole-body knockout of RGS6 in male and female mice attenuated the effects of chronic alcohol consumption (5% v/v liquid diet, 2 month) on cardiac apoptosis; it reduced the number of TUNEL-stained nuclei in the right ventricle and prevented the accompanying increase in heart weight, fibrosis and myofilament disarray (92). The RGS6-dependent decrease in apoptosis was due in part to a reduction in NADPH oxidase-induced ROS generation that had subsequently increased cardiac myocyte apoptosis (89, 92).
Most recently, Zhu et al. (95) linked the induction of apoptosis by alcohol with the ROS-mediated increase in autophagy (i.e., cleaved caspase 3, TUNEL, Bax), as the inhibition of autophagy prevented the alcohol-induced increase in apoptosis in vivo and in vitro. The protein c-JUN N-terminal kinase (JNK) was identified as the mechanistic link between the alcohol-mediated induction in ROS and activation of autophagy and apoptosis by alcohol. Specifically, JNK inhibition prevented bcl-2 phosphorylation and the dissociation of the Beclin1-Bcl-2 complex thereby reducing markers of apoptosis (TUNEL, cleaved caspase-3, annexin staining).
Overall, excessive alcohol enhances apoptotic signaling in the heart of rodents and the majority of in vitro experiments. However, findings in humans with alcoholic cardiomyopathy provided by Fernandez-Sola et al. (82) are not as unequivocal as both anti-and pro-apoptotic signals were detected. It will be important to continue to assess apoptosis in the context of other causative mechanisms contributing to ACM, including oxidative stress and mitochondrial dysfunction. The lack of clinical studies in this area represents an important limitation as it precludes appropriate preclinical model development and forward progress in the design of rational treatment strategies.
Conclusion
In summary, recently published studies emphasized the negative effects of alcohol on mitochondria and related processes within the heart. Alcohol decreased mitochondria membrane potential, respiration and complex activity along with inducing DNA damage. It is likely that these mitochondrial perturbations contributed to the increased mediators of oxidative stress and apoptosis and subsequent contractility defects. In some instances alcohol metabolism, via the production of acetaldehyde, also contributed to these effects, while in others the presence of estrogen appeared to influence the response. Several of these alcohol-induced changes were also dose-dependent potentially indicating a personalized medicine approach will be necessary to most successfully identify and treat patients with ACM. Overall, increased markers of oxidative stress and apoptosis along with mitochondrial dysfunction appear to comprise primary processes implicated in the etiology of ACM. With the advances in proteomic and genomic methodology other mechanisms will likely be identified as important aspects of disease development.
Acknowledgments
Support: This work was supported by a grant from NIAAA R37 AA011290 (C.H.L) and F32 AA023422 (J.L.S).
Abbreviations
- ACM
alcoholic cardiomyopathy
- ALDH
aldehyde dehydrogenase
- ADH
alcohol dehydrogenase
- PKC-β
protein kinase C (PKC)-beta
- TCA
tricarboxylic acid cycle
- ETC
electron transport chain
- RCI
mitochondrial respiratory control index/ratio
- FAD+
flavin adenine dinucleotide
- CoQ
Coenzyme Q
- COX IV
cytochrome c oxidase
- AMPK
AMP-activated protein kinase
- ANT1
adenine nucleotide translocator 1
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- UCP2
uncoupling protein-2
- ERRα
estrogen related receptor alpha
- mtDNA
mitochondria DNA
- ROS
reactive oxygen species
- O2·−
superoxide anion radical
- ˙OH
hydroxyl radical
- H2O2
hydrogen peroxide
- 1O2
singlet oxygen
- GSH
glutathione
- SOD
superoxide dismutase
- E2
estrogen
- IGF-I
insulin like growth factor – I
- CYP2E1
cytochrome P450 2E1
- Cu/ZnSOD, SOD1
copper/zinc-containing SOD
- MnSOD, SOD2
manganese SOD
- ECSOD, SOD3
extracellular superoxide dismutase
- kcal
kilocalories
- NaHS
sodium hydrosulfide
- H2S
hydrogen sulfide
- GSH
glutathione
- GPx
glutathione peroxidase
- GSSG
glutathione disulfide
- SPH
sardinelle heads
- GST
glutathione S-transferase
- MDA
Malondialdehyde
- TBARS
thiobarbituric acid reactive substances
- 4-HNE
4-hydroxynonenal
- Hsp
Heat shock proteins
- HSF1
heat shock factor 1
- HO-1
heme oxygenase-1
- dP/dt max
maximal rise of (ventricular) pressure over time
- ±dL/dt
maximal velocity of shortening/relengthening
- TR90
time to 90% of relengthening
- AIF
apoptosis inducing factor
- TNF-α
tumor necrosis factor alpha
- FasL
Fas ligand
- AT1
angiotensin II type I
- RGS6
regulator of G protein signaling 6 gene
- LV
left ventricular
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- GR
glutathione reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernández-Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol. 2015;12(10):576–87. doi: 10.1038/nrcardio.2015.91. Epub 2015/06/23. [DOI] [PubMed] [Google Scholar]
- 2.Graves EJ. Detailed diagnoses and procedures, National Hospital Discharge Survey. Vital Health Stat 13. 1993;1995(122):1–288. [PubMed] [Google Scholar]
- 3.Kasper EK, Agema WR, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol. 1994;23(3):586–90. doi: 10.1016/0735-1097(94)90740-4. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Fleury B, Gueguen A, Bonnet J, Lorente P, Nakache JP, et al. Mortality of dilated myocardiopathies as a function of continuation of alcohol drinking Multivariate analysis concerning 236 patients. Presse Med. 1989;18(14):711–4. [PubMed] [Google Scholar]
- 5.Gavazzi A, De Maria R, Parolini M, Porcu M. Alcohol abuse and dilated cardiomyopathy in men. Am J Cardiol. 2000;85(9):1114–8. doi: 10.1016/s0002-9149(00)00706-2. [DOI] [PubMed] [Google Scholar]
- 6.Fauchier L, Babuty D, Poret P, Casset-Senon D, Autret ML, Cosnay P, et al. Comparison of longterm outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000;21(4):306–14. doi: 10.1053/euhj.1999.1761. [DOI] [PubMed] [Google Scholar]
- 7.Prazak P, Pfisterer M, Osswald S, Buser P, Burkart F. Differences of disease progression in congestive heart failure due to alcoholic as compared to idiopathic dilated cardiomyopathy. Eur Heart J. 1996;17(2):251–7. doi: 10.1093/oxfordjournals.eurheartj.a014842. [DOI] [PubMed] [Google Scholar]
- 8.Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol. 2014;14(4):291–308. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzzo-Merello G, Cobo-Marcos M, Gallego-Delgado M, Garcia-Pavia P. Alcoholic cardiomyopathy. World J Cardiol. 2014;6(8):771–81. doi: 10.4330/wjc.v6.i8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent D, Edwards JG. Alcoholic Cardiomyopathy: Multigenic Changes Underlie Cardiovascular Dysfunction. J Cardiol Clin Res. 2014;2(1) Epub 2014/01/24. [PMC free article] [PubMed] [Google Scholar]
- 11.Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, et al. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013;37(8):1253–60. doi: 10.1111/acer.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsiplenkova VG, Vikhert AM, Cherpachenko NM. Ultrastructural and histochemical observations in human and experimental alcoholic cardiomyopathy. J Am Coll Cardiol. 1986;8(1 Suppl A):22–32. doi: 10.1016/s0735-1097(86)80025-0. [DOI] [PubMed] [Google Scholar]
- 13.Hibbs RG, Ferrans VJ, Black WC, Weilbaecher DG, Burch GE. ALCOHOLIC CARDIOMYOPATHY; AN ELECTRON MICROSCOPIC STUDY. Am Heart J. 1965;69:766–79. doi: 10.1016/0002-8703(65)90450-3. [DOI] [PubMed] [Google Scholar]
- 14.Jing L, Zhou LJ, Li WM, Zhang FM, Yuan L, Li S, et al. Carnitine regulates myocardial metabolism by Peroxisome Proliferator-Activated Receptor-alpha (PPARalpha) in alcoholic cardiomyopathy. Med Sci Monit. 2011;17(1):1–9. doi: 10.12659/MSM.881311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, et al. Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: Roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 2010;49(7):1238–53. doi: 10.1016/j.freeradbiomed.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–17. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 17.Laurent D, Mathew JE, Mitry M, Taft M, Force A, Edwards JG. Chronic ethanol consumption increases myocardial mitochondrial DNA mutations: a potential contribution by mitochondrial topoisomerases. Alcohol Alcohol. 2014;49(4):381–9. doi: 10.1093/alcalc/agu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li Z, Zhang Y, Yang W, Sun J, Shan L, et al. Targeting Pin1 Protects Mouse Cardiomyocytes from High-Dose Alcohol-Induced Apoptosis. Oxid Med Cell Longev. 2016;2016:4528906. doi: 10.1155/2016/4528906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhao J, Yang W, Bi Y, Chi J, Tian J, et al. High-dose alcohol induces reactive oxygen species-mediated apoptosis via PKC-β/p66Shc in mouse primary cardiomyocytes. Biochem Biophys Res Commun. 2015;456(2):656–61. doi: 10.1016/j.bbrc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, Ren J. Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One. 2010;5(1):8757. doi: 10.1371/journal.pone.0008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Yu L, Byra EA, Hu N, Kitagawa K, Nakayama KI, et al. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol. 2010;49(2):322–9. doi: 10.1016/j.yjmcc.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bing RJ, Tillmanns H, Fauvel JM, Seeler K, Mao JC. Effect of prolonged alcohol administration on calcium transport in heart muscle of the dog. Circ Res. 1974;35(1):33–8. doi: 10.1161/01.res.35.1.33. [DOI] [PubMed] [Google Scholar]
- 23.Weishaar R, Sarma JS, Maruyama Y, Fischer R, Bertuglia S, Bing RJ. Reversibility of mitochondrial and contractile changes in the myocardium after cessation of prolonged ethanol intake. Am J Cardiol. 1977;40(4):556–62. doi: 10.1016/0002-9149(77)90071-6. [DOI] [PubMed] [Google Scholar]
- 24.Williams ES, Li TK. The effect of chronic alcohol administration on fatty acid metabolism and pyruvate oxidation of heart mitochondria. J Mol Cell Cardiol. 1977;9(12):1003–11. doi: 10.1016/s0022-2828(77)80052-7. [DOI] [PubMed] [Google Scholar]
- 25.Sarma JS, Ikeda S, Fischer R, Maruyama Y, Weishaar R, Bing RJ. Biochemical and contractile properties of heart muscle after prolonged alcohol administration. J Mol Cell Cardiol. 1976;8(12):951–72. doi: 10.1016/0022-2828(76)90077-8. [DOI] [PubMed] [Google Scholar]
- 26.Pachinger OM, Tillmanns H, Mao JC, Fauvel JM, Bing RJ. The effect of prolonged administration of ethanol on cardiac metabolism and performance in the dog. J Clin Invest. 1973;52(11):2690–6. doi: 10.1172/JCI107463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segel LD, Rendig SV, Choquet Y, Chacko K, Amsterdam EA, Mason DT. Effects of chronic graded ethanol consumption on the metabolism, ultrastructure, and mechanical function of the rat heart. Cardiovasc Res. 1975;9(5):649–63. doi: 10.1093/cvr/9.5.649. [DOI] [PubMed] [Google Scholar]
- 28.Mihailovic D, Nikolic J, Bjelakovic BB, Stankovic BN, Bjelakovic G. Morphometric and biochemical characteristics of short-term effects of ethanol on rat cardiac muscle. Exp Toxicol Pathol. 1999;51(6):545–7. doi: 10.1016/S0940-2993(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 29.Valls-Belles V, Torres C, Muñiz P, Codoñer-Franch P. Effect of beer consumption on levels of complex I and complex IV liver and heart mitochondrial enzymes and coenzymes Q9 and Q10 in adriamycin-treated rats. Eur J Nutr. 2010;49(3):181–7. doi: 10.1007/s00394-009-0064-4. [DOI] [PubMed] [Google Scholar]
- 30.Mátyás C, Varga ZV, Mukhopadhyay P, Paloczi J, Lajtos T, Erdelyi K, et al. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction and steatosis. Am J Physiol Heart Circ Physiol. 2016 doi: 10.1152/ajpheart.00214.2016. ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong L, Zhu J, Lv T, Chen G, Sun H, Yang X, et al. Ethanol and its metabolites induce histone lysine 9 acetylation and an alteration of the expression of heart development-related genes in cardiac progenitor cells. Cardiovasc Toxicol. 2010;10(4):268–74. doi: 10.1007/s12012-010-9081-z. [DOI] [PubMed] [Google Scholar]
- 32.Kandadi MR, Hu N, Ren J. ULK1 plays a critical role in AMPK-mediated myocardial autophagy and contractile dysfunction following acute alcohol challenge. Curr Pharm Des. 2013;19(27):4874–87. doi: 10.2174/1381612811319270010. [DOI] [PubMed] [Google Scholar]
- 33.Mashimo K, Arthur PG, Ohno Y. Ethanol Dose- and Time-dependently Increases α and β Subunits of Mitochondrial ATP Synthase of Cultured Neonatal Rat Cardiomyocytes. J Nippon Med Sch. 2015;82(5):237–45. doi: 10.1272/jnms.82.237. [DOI] [PubMed] [Google Scholar]
- 34.Hu C, Ge F, Hyodo E, Arai K, Iwata S, Lobdell H, et al. Chronic ethanol consumption increases cardiomyocyte fatty acid uptake and decreases ventricular contractile function in C57BL/6J mice. J Mol Cell Cardiol. 2013;59:30–40. doi: 10.1016/j.yjmcc.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan F, Lei Y, Wang Q, Esberg LB, Huang Z, Scott GI, et al. Moderate ethanol administration accentuates cardiomyocyte contractile dysfunction and mitochondrial injury in high fat diet-induced obesity. Toxicol Lett. 2015;233(3):267–77. doi: 10.1016/j.toxlet.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Heart mitochondria response to alcohol is different than brain and liver. Alcohol Clin Exp Res. 1995;19(6):1463–6. doi: 10.1111/j.1530-0277.1995.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 38.Kou SY, Cohen NS. Ethanol feeding produces deficiencies in left ventricle total RNA, total DNA and mitochondrial ribosomal RNA. Int J Biochem Cell Biol. 1998;30(4):475–85. doi: 10.1016/s1357-2725(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 39.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298(2):737–43. [PubMed] [Google Scholar]
- 40.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70(2):200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 41.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–8. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 42.Vikhert AM, Tsiplenkova VG, Cherpachenko NM. Alcoholic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 1986;8(1 Suppl A):3–11. doi: 10.1016/s0735-1097(86)80023-7. [DOI] [PubMed] [Google Scholar]
- 43.Fahimi HD, Kino M, Hicks L, Thorp KA, Abelman WH. Increased myocardial catalase in rats fed ethanol. Am J Pathol. 1979;96(2):373–90. [PMC free article] [PubMed] [Google Scholar]
- 44.Kino M. Chronic effects of ethanol under partial inhibition of catalase activity in the rat heart: light and electron microscopic observations. J Mol Cell Cardiol. 1981;13(1):5–21. doi: 10.1016/0022-2828(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 45.Edes I, Piros G, Forster T, Csanady M. Alcohol-induced congestive cardiomyopathy in adult turkeys: effects on myocardial antioxidant defence systems. Basic Res Cardiol. 1987;82(6):551–6. doi: 10.1007/BF01907225. [DOI] [PubMed] [Google Scholar]
- 46.Ribière C, Hininger I, Rouach H, Nordmann R. Effects of chronic ethanol administration on free radical defence in rat myocardium. Biochem Pharmacol. 1992;44(8):1495–500. doi: 10.1016/0006-2952(92)90463-s. [DOI] [PubMed] [Google Scholar]
- 47.Seiva FR, Amauchi JF, Rocha KK, Ebaid GX, Souza G, Fernandes AA, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649–56. doi: 10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Edes I, Tószegi A, Csanády M, Bozóky B. Myocardial lipid peroxidation in rats after chronic alcohol ingestion and the effects of different antioxidants. Cardiovasc Res. 1986;20(7):542–8. doi: 10.1093/cvr/20.7.542. [DOI] [PubMed] [Google Scholar]
- 49.Preedy VR, Patel VB, Reilly ME, Richardson PJ, Falkous G, Mantle D. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999;4:58–66. doi: 10.2741/A480. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32(3):175–86. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Umoh NA, Walker RK, Al-Rubaiee M, Jeffress MA, Haddad GE. Acute alcohol modulates cardiac function as PI3K/Akt regulates oxidative stress. Alcohol Clin Exp Res. 2014;38(7):1847–64. doi: 10.1111/acer.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue L, Xu F, Meng L, Wei S, Wang J, Hao P, et al. Acetylation-dependent regulation of mitochondrial ALDH2 activation by SIRT3 mediates acute ethanol-induced eNOS activation. FEBS Lett. 2012;586(2):137–42. doi: 10.1016/j.febslet.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 53.McGee MA, Abdel-Rahman AA. Ethanol attenuates peripheral NMDAR-mediated vascular oxidative stress and pressor response. Alcohol. 2015;49(5):499–506. doi: 10.1016/j.alcohol.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Mas MM, Abdel-Rahman AA. Estrogen modulation of the ethanol-evoked myocardial oxidative stress and dysfunction via DAPK3/Akt/ERK activation in male rats. Toxicol Appl Pharmacol. 2015;287(3):284–92. doi: 10.1016/j.taap.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibrahim BM, Fan M, Abdel-Rahman AA. Oxidative stress and autonomic dysregulation contribute to the acute time-dependent myocardial depressant effect of ethanol in conscious female rats. Alcohol Clin Exp Res. 2014;38(5):1205–15. doi: 10.1111/acer.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Mas MM, Abdel-Rahman AA. Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab. 2014;306(7):740–7. doi: 10.1152/ajpendo.00465.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, Wang XM, et al. Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochim Biophys Acta. 2013;1832(1):128–41. doi: 10.1016/j.bbadis.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yayalacı Y, Celik I, Batı B. Hepatoprotective and antioxidant activity of linden (Tilia platyphyllos L.) infusion against ethanol-induced oxidative stress in rats. J Membr Biol. 2014;247(2):181–8. doi: 10.1007/s00232-013-9622-z. [DOI] [PubMed] [Google Scholar]
- 59.Rajbanshi SL, Pandanaboina CS. Alcohol stress on cardiac tissue - ameliorative effects of Thespesia populnea leaf extract. J Cardiol. 2014;63(6):449–59. doi: 10.1016/j.jjcc.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Lu X, Xu W, Chen J. Protective effects of hydrogen sulfide against chronic alcohol intake-induced left ventricular remodeling in rats. Cardiovasc Drugs Ther. 2013;27(3):221–7. doi: 10.1007/s10557-013-6441-5. [DOI] [PubMed] [Google Scholar]
- 61.Islam A, Abraham P, Hapner CD, Deuster PA, Chen Y. Tissue-specific upregulation of HSP72 in mice following short-term administration of alcohol. Cell Stress Chaperones. 2013;18(2):215–22. doi: 10.1007/s12192-012-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamoun Z, Kamoun AS, Bougatef A, Chtourou Y, Boudawara T, Nasri M, et al. Efficacy of sardinelle protein hydrolysate to alleviate ethanol-induced oxidative stress in the heart of adult rats. J Food Sci. 2012;77(8):156–62. doi: 10.1111/j.1750-3841.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 63.Ojeda ML, Barrero MJ, Nogales F, Murillo ML, Carreras O. Oxidative effects of chronic ethanol consumption on the functions of heart and kidney: folic acid supplementation. Alcohol Alcohol. 2012;47(4):404–12. doi: 10.1093/alcalc/ags056. [DOI] [PubMed] [Google Scholar]
- 64.Kalaz EB, Evran B, Develi S, Erata G, Uysal M, Koçak-Toker N. Effect of binge ethanol treatment on prooxidant-antioxidant balance in rat heart tissue. Pathophysiology. 2012;19(1):49–53. doi: 10.1016/j.pathophys.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Yao Z, Zhang Y, Li H, Deng Z, Zhang X. Synergistic effect of Se-methylselenocysteine and vitamin E in ameliorating the acute ethanol-induced oxidative damage in rat. J Trace Elem Med Biol. 2015;29:182–7. doi: 10.1016/j.jtemb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Vucevic D, Mladenovic D, Ninkovic M, Aleksic V, Stankovic MN, Stankovic M, et al. The effects of caloric restriction against ethanol-induced oxidative and nitrosative cardiotoxicity and plasma lipids in rats. Exp Biol Med (Maywood) 2013;238(12):1396–405. doi: 10.1177/1535370213506806. [DOI] [PubMed] [Google Scholar]
- 67.Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, et al. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation. 2004;109(1):59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 68.Yamada H, Yamada Y, Adachi T, Fukatsu A, Sakuma M, Futenma A, et al. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron. 2000;84(3):218–23. doi: 10.1159/000045580. doi: 45580. [DOI] [PubMed] [Google Scholar]
- 69.van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, et al. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44(7):1305–13. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landmesser U, Merten R, Spiekermann S, Büttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101(19):2264–70. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 71.Singh K, Ahluwalia P. Effect of monosodium glutamate on lipid peroxidation and certain antioxidant enzymes in cardiac tissue of alcoholic adult male mice. J Cardiovasc Dis Res. 2012;3(1):12–8. doi: 10.4103/0975-3583.91595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fogle RL, Hollenbeak CS, Stanley BA, Vary TC, Kimball SR, Lynch CJ. Functional proteomic analysis reveals sex-dependent differences in structural and energy-producing myocardial proteins in rat model of alcoholic cardiomyopathy. Physiol Genomics. 2011;43(7):346–56. doi: 10.1152/physiolgenomics.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balasubramaniyan V, Nalini N. Effect of leptin on peroxidation and antioxidant defense in ethanol-supplemented Mus musculus heart. Fundam Clin Pharmacol. 2007;21(3):245–53. doi: 10.1111/j.1472-8206.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 74.Lam PY, Chiu PY, Leung HY, Chen N, Leong PK, Ko KM. Schisandrin B co-treatment ameliorates the impairment on mitochondrial antioxidant status in various tissues of long-term ethanol treated rats. Fitoterapia. 2010;81(8):1239–45. doi: 10.1016/j.fitote.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–91. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Navarro-Zaragoza J, Ros-Simó C, Milanés MV, Valverde O, Laorden ML. Binge Ethanol and MDMA Combination Exacerbates Toxic Cardiac Effects by Inducing Cellular Stress. PLoS One. 2015;10(10):0141502. doi: 10.1371/journal.pone.0141502. Epub 2015/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richardson PJ, Patel VB, Preedy VR. Alcohol and the myocardium. Novartis Found Symp. 1998;216:35–45. doi: 10.1002/9780470515549.ch4. discussion −50. [DOI] [PubMed] [Google Scholar]
- 78.Vary TC, Deiter G. Long-term alcohol administration inhibits synthesis of both myofibrillar and sarcoplasmic proteins in heart. Metabolism. 2005;54(2):212–9. doi: 10.1016/j.metabol.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Su CY, Chong KY, Owen OE, Dillmann WH, Chang C, Lai CC. Constitutive and inducible hsp70s are involved in oxidative resistance evoked by heat shock or ethanol. J Mol Cell Cardiol. 1998;30(3):587–98. doi: 10.1006/jmcc.1997.0622. [DOI] [PubMed] [Google Scholar]
- 80.Capasso JM, Li P, Guideri G, Malhotra A, Cortese R, Anversa P. Myocardial mechanical, biochemical, and structural alterations induced by chronic ethanol ingestion in rats. Circ Res. 1992;71(2):346–56. doi: 10.1161/01.res.71.2.346. [DOI] [PubMed] [Google Scholar]
- 81.Chen DB, Wang L, Wang PH. Insulin-like growth factor I retards apoptotic signaling induced by ethanol in cardiomyocytes. Life Sci. 2000;67(14):1683–93. doi: 10.1016/s0024-3205(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 82.Fernández-Solà J, Fatjó F, Sacanella E, Estruch R, Bosch X, Urbano-Márquez A, et al. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006;37(8):1100–10. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 83.Guan Z, Lui CY, Morkin E, Bahl JJ. Oxidative stress and apoptosis in cardiomyocyte induced by high-dose alcohol. J Cardiovasc Pharmacol. 2004;44(6):696–702. doi: 10.1097/00005344-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Johnson S, Williams AN, Johnson C, Ou XM. The effects of antidepressant drug on ethanol-induced cell death. Drug Discov Ther. 2007;1(2):130–5. [PubMed] [Google Scholar]
- 85.Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54(23):2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 86.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119(14):1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Hajnóczky G, Buzas CJ, Pacher P, Hoek JB, Rubin E. Alcohol and mitochondria in cardiac apoptosis: mechanisms and visualization. Alcohol Clin Exp Res. 2005;29(5):693–701. doi: 10.1097/01.alc.0000163493.45344.7a. [DOI] [PubMed] [Google Scholar]
- 88.Jing L, Jin CM, Li SS, Zhang FM, Yuan L, Li WM, et al. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem. 2012;359(1–2):283–92. doi: 10.1007/s11010-011-1022-z. [DOI] [PubMed] [Google Scholar]
- 89.Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol. 2012;59(16):1477–86. doi: 10.1016/j.jacc.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernández-Solà J, Lluis M, Sacanella E, Estruch R, Antúnez E, Urbano-Márquez A. Increased myostatin activity and decreased myocyte proliferation in chronic alcoholic cardiomyopathy. Alcohol Clin Exp Res. 2011;35(7):1220–9. doi: 10.1111/j.1530-0277.2011.01456.x. [DOI] [PubMed] [Google Scholar]
- 91.Borrisser-Pairó F, Antúnez E, Tobías E, Fernández-Solà J. Insulin-like growth factor 1 myocardial expression decreases in chronic alcohol consumption. Regen Med Res. 2013;1(1):3. doi: 10.1186/2050-490X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stewart A, Maity B, Anderegg SP, Allamargot C, Yang J, Fisher RA. Regulator of G protein signaling 6 is a critical mediator of both reward-related behavioral and pathological responses to alcohol. Proc Natl Acad Sci U S A. 2015;112(7):786–95. doi: 10.1073/pnas.1418795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raymond AR, Becker J, Woodiwiss AJ, Booysen HL, Norton GR, Brooksbank RL. Ethanol-Associated Cardiomyocyte Apoptosis and Left Ventricular Dilation Are Unrelated to Changes in Myocardial Telomere Length in Rats. J Card Fail. 2016;22(4):294–302. doi: 10.1016/j.cardfail.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, Song J, Zhang L, Huang S, Bao L, Chen F, et al. Increased expression of microRNA-378a-5p in acute ethanol exposure of rat cardiomyocytes. Cell Stress Chaperones. 2017;22(2):245–52. doi: 10.1007/s12192-016-0760-y. Epub 2017/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Z, Huang Y, Lv L, Tao Y, Shao M, Zhao C, et al. Acute Ethanol Exposure-induced Autophagy-mediated Cardiac Injury via Activation of the ROS-JNK-Bcl-2 Pathway. J Cell Physiol. 2017 doi: 10.1002/jcp.25934. Epub 2017/03/28. [DOI] [PubMed] [Google Scholar]
- 96.Kartkaya K, Kanbak G, Oğlakçı A, Burukoğlu D, Ozer MC. Protective effect of calpain inhibitor N-acetyl-L-leucyl-L-leucyl-L-norleucinal on acute alcohol consumption related cardiomyopathy. Mol Biol Rep. 2014;41(10):6743–53. doi: 10.1007/s11033-014-3560-4. [DOI] [PubMed] [Google Scholar]
- 97.Law BA, Carver WE. Activation of cardiac fibroblasts by ethanol is blocked by TGF-β inhibition. Alcohol Clin Exp Res. 2013;37(8):1286–94. doi: 10.1111/acer.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82(11):1111–29. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez A, Chawla K, Umoh NA, Cousins VM, Ketegou A, Reddy MG, et al. Alcohol and Apoptosis: Friends or Foes? Biomolecules. 2015;5(4):3193–203. doi: 10.3390/biom5043193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mackie AR, Krishnamurthy P, Verma SK, Thorne T, Ramirez V, Qin G, et al. Alcohol consumption negates estrogen-mediated myocardial repair in ovariectomized mice by inhibiting endothelial progenitor cell mobilization and function. J Biol Chem. 2013;288(25):18022–34. doi: 10.1074/jbc.M113.468009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li M, Gao P, Zhang J. Crosstalk between Autophagy and Apoptosis: Potential and Emerging Therapeutic Targets for Cardiac Diseases. Int J Mol Sci. 2016;17(3) doi: 10.3390/ijms17030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lang CH, Korzick DH. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol. 2014;306(1):23–33. doi: 10.1152/ajpregu.00414.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lang CH, Derdak Z, Wands JR. Strain-dependent differences for suppression of insulin-stimulated glucose uptake in skeletal and cardiac muscle by ethanol. Alcoholism, clinical and experimental research. 2014;38(4):897–910. doi: 10.1111/acer.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sorrentino G, Comel A, Mantovani F, Del Sal G. Regulation of mitochondrial apoptosis by Pin1 in cancer and neurodegeneration. Mitochondrion. 2014;19(Pt A):88–96. doi: 10.1016/j.mito.2014.08.003. Epub 2014/08/15. [DOI] [PubMed] [Google Scholar]
- 105.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315(5812):659–63. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 106.Galimov ER. The Role of p66shc in Oxidative Stress and Apoptosis. Acta Naturae. 2010;2(4):44–51. [PMC free article] [PubMed] [Google Scholar]