The dopamine (DA) transporter (DAT), which mediates the inactivation of released DA through its reuptake, is a primary molecular target for psychostimulants.1,2 Cocaine and methylphenidate (MPH) exert their psychostimulant properties by blocking DA reuptake, leading to the elevation of extracellular DA.2 In contrast, amphetamine (AMPH) acts as a substrate for DAT and subsequently induces non-exocytotic DAT-mediated release of DA (DA efflux).2 Here we use a Drosophila behavioral assay to delineate the signaling mechanisms that modulate DAT-mediated AMPH-induced behavior in vivo. Understanding these mechanisms is critical to understanding how the actions of AMPH might be blocked therapeutically, while simultaneously preserving DA transport.
Multiple lines of evidence suggest that kinase activity can modulate DAT function and, more specifically, AMPH action on DAT. Both calcium/calmodulin kinase II alpha (CaMKII) and protein kinase C (PKC) can phosphorylate an N-terminal DAT peptide in vitro3 and inhibiting the activity of either kinase attenuates AMPH-induced DA efflux in rodent striatial slices.3,4 Data from CaMKII and PKC knockout mice suggest a role for these kinases in regulating AMPH action in vivo.5,6 However, it is difficult to ascertain from these studies using global knockout strategies whether the observed effects on AMPH-induced DA efflux are mediated directly through alterations in DAT phosphorylation or whether they arise indirectly through phosphorylation of other targets or through circuit effects.
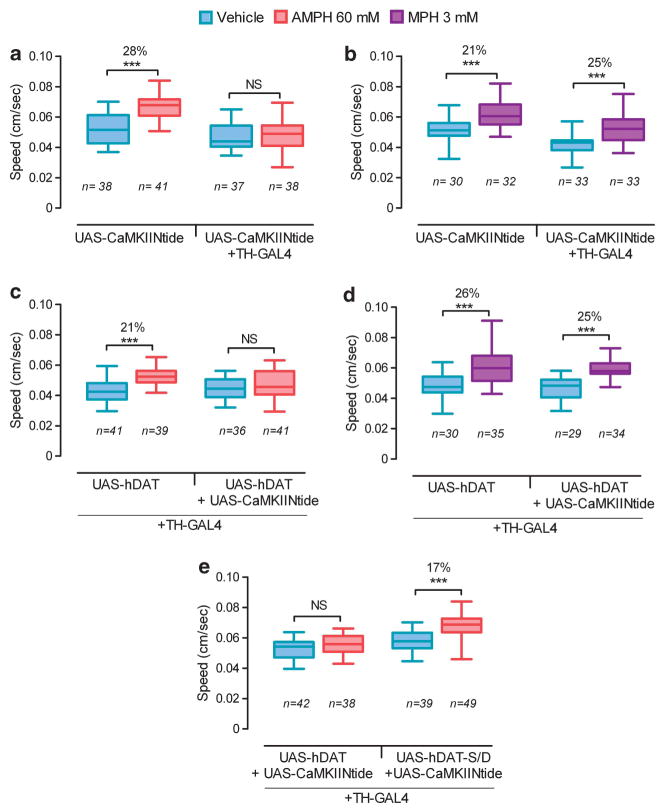
Recently we showed that phosphorylation of the N-terminus of DAT is essential for AMPH-induced, but not MPH-induced hyperlocomotion in Drosophila. Larvae respond to either AMPH or MPH by increasing their crawling velocity, and a null mutation in Drosophila DAT (dDATfmn) abolishes these locomotor responses.7 Expression of wild-type human (hDAT) in DA neurons of dDATfmn mutants rescues the response to either psychostimulant.7 In contrast, expression of a phospho-deficient mutant hDAT (hDAT-S/A) rescued only the response to MPH but not to AMPH,7 consistent with studies showing that DAT phosphorylation is required for AMPH-induced DA efflux but not for DA reuptake in heterologous cultured cells.3,8 Using the tractable Drosophila system, we have now determined whether CaMKII is required specifically in DA neurons for AMPH-induced behavior. To inhibit the activity of CaMKII, we used a UAS-driven highly selective inhibitory peptide (CaMKIINtide).9 We expressed CaMKIINtide in DA neurons of larvae using the tyrosine hydroxylase (TH) GAL4 driver.10 These larvae were fed either vehicle or AMPH and their speed of locomotion was measured as previously described.7 Larvae expressing CaMKIINtide in DA neurons (UAS-CaMKIINtide(2x)/TH-GAL4) failed to increase their crawling velocity in response to AMPH in contrast to control larvae without TH-Gal4 (UAS-CaMKIINtide(2x)/+), which exhibited significant hyperlocomotion (Figure 1a). Expression of CaMKIINtide in DA neurons did not inhibit MPH-induced hyperlocomotion (Figure 1b), consistent with our previous finding that phosphorylation of DAT is not required for the behavioral response to this DA uptake inhibitor.7 We also found that co-expression of CaMKIINtide with hDAT in DA neurons of mutant larvae that lack dDAT (dDATfmn; TH-GAL4, UAS-hDAT/UAS-CaMKIINtide(2x)) blunted AMPH-induced but not MPH-induced hyperlocomotion (Figures 1c and d, respectively). These data show that CaMKII is essential specifically in DA neurons for both dDAT-mediated and hDAT-mediated AMPH-induced hyperlocomotion in Drosophila. They also suggest that CaMKII activity is not required for DA uptake in vivo, as the response to MPH was unaffected in its absence.
Figure 1.
(a) Control larvae carrying non-driven UAS-CaMKIINtide responded to amphetamine (AMPH) by significantly increasing their crawling speed. CaMKIINtide driven by TH-GAL4 blunted the locomotor response. (b) Control larvae with either non-driven CaMKIINtide or CaMKIINtide driven by TH-GAL4 responded to methylphenidate (MPH) by significantly increasing their locomotor speed. (c–e) Experiments were performed in a dDATfmn null mutant background. (c) CaMKIINtide driven by TH-GAL4 inhibited the AMPH response mediated by human DAT (hDAT). (d) CaMKIINtide driven by TH-GAL4 did not inhibit the MPH response mediated by hDAT. (e) Expression of mutant hDAT-S/D rescued the response to AMPH that was blunted by CaMKIINtide. In all panels, UAS-CaMKIINtide indicates larvae carrying 2 copies of the transgene. Boxplots represent the median as the middle line, with the lower and upper edges of the boxes representing the 25% and 75% quartiles, respectively, and the whiskers representing the 5% and 95% quantiles. Asterisks indicate the statistical significance of the difference in mean velocity of larvae-fed drug as compared to larvae of the same genotype-fed vehicle, as determined using the nonparametric Mann–Whitney–Wilcoxon test using GraphPad Prism (NS P>0.05, ***P<0.001).
To examine whether the role of CaMKII is mediated via DAT phosphorylation, we determined whether pseudophosphorylation of the DAT N-terminus can decouple the AMPH-induced behavior from the activation state of CaMKII. Previously we demonstrated that a mutant hDAT (hDAT-S/D), which mimics constitutive phosphorylation by mutation of the five N-terminal serines to aspartates, could restore AMPH-induced hyperlocomotion in dDAT mutant larvae.7 When we expressed hDAT-S/D in animals where CaMKII was inhibited (dDATfmn; TH-GAL4, UAS-hDAT-S/D/UAS-CaMKIINtide(2x)) we found that the response to AMPH was restored (Figure 1e). In contrast, larvae expressing wild-type hDAT (dDATfmn; TH-GAL4, UAS-hDAT/UAS-CaMKIINtide(2x)) failed to respond to AMPH (Figure 1e). These data, combined with in vitro studies that show that CaMKII associates with the C-terminus of DAT and phosphorylates an N-terminal DAT peptide,3 suggest that CaMKII mediates AMPH-induced hyperlocomotion by promoting DAT phosphorylation, although further experiments will be required to confirm CaMKII-mediated DAT phosphorylation in vivo.
The role of PKC in DAT phosphorylation and AMPH-induced DA efflux remains unclear. The two kinases might act in parallel given their demonstrated ability to phosphorylate DAT,3 although our data suggest a more complex interaction, as AMPH-induced hyperlocomotion is completely blocked by CaMKIINtide. A PKC-mediated mechanism should not be blocked unless CaMKII functions downstream of the actions of PKC. Furthermore, expressing a PKC inhibitor peptide11 in DA neurons of larvae (UAS-PKCi/TH-GAL4) did not inhibit the AMPH-induced hyperlocomotion; larvae exhibited a 24% increase in speed (P<0.001) upon AMPH treatment (data not shown). Still, although this construct has previously been shown to inhibit PKC function,11 it is unclear whether it targets all fly PKC isoforms, of which there are at least five that may play redundant and/or compensatory roles.12 Therefore, further experiments will be required to determine the precise role of PKC in AMPH-induced behavior.
Understanding the mechanisms that modulate CaMKII activity in response to AMPH will be critical for understanding the actions of AMPH and how they can be blocked therapeutically. This may be particularly relevant in the case of AMPH-induced sensitization, as signaling through CaMKII can play a key role in regulating synaptic plasticity.13 Furthermore, as the signaling pathways that regulate efflux become clearer, it is possible that a physiological role for DA efflux through DAT, independent of its role in psychostimulant action, might be revealed.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Sora I, Li B, Igari M, Hall FS, Ikeda K. Ann N Y Acad Sci. 2010;1187:218–246. doi: 10.1111/j.1749-6632.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- 2.Sulzer D, Sonders MS, Poulsen NW, Galli A. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, et al. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, 4th, Leitges M, et al. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinkellner T, Yang JW, Montgomery TR, Chen WQ, Winkler MT, Sucic S, et al. J Biol Chem. 2012;287:29627–29635. doi: 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, et al. Mol Psychiatry. 2012 Jun 19; doi: 10.1038/mp.2012.82. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoshbouei H, Sen N, Guptaroy B, Johnson L’, Lund D, Gnegy ME, et al. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- 10.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 11.Broughton SJ, Kane NS, Arthur B, Yoder M, Greenspan RJ, Robichon A. J Cell Biochem. 1996;60:584–599. doi: 10.1002/(sici)1097-4644(19960315)60:4<584::aid-jcb14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Shieh BH, Parker L, Popescu D. J Biochem. 2002;132:523–527. doi: 10.1093/oxfordjournals.jbchem.a003252. [DOI] [PubMed] [Google Scholar]
- 13.Lee AM, Messing RO. Ann N Y Acad Sci. 2008;1141:22–57. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]