Abstract
Purpose
An outbreak of pneumococcal conjunctivitis occurred at Dartmouth College in 2002. We describe the clinical features, outcomes, and costs associated with this outbreak.
Methods
Six hundred ninety-eight students were diagnosed with conjunctivitis; culture of conjunctival discharge was obtained for 254. A screening protocol was used to evaluate 67 patients. A retrospective survey was offered to all 698 cases and follow-up clinical examination to all patients with culture-confirmed infection (n = 110). Local ophthalmology offices were contacted to develop a cost analysis. The college health service provided conjunctivitis data for nonoutbreak years.
Results
Of 67 patients evaluated using the screening protocol, findings associated with culture-confirmed Streptococcus pneumoniae conjunctivitis (P < 0.01) were red eye visible from 2 feet, any type of conjunctival discharge, obscuration of tarsal conjunctival blood vessels, and chemosis. Two hundred thirty-two students responded to our retrospective survey; 89% reported bilateral eye involvement; 96% received topical antibiotics and noted symptom improvement within 3 days of treatment. No ocular sequelae were identified as a result of this infection. No recurrent outbreaks have occurred at Dartmouth since the initial event. The estimated cost of this outbreak including evaluations, cultures, and antibiotics ranged from $66,468 to $120,583.
Conclusions
The ST448 strain of S. pneumoniae caused a disruptive outbreak of conjunctivitis at Dartmouth College. A screening protocol was effective at identifying culture-positive cases. Although most culture-positive patients experienced bilateral conjunctivitis, the clinical course was mild with quick resolution of symptoms after initiating antibiotics and no ocular sequelae.
Keywords: conjunctivitis, pneumococcus, ST448, Streptococcus pneumoniae, epidemic
From January 1 until April 12, 2002, a pneumococcal conjunctivitis outbreak occurred at Dartmouth College; 698 patients were diagnosed by the college health service with conjunctivitis (Fig. 1). We have previously described the microbiology and epidemiology of this outbreak.1 The causative organism was a nontypeable unencapsulated strain of Streptococcus pneumoniae. This strain had the same pulsed-field gel electrophoresis pattern as strains of pneumococcus isolated from conjunctivitis outbreaks in the early 1980s.2,3 During these earlier outbreaks, no detailed clinical descriptions were published. Since 2002, there have been 4 additional well-described conjunctivitis outbreaks caused by an unencapsulated strain of S. pneumoniae similar or identical to the Dartmouth outbreak strain, but none reported in the ophthalmic literature.4–7 The purpose of this report is to more fully describe the clinical features, outcomes, and estimated costs associated with an outbreak of this strain of S. pneumoniae as seen at Dartmouth College in 2002.
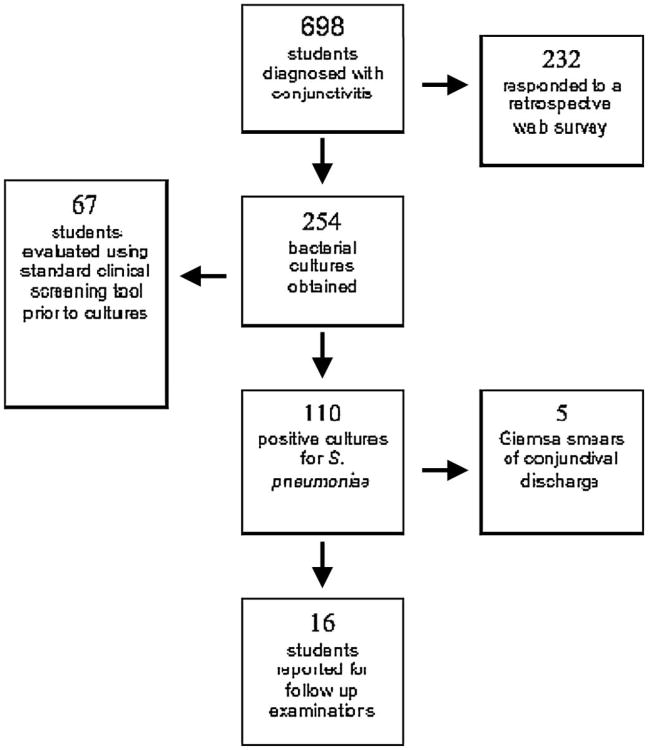
Figure 1.
Outline of data collected during and after the outbreak.
Materials and Methods
Prospective Series of Clinical Evaluations and Conjunctival Cultures
Clinical information and conjunctival cultures were obtained prospectively using a screening protocol from a consecutive series of 67 patients presenting to the college health service complaining of conjunctivitis from March 1 to 7, 2002. The protocol (Tables 1, 2) was designed to provide a platform for primary care providers (PCPs) at the college health service to gather information without the use of a slit lamp, to link clinical descriptions with culture results, and to serve as part of the medical record. PCPs were trained by an ophthalmologist (M.E.Z.) to use the protocol and to obtain conjunctival cultures. Cultures were obtained on all patients using a rayon-tipped applicator and transported in modified Stuart medium to the microbiology laboratory at Dartmouth-Hitchcock Medical Center (DHMC). Figure 1 summarizes how the data were collected during and after the outbreak.
Table 1.
Clinical Characteristics of a Consecutive Series of Patients With Cultures That Grew S. Pneumoniae Compared With Those, Whose Cultures Had No Growth or Grew Only Coagulase-Negative Staphylococcus
| S. pneumoniae n (%) n = 25 | No Growth or Coagulase-Negative Staphylococcus* n (%) n = 34 | P† | |
|---|---|---|---|
| Red at 2 feet | 24 (96) | 22 (65) | <0.01 |
| PA lymph nodes | 5 (20) | 4 (12) | 0.47 |
| SM lymph nodes | 1 (4) | 0 | 0.42 |
| Discharge | — | — | — |
| None | 3 (12) | 15 (44) | 0.01‡ |
| Some | 22 (88) | 19 (56) | — |
| Watery | 3 (14) | 9 (47) | 0.04 |
| Mucoid | 11 (50) | 8 (42) | 0.76 |
| Purulent | 8 (36) | 2 (11) | 0.08 |
| Tarsal vessels | — | — | — |
| Normal | 2 (8) | 13 (41) | 0.01§ |
| Abnormal | 23 (92) | 21 (62) | — |
| Partially obscured | 15 (65) | 19 (90) | 0.071¶ |
| Completely obscured | 8 (35) | 2 (10) | — |
| Chemosis | 7 (28) | 0 | <0.01 |
| Sharp corneal reflex | 25 (100) | 33 (97) | 1.00 |
PA, preauricular; SM, submandibular.
Eight patients grew H. influenzae on conjunctival culture and were not included in the analysis.
P value (Fisher Exact) of <0.05 was considered statistically significant.
Comparison of any type of discharge and the presence of positive culture for S. pneumoniae.
Comparison of any category of tarsal vessels (normal, partially obscured, or completely obscured) and the presence of positive culture for S. pneumoniae.
Comparison of partially obscured versus completely obscured tarsal vessels and the presence of positive culture for S. pneumoniae.
Table 2. Sensitivity and Specificity of Clinical Findings: Red Eye from 2 Feet, Any Discharge, and a Combination of Red Eye or Any Discharge.
| Formulae | Red Eye | Any Discharge | Red Eye or Any Discharge | |
|---|---|---|---|---|
| Sensitivity | TP/(TP + FN) | 0.96 | 0.88 | 1.00 |
| Specificity | TN/(TN + FP) | 0.35 | 0.44 | 0.18 |
| Positive predictive value | TP/(TP + FP) | 0.52 | 0.34 | 0.47 |
| Negative predictive value | TN/(TN + FN) | 0.92 | 0.83 | 1.00 |
| Accuracy | (TN + TP)/all | 0.61 | 0.63 | 0.53 |
FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Cytology of Conjunctival Discharge
The conjunctival discharge of a nonrandomized series of 5 patients was examined by Giemsa staining and microscopy. No criteria were used to select these patients except the presence of purulent discharge (Fig. 2). Slides were prepared by spreading conjunctival discharge over a glass microscope slide with a rayon-tipped applicator and examined by a pathologist (J.D.S.) at DHMC.
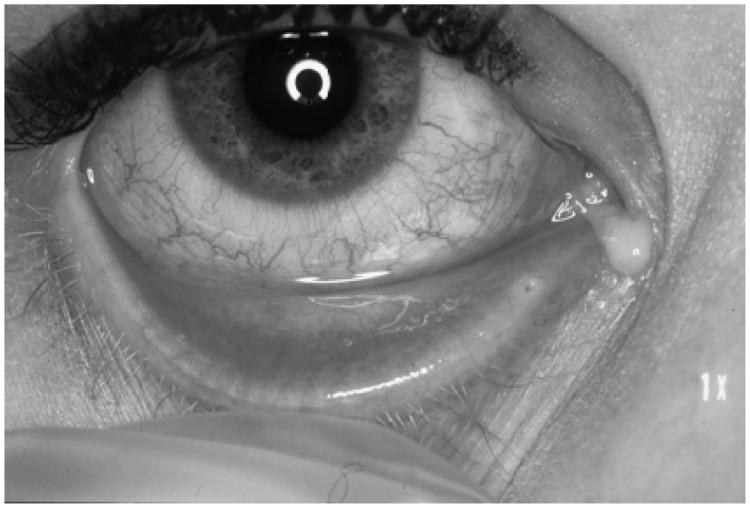
Figure 2.
Example of a patient with S. pneumoniae conjunctivitis showing a red eye and purulent discharge.
Follow-up Clinical Examinations of Patients With Culture-Proven Pneumococcal Conjunctivitis
After the outbreak, a follow-up visit with an ophthalmologist on April 20, 2002 was offered via email to all patients (n = 110) whose conjunctival cultures grew S. pneumoniae to identify infection-associated sequelae. Twenty-one patients scheduled appointments; 16 of these patients (76%) reported to their appointments and were examined a minimum of 2 weeks after initiating treatment. A Snellen test was used to measure visual acuity. A slit-lamp examination of the anterior segment of each eye was performed on each patient by the same ophthalmologist.
Retrospective Survey of All Students Diagnosed With Conjunctivitis During the Outbreak
On April 26, 2002, all patients who had office visits coded as conjunctivitis from January 1 to April 12, 2002 (n = 698) were contacted via email and invited to complete an Internet-based survey regarding the clinical features, course, and outcome of their episode of conjunctivitis. This 17-item survey was available on a college Web site in a similar fashion to surveys reported previously by our group.1,8 The Web site was password protected and open for 7 days.
Cost Analysis
A retrospective cost analysis of the outbreak was performed, including costs of office visits, cultures, and antibiotic treatment. A range of total cost estimates was created based on type of office visit and antibiotic used. The cost of an office visit for International Classification of Diseases, 9th edition (ICD-9)-9 codes 372.30 (conjunctivitis, unspecified) and 372.03 (other mucopurulent conjunctivitis) varies according to the type of provider (PCP vs ophthalmologist), complexity, geographic region, and time spent with each patient. Local ophthalmology offices were contacted to establish a regional average cost for these ICD-9 codes. Charges ranged from $78 to $148 (average cost $113) per visit with $112 being the most common. The cost of $112 per visit to see an ophthalmologist was used as the baseline ophthalmology cost in this analysis. Costs from 7570 insurance claims were used to establish a baseline cost for a patient to see a PCP (average cost $73.72). These costs ($112 and $73.72) were multiplied by the total number of patients (N = 698) diagnosed with conjunctivitis at the college health service from January 1 to April 12, 2002. The total cost of bacterial cultures was calculated by multiplying the cost of each culture ($40) by the total number of cultures performed (n = 254). A range of cost estimates for treatment was obtained by multiplying the cost of the 4 most commonly prescribed antibiotics in this outbreak [bacitracin 500 U/g ointment: $7.24, ciprofloxacin 0.3% (5 mL): $48.13, ofloxacin 0.3% (5 mL): $43.05, and levofloxacin 0.5% (5 mL): $43.60] by 670 (96% of 698; see Results from retrospective survey). The cost estimates assume that each antibiotic was used on the total population of patients and do not account for costs associated with use of other antibiotics or antibiotics used in combination with each other.
Conjunctivitis Cases Before and After the 2002 Outbreak
The college health service provided data on the number of cases coded as conjunctivitis between January 1 and April 12 for the years 2000 through 2007.
Statistical Analysis
χ2 and exact tests were used for the prospective screening protocol and the retrospective clinical survey. All analyses were conducted using Stata 10.0 (StataCorp LP, College Station, TX). P values of 0.05 or less (Fisher exact) were considered to be statistically significant.
Results
Prospective Series of Clinical Evaluations and Conjunctival Cultures
PCPs collected clinical data and conjunctival cultures on 67 patients presenting with ocular complaints at the college health service from March 1 to 7, 2002. Of 67 conjunctival cultures obtained, 25 (37%) grew S. pneumoniae, 34 (51%) had no growth or grew only coagulase-negative Staphylococcus and 8 (12%) grew Haemophilus influenzae. These proportions are similar when compared with culture data gathered from the entire outbreak [of 254 conjunctival cultures, 110 (43%) grew S. pneumoniae, 125 (49%) had no growth or grew only coagulase-negative Staphylococcus, and 19 (8%) grew H. influenzae].1
Table 1 summarizes the clinical characteristics of patients with cultures that grew S. pneumoniae (n = 25) and those, which had no growth or grew only coagulase-negative Staphylococcus (n = 34). Patients with positive S. pneumoniae cultures were more likely to have a red eye observed from 2 feet (P < 0.01), any discharge (P = 0.01), obscured tarsal blood vessels (P = 0.01), and chemosis (P < 0.01). Purulent discharge compared with other forms of discharge and completely obscured tarsal vessels compared with partially obscured tarsal vessels were near statistical significance for association with positive cultures for S. pneumoniae cultures but did not reach a P value of 0.05 likely owing to the small number of patients with these findings. All 7 patients reported to have chemosis had S. pneumoniae-positive conjunctival cultures. The presence of palpable preauricular lymph nodes (P = 0.31), watery discharge, mucoid discharge, partially obscured tarsal vessels, or a sharp corneal reflex were not statistically significantly different between the 2 groups. Table 2 summarizes the sensitivity, specificity, positive predictive value, and negative predictive value of a red eye observed from 2 feet, any discharge, and a combination of red eye or any discharge for positive S. pneumoniae cultures.
Cytology of Conjunctival Discharge
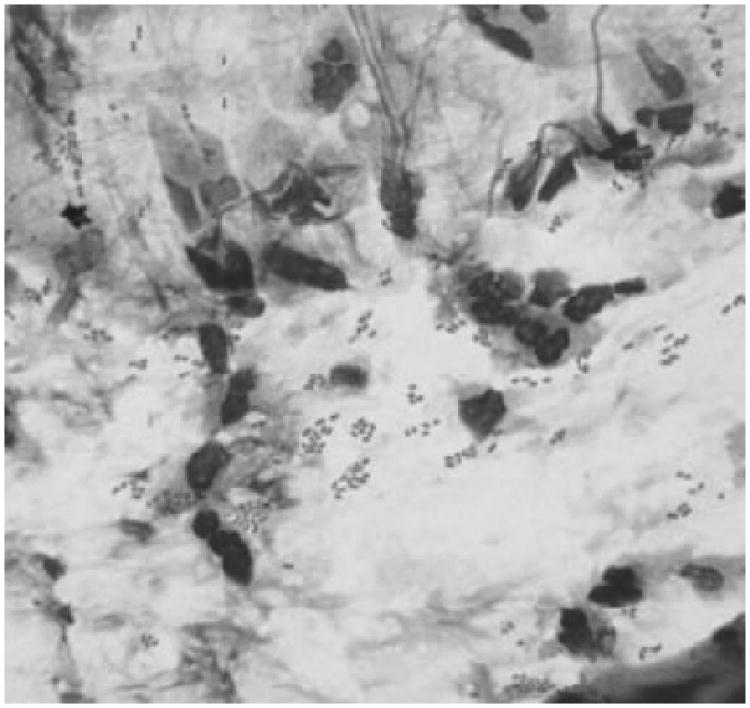
Giemsa smears of conjunctival discharge were obtained from 5 students during the course of the epidemic. A neutrophilic response and organisms consistent with S. pneumoniae (lancet-shaped diplococci) were identified on all 5 smears (Fig. 3). All 5 patients had cultures confirming the presence of S. pneumoniae.
Figure 3.
Giemsa stain smear of conjunctival discharge from a patient with culture-confirmed S. pneumoniae infection showing neutrophilic response and lancet-shaped diplococci. No viral inclusion bodies were noted.
Follow-up Clinical Examinations on Patients With Culture-Positive S. pneumoniae Conjunctivitis
Of 110 S. pneumoniae culture-positive patients invited for clinical follow up, 16 chose to report for examination. The best-corrected visual acuity was 20/20 in 31 of 32 eyes. One eye was 20/32 in a patient with a history of amblyopia. Of 32 eyes, 31 showed no clinical evidence of current bacterial conjunctivitis. One patient had clinical evidence of recurrent infection confirmed by culture. No corneal or conjunctival scarring was observed.
Retrospective Survey of Students Diagnosed With Conjunctivitis During the Outbreak
During the period from April 26 to May 2, 2002, 232 of 698 students (33%) with conjunctivitis responded to our follow-up survey. Eyes were reported as “normal” by 203 of 232 respondents (88%). The following symptoms were reported by the 29 respondents (Table 3), which indicated that their eyes were not back to normal: red eyes [27 of 29 (93%)], pruritus [22 of 29 (76%)], dry eyes [21 of 29 (72%)], crusting [16 of 29 (55%)], discharge [9 of 29 (31%)], periorbital swelling [5 of 29 (17%)], vision changes [5 of 29 (17%)], pain [4 of 29 (14%)], gritty sensation [3 of 29 (10%)], and/or other [1 of 29 (3%)]. Total symptom duration for all 232 respondents ranged from 1 to 60 days with a median of 7 days. No significant difference in symptom duration was noted when stratified by culture results. All respondents rated their level of discomfort as moderate (median 4 of 10 on a standard 1–10 pain scale). Bilateral disease was reported in 206 of 232 respondents (89%), with 91 (39%) having both eyes involved at onset and 115 (50%) having the second eye become symptomatic in a median of 2 days (range 1–12 days). Furthermore, patients who had 1 eye involved at onset and had S. pneumoniae-positive cultures were more likely to have the second eye become symptomatic [34 of 38 (90%)] than those without positive S. pneumoniae cultures [81 of 114 (71%); P = 0.028]. Of 229 respondents, 220 (96%) received topical antibiotics and noted improvement in 1–25 days (median of 3 days). Of 232 respondents, 214 (92%) received an alcohol-based hand sanitizer (Endure); of these, 174 (81%) reported using 0%–25% of the 4-ounce bottle.
Table 3. Symptoms Reported by 29 Students With Persistent Symptoms of Conjunctivitis Among 232 Respondents to the Retrospective Clinical Survey, April 26–May 2, 2002.
| Reported Symptom | Patients Reporting n (%) n = 29 |
|---|---|
| Red eyes | 27 (93) |
| Pruritus | 22 (76) |
| Dry eyes | 21 (72) |
| Crusting | 16 (55) |
| Discharge | 9 (31) |
| Periorbital swelling | 5 (17) |
| Vision changes | 5 (17) |
| Pain | 4 (14) |
| Gritty sensation | 3 (10) |
| Other | 1 (3) |
Cost Analysis
Table 4 summarizes the cost analysis results. The cost of 698 PCP office visits was $51,457. Likewise, the cost of 698-ophthalmology office visits for conjunctivitis was $78,176. The cost of the 254 cultures performed was $10,160. The estimated cost of individual antibiotic treatment for 670 patients was $4851 (bacitracin), $28,844 (ofloxacin), $29,212 (levofloxacin), and $32,247 (ciprofloxacin). The total cost estimate (sum of office visits, cultures, and antibiotic treatment) of the outbreak depends on antibiotic choice and provider type: bacitracin (PCP-$66,468; ophthalmologist-$93,187), ofloxacin (PCP-$90,461; ophthalmologist-$117,180), levofloxacin (PCP-$90,829; ophthalmologist-$117,548), and ciprofloxacin (PCP-$93,864; ophthalmologist-$120,583).
Table 4. Retrospective Cost Analysis of the Outbreak.
| Antibiotic | Cost to Treat ($) n = 670 | Cost of Culture ($40) n = 254 | Cost of PCP Office Visits ($73.72) n = 698 | Cost of Ophthalmology Office Visits ($112) n = 698 | Total Cost Estimate ($) |
|---|---|---|---|---|---|
| Bacitracin $7.24 | 4851 | 10,160 | 51,457 | 78,176 | 66,468* 93,187† |
| Ofloxacin $43.05 | 28,844 | 10,160 | 51,457 | 78,176 | 90,461* 117,180† |
| Levofloxacin $43.60 | 29,212 | 10,160 | 51,457 | 78,176 | 90,829* 117,548† |
| Ciprofloxacin $48.13 | 32,247 | 10,160 | 51,457 | 78,176 | 93,864* 120,583† |
Total cost = cost to treat + cost to culture + cost of PCP office visit.
Total cost = cost to treat + cost to culture + cost of ophthalmologist office visit.
Cases of Conjunctivitis During, Before, and After the 2002 Outbreak
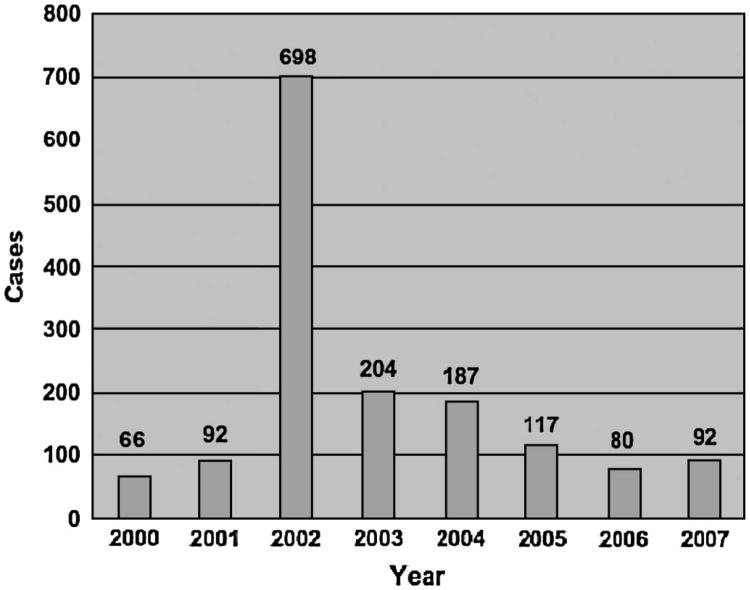
The number of conjunctivitis cases seen at the college health service between January 1 and April 12 in the years 2000–2007 is presented in Figure 4. The number of diagnoses of conjunctivitis made was 7- to 10-fold higher in 2002 than in the 2 preceding nonoutbreak years (2000 and 2001), remained 2–3 times baseline in 2003, and has returned to baseline since 2006.
Figure 4.
Number of patients diagnosed with conjunctivitis at the Dartmouth College Health Service between January 1 and April 12 for the years 2000 through 2007.
Discussion
The ST448 strain of S. pneumoniae that caused the Dartmouth outbreak of conjunctivitis has been associated with other conjunctivitis outbreaks before and since the Dartmouth outbreak,1,4–7 and it is likely that additional outbreaks will occur in the future. Because of its setting on a college campus near a medical school, we were able to collect a large amount of both prospective and retrospective data regarding the clinical characteristics of this form of bacterial conjunctivitis during and after the outbreak (Fig. 1). Our data regarding the diagnosis, symptoms, and prognosis of conjunctivitis associated with this strain of S. pneumoniae will be useful to clinicians in managing future outbreaks.
The role of antibiotics in the treatment of bacterial conjunctivitis is controversial. Some argue that bacterial conjunctivitis is a benign self-limiting condition and its widespread treatment adds to the selection of drug-resistant microorganisms. In practice, patients and clinicians often feel uncomfortable withholding treatment. A recent Cochrane Review concluded that there is a moderate benefit to antibiotic treatment.9 During an outbreak, increased anxiety among the affected population and a desire to control disease spread provide additional pressures to treat with antibiotics. This is reflected in our experience where 96% of survey respondents indicated that they had been treated with antibiotics. Because of this high treatment rate, we cannot draw conclusions about the effectiveness of antibiotics, either to the individual patient or in quelling the outbreak. However, our data about the presentation and sequelae of conjunctivitis associated with the ST448 strain do offer insights when deciding whether or how to employ topical antibiotics in the management of epidemic bacterial conjunctivitis.
In our retrospective survey, only 39% of patients reported bilateral disease at presentation but 89% developed bilateral disease, most within 2 days of presentation. There may be a window where antibiotics could prevent spread to the second eye; nevertheless, most patients seen during the outbreak were treated with antibiotics and still progressed to bilateral disease. We do not have data about whether students were using antibiotics in the symptomatic eye only or in both eyes, but given the high rate of bilateral disease associated with this strain of S. pneumoniae, if antibiotics are used, bilateral treatment may be a reasonable approach even among patients presenting with unilateral disease.
A screening protocol was developed during the outbreak and used in a series of patients presenting to the college health service with ocular complaints. The exam criteria were designed to be used by PCPs without ophthalmic equipment. We found that a red eye visible from 2 feet, the presence of any discharge, abnormal tarsal vessels, and chemosis were more likely to be present in patients with culture-positive S. pneumoniae infection. The presence of a red eye visible from 2 feet had a sensitivity of 96% for predicting culture-positive S. pneumoniae infection but was only 35% specific. Thus, if we used red eye visible at 2 feet as a criterion for initiating antibiotic treatment, we would treat most of the culture-positive patients and 65% of the culture-negative patients. This may be appropriate because in the setting of an outbreak, the cohort of culture-negative patients may include a significant number of patients infected with the outbreak strain who have false-negative culture results. Given that 43% of the ocular cultures obtained during the outbreak and 37% in our prospective series grew S. pneumoniae, developing clinical criteria to guide treatment is desirable because well patients may have sought treatment secondary to anxiety about infection. Increases in symptoms among well patients are common during disease outbreaks.11,12 Although our study did not reveal a clinical sign with both high sensitivity and specificity for culture-positive pneumococcal disease, even a small reduction in the number of patients treated in the setting of a large outbreak could yield significant cost and manpower savings.
Of 110 students offered follow-up examinations with cultures positive for S. pneumoniae, 16 reported to clinic. This low attendance rate suggests a low rate of ocular complications associated with the outbreak. Among the 16 students examined, there was no evidence of damage to the ocular surface or other ocular pathology attributable to their infection. One patient was diagnosed with recurrent pneumococcal conjunctivitis. The Corneal/External Disease Service at DHMC has not received any referrals for ocular complications related to the outbreak. Since 2002, there have been no recurrent outbreaks at Dartmouth. Conjunctivitis visits to the college health service were dramatically reduced during the winter of 2003 compared with 2002 and have continued to fall up through 2006 (Fig. 4). Thus, there is no evidence that this strain caused permanent damage to infected eyes or of recurrent outbreaks in the population. The apparently benign ocular prognosis for this infection may be helpful in reassuring exposed populations during future outbreaks.
In the Dartmouth outbreak our public health interventions included education of the community about the epidemic, as well as the distribution of an alcohol based hand sanitizer. While more than 92% of students responding to our retrospective survey reported that they received the hand sanitizer, only 19% used more than 25%, leaving unanswered the question of whether these measures might be effective in the community as they have been found to be in healthcare settings.10 Perhaps, the hand sanitizer functioned less as a disinfectant and more as an education mnemonic to remind students about the epidemic and importance of hand washing.
Cost estimates for the outbreak range from $66,468 to $120,583 depending on whether the initial evaluation was done by a PCP in the student health center or by an ophthalmologist and which antibiotic was selected. The cost analysis does not account for missed school or workdays or the extra hours worked by the medical staff. Our microbiology laboratory identified this S. pneumoniae strain as susceptible to all 4 antibiotics used in the cost analysis; therefore, prescribing the least expensive antibiotic (unless contraindicated) would be 1 approach to contain costs in future outbreaks—in our case bacitracin. A limitation to our cost analysis is that we were unable to obtain the proportion of students who saw a PCP versus and ophthalmologist, and the exact distribution of antibiotics used.
Additionally, recent outbreaks of nontypeable unencapsulated pneumococcal conjunctivitis have been reported. The ST448 strain of S. pneumoniae was identified as the pathogen in an outbreak among elementary school children in Maine.5 As in the Dartmouth outbreak, systemic pneumococcal infections were not diagnosed and the outbreak subsided after a school holiday approximately 4 months after its onset. Another outbreak of S. pneumoniae conjunctivitis began in December of 2003 among 92 individuals at a military base in San Diego.4 Multilocus sequence typing (MLST) revealed a unique profile, ST1186, but the most closely related strain was ST448.4 The ocular findings were similar to those we have described, and there were no infections among health care workers [Nancy Crum-Cianflone (MD, MPH, personal oral communication)]. Importantly, unlike the other outbreaks, 4 patients were identified with concurrent pneumococcal pneumonia and 1 with bacterial sinusitis. Another outbreak occurred in late 2003 in Minnesota. In contrast to the other reports, cases were widely distributed in the community and not localized to an institution. Multilocus sequence typing revealed that the majority of isolates were the ST448 strain.6 Finally, an outbreak occurred in March and April 2005 in Saskatchewan, Canada. Fluorescence-based amplified fragment length polymorphism analysis was used to characterize the isolates rather than pulsed-field gel electrophoresis or MLST, so it is unknown if the ST448 strain caused this outbreak, however, like the Dartmouth outbreak, there were no associated cases of pneumonia, and the majority of cases had bilateral eye involvement.7
Consistent with our experience at Dartmouth, there were no reports of health care worker infection or transmission during these recent outbreaks, in contrast to adenoviral conjunctivitis where health care worker infection is often integral to transmission to patients.13,14 Interestingly, all 4 outbreaks occurred in the autumn or winter and all subsided after holiday vacations. The physical dispersal of the community may have played a role in ending these outbreaks as it appeared to during the Dartmouth outbreak. If practical, future outbreaks occurring among groups living or working in close association may benefit from dispersal of the affected community.
In summary, we have presented data on the clinical characteristics of a large outbreak of conjunctivitis caused by the ST448 of S. pneumoniae. A screening tool administered by PCPs was effective at prospectively identifying patients from whom the outbreak strain could be isolated. This may be useful for targeting antibiotic treatment in future outbreaks. The cost of care for the outbreak was significant, and antibiotic treatment contributed considerably to this cost. There were no long-term ocular sequelae identified. Data from recent outbreaks of S. pneumoniae conjunctivitis confirm the relatively benign ocular clinical course and suggest that dispersal of the community plays a substantial role in suppressing these outbreaks.
Acknowledgments
Author Contributions: Design of the study: Michael E. Zegans, Michael T. Martin, Cynthia G. Whitney, and Paul A. Sanchez. Conduct of study: Michael E. Zegans, Michael T. Martin, Joseph D. Schwartzman, Cynthia G. Whitney, John H. Turco, and Paul A. Sanchez. Collection and management: Michael E. Zegans, John H. Turco, Joseph D. Schwartzman, John H. Pryor, and Paul A. Sanchez. Analysis and interpretation of the data: Michael E. Zegans, Cynthia G. Whitney, Donald S. Likosky, Rory T. Allar, and Paul A. Sanchez. Preparation, review, and approval of the article: Michael E. Zegans, Cynthia G. Whitney, Donald S. Likosky, Paul A. Sanchez, and Rory T. Allar. We are indebted to Susan Pepin, MD, Angela D. Sanchez, MS, Jessica Greenwood, MS, Mark Nunlist, MD, Patrick Herson, MD, Claudia Zegans, MD, Peter Mahar, MD, Steven Woloshin, MD, Lisa Schwartz, MD, Sandra Robinson, Laurie Needham, Carrie Morrison, CPC, Diane Minard, CPC, Yolanda Baumgartner, Charlene Bradley, Sam Power, Rachel E. Woolley, ARNP, Nikole Eyre, MS, clinicians and other staff members at the Dartmouth College Health Service.
Supported in part by a grant (1 K08 EY13977-01 to Dr. Zegans) from the National Eye Institute.
The Institutional Review Board Office at Dartmouth College was alerted to the Centers for Disease Control and Prevention investigation and our additional investigations. The Institutional Review Board Office designated both investigations as “not research” as defined by federal regulations 45CFR46. The study protocol adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996.
Footnotes
The authors indicate no financial conflict of interest.
References
- 1.Martin M, Turco JH, Zegans ME, et al. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N Engl J Med. 2003;348:1112–1121. doi: 10.1056/NEJMoa022521. [DOI] [PubMed] [Google Scholar]
- 2.Shayegani M, Parsons LM, Gibbons WE, Jr, et al. Characterization of nontypable Streptococcus pneumoniae-like organisms isolated from outbreaks of conjunctivitis. J Clin Microbiol. 1982;16:8–14. doi: 10.1128/jcm.16.1.8-14.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ertugrul N, Rodriguez-Barradas MC, Musher DM, et al. BOX-polymerase chain reaction-based DNA analysis of nonserotypeable Streptococcus pneumoniae implicated in outbreaks of conjunctivitis. J Infect Dis. 1997;176:1401–1405. doi: 10.1086/517331. [DOI] [PubMed] [Google Scholar]
- 4.Crum NF, Barrozo CP, Chapman FA, et al. An outbreak of conjunctivitis due to a novel unencapsulated Streptococcus pneumoniae among military trainees. Clin Infect Dis. 2004;39:1148–1154. doi: 10.1086/424522. [DOI] [PubMed] [Google Scholar]
- 5.Leighton C, Piper D, Gunerman-King J, et al. Pneumococcal conjunctivitis at an elementary school—Maine, September 20–December 6, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:64–66. [PubMed] [Google Scholar]
- 6.Buck JM, Lexau C, Shapiro M, et al. A community outbreak of conjunctivitis caused by nontypeable Streptococcus pneumoniae in Minnesota. Pediatr Infect Dis J. 2006;25:906–911. doi: 10.1097/01.inf.0000238143.96607.ec. [DOI] [PubMed] [Google Scholar]
- 7.Hennink M, Abbas Z, McDonald RR, et al. Streptococcus pneumoniae outbreak in a rural Regina community. Can Commun Dis Rep. 2006;32:181–186. [PubMed] [Google Scholar]
- 8.Pryor JH, Martin MT, Whitney CG, et al. Rapid response to a conjunctivitis outbreak: the use of technology to leverage information. J Am Coll Health. 2002;50:267–271. doi: 10.1080/07448480209603444. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006(2):CD001211. doi: 10.1002/14651858.CD001211.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the healthcare infection control practices advisory committee and the HIPAC/SHEA/APIC/IDSA hand hygiene task force. Am J Infect Control. 2002;30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 11.Jones TF. Mass psychogenic illness: role of the individual physician. Am Fam Physician. 2000;62:2649–2653. 2655–2656. [PubMed] [Google Scholar]
- 12.Radford B, Bartholomew R. Pokemon contagion: photosensitive epilepsy or mass psychogenic illness? South Med J. 2001;94:197–204. [PubMed] [Google Scholar]
- 13.Jernigan JA, Lowry BS, Hayden FG, et al. Adenovirus type 8 epidemic keratoconjunctivitis in an eye clinic: risk factors and control. J Infect Dis. 1993;167:1307–1313. doi: 10.1093/infdis/167.6.1307. [DOI] [PubMed] [Google Scholar]
- 14.Warren D, Nelson KE, Farrar JA, et al. A large outbreak of epidemic keratoconjunctivitis: problems in controlling nosocomial spread. J Infect Dis. 1989;160:938–943. doi: 10.1093/infdis/160.6.938. [DOI] [PubMed] [Google Scholar]