Abstract
Alzheimer’s disease (AD) was originally conceived as a rare disease that caused presenile dementia but has come to be understood as the most prevalent cause of dementia at any age worldwide. It has an extended preclinical phase characterized by sequential changes in imaging and cerebrospinal fluid biomarkers with subtle memory decline beginning more than a decade before the emergence of symptomatic memory loss heralding the beginning of the mild cognitive impairment stage. The apolipoprotein Eε4 allele is a prevalent and potent risk factor for AD that has facilitated research into its preclinical phase. Cerebral Aβ amyloid levels build from preclinical through early dementia stages followed by hyperphosphorylated tau-related pathology, the latter driving cognitive deficits and dementia severity. Structural and molecular imaging can now recapitulate the neuropathology of AD antemortem. Autosomal dominant forms of early onset familial AD gave rise to the amyloid hypothesis of AD that in turn has led to therapeutic trials of immunotherapy designed to clear cerebral amyloid but to date results have been disappointing. Genome wide association studies have identified multiple additional risk factors but to date none have yielded an effective alternate therapeutic target. Current and future trials aimed at presymptomatic individuals either harboring cerebral amyloid or at genetically high risk offer the hope that earlier intervention might yet succeed where trials in patients with established dementia have failed. A major looming challenge will be that of expensive, incompletely effective disease modifying therapy: who and when to treat, and how to pay for it.
I. Introduction
Much has changed since November 3, 1906 the day Alois Alzheimer first presented the unusual case of Auguste Deter to the Society of Southwest German Psychiatrists. Her symptoms of delusional jealousy, paranoia, and memory loss began insidiously at age 51, ended with her death at age 55, and led to the original conception of Alzheimer’s disease (AD) as a rare cause of presenile dementia.1 The first major change occurred seventy years later when Katzman and Terry argued that the disease bearing Alzheimer’s name was also the cause of senile dementia in the elderly, a much more prevalent condition,2,3 and so far from previous conceptions AD came to be understood as highly prevalent, the major cause of dementia at any age, and a major cause of death. A second and more recent major change has been to dispel the notion that AD can only be confirmed at autopsy. Advances in brain imaging have made antemortem confirmation a reality (within the research arena). Genomics and many more advances have further led to our current concept of AD and constitute the bulk of this review.
II. Clinical Background
When symptoms first become apparent, patients are forgetful but still functioning independently. The diagnostic term "mild cognitive impairment" (MCI) was originally introduced to define a nondisabling but progressive monosymptomatic amnestic syndrome,4–6 and evolved into a broader classification of early, nondisabling cognitive deficits.7,8 Longitudinal studies of patients with MCI have shown that roughly 10–15% per year lose their ability to function reasonably independently, the defining characteristic of dementia.4,5,9,10 After five years, about half of all patients with MCI will meet criteria for dementia, particularly AD, and after 10 years, most will have AD or another dementia syndrome. At autopsy, 70–80% of patients originally diagnosed with MCI prove to have AD as the major component of the dementia.10,11
The latest version of the National Institute on Aging Alzheimer’s Disease Center’s Uniform Data Set, characterizes AD dementia as an “amnestic multidomain dementia syndrome”12 meaning progressive memory loss over months to years with the gradual emergence of executive, language, visuospatial and other deficits with or without behavioral features such as sundowning and paranoia. Diagnostic criteria are summarized in table 1.13 Alzheimer’s disease does not always follow the canonical neuropathological pattern, however. Variant syndromes reflect a different pathological topography. Visual variant AD, or posterior cortical atrophy reflects progressive visual impairment related to early degenerative involvement of visual cortices.14 Other focal variants of Alzheimer’s disease affect language, motor, and executive functions.15–18
Table 1.
NIA-AA Diagnostic Guidelines for Alzheimer’s Disease
A. Criteria for “all-cause dementia” include cognitive or behavioral symptoms that:
|
B. Criteria for probable Alzheimer’s disease dementia includes criteria for all cause dementia plus:
|
C. Criteria for probable Alzheimer’s disease dementia with “increased level of certainty”
|
D. Criteria for Possible Alzheimer’s disease dementia
|
| E. Probable Alzheimer’s disease dementia with evidence of the pathophysiological process (biomarkers) |
| F. Possible Alzheimer’s disease dementia with evidence of the pathophysiological process (biomarkers) |
| G. Pathophysiologically proved Alzheimer’s disease dementia (Neuropathologically confirmed) |
H. Dementia unlikely to be due to Alzheimer’s disease
|
Data from Alz Dem.13
III. Neuropathology
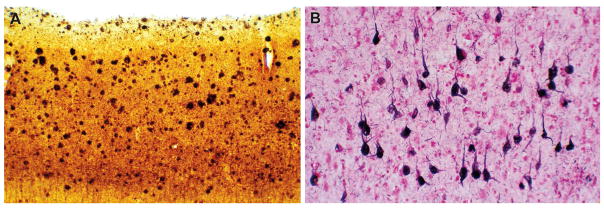
Neuropathologically, AD is characterized by two hallmark features: amyloid plaques and neurofibrillary tangles (NFT) (figure 1). Morphologically amyloid plaques are described as either diffuse or neuritic. Both types may be seen in nondemented individuals, where they may indicate an increased risk of progression to dementia. Neuritic plaque density has a significant association with cognitive impairment while for diffuse plaques this relationship is tenuous. The primary event in plaque formation is the deposition of insoluble Aβ amyloid while the “neuritic” elements (dystrophic axons and dendrites) are a reaction to this, and contain pathological bundles of tau proteins that are identical to the NFT’s found within neuronal perikarya. Tau is a cytoskeletal protein whose function is to stabilize microtubules that comprise the neuronal cytoskeleton. Tau within dystrophic neurites and NFTs is abnormally phosphorylated. This may impair microtubule binding and facilitate aggregation of tau into paired helical filaments, which are likely to impair the neuron’s ability to maintain extensive dendritic and axonal arborizations, ultimately leading to loss of synaptic connectivity and neuronal death.
Figure 1.
Photomicrographs of frequent diffuse and neuritic plaques (Campbell-Switzer silver stain, middle frontal gyrus, 100x), and neurofibrillary tangles (Gallyas silver stain, cerebral cortex, 100x) in a patient with Alzheimer’s disease.
Because both plaques and tangles can also occur in nondemented individuals, their mere presence is insufficient to diagnose AD, and so neuropathological criteria have been developed to define the likelihood that dementia is a consequence of AD. In 1985, the recommendations of an NIA consensus panel focused on neocortical total plaque density as a function of age,19 a recommendation echoed with a focus on neuritic plaques in 1991 by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD).20 Around this time, Braak and Braak demonstrated that there is a predictable six stage march of NFT pathology from paralimbic to neocortical regions summarized, from earliest to latest, as transentorhinal (stage I/II), limbic (stage III/IV), and neocortical (stage V/VI).21,22 In 1997 an NIA and Reagan Institute working group combined CERAD amyloid plaque and Braak NFT staging to define the likelihood that dementia results from AD. High likelihood is reflected by frequent CERAD neuritic plaque score and Braak Stage V/VI NFT; intermediate likelihood by moderate CERAD plaque score and Braak NFT stage III/IV; and low likelihood AD if sparse CERAD plaque score and Braak NFT stage I/II.23 Essential areas to be sampled differ somewhat between criteria but all include limbic, neocortical, and subcortical areas.
Amyloid plaque distribution, in contrast to NFT’s, begins in neocortical regions and even in the earliest dementia stages has usually progressed to involve diencephalic regions.24 It continues to build through Braak stages IV and V with progressively increasing subcortical and eventually brainstem and cerebellar involvement, but the rate of deposition may decline in the late stages of dementia.25 As will be discussed, molecular imaging in living patients with PET ligands for amyloid and tau have made it possible to demonstrate this neuropathological evolution in real time.
At autopsy, AD is often found not to be a unitary neuropathological diagnosis but includes prevalent comorbidities, particularly vascular and Lewy body pathology which may contribute to dementia severity.26–31 Table 2 shows the commonly identified major neuropathological comorbidities from the Banner Sun Health Research Institute brain bank.32 The proportion of patients with relatively pure AD drops from 38% in sexagenarians to 25% in nonagenarians. This heterogeneity is also seen in patients dying during the MCI stage.26 Even in those older patients who come to autopsy without cognitive problems, AD, vascular, and Parkinson’s disease related pathologies are frequent findings (table 3).
Table 2.
Decadal counts of neuropathological Alzheimer’s disease total comorbidities, Banner Sun Health Research Institute Brain and Body Donation Program/Arizona Study of Aging and Neurodegenerative Disorders.
| Decade | AD “pure”1 | AD all | AD/VaD | AD/PD | AD/DLB | AD/LB | AD/PSP | AD/HS | AD/FTLD-TDP |
|---|---|---|---|---|---|---|---|---|---|
| 50’s - 60’s | 20 (38%) | 53 | 0 | 1 | 11 | 20 | 0 | 0 | 0 |
| 70’s | 55 (31%) | 177 | 14 | 23 | 33 | 45 | 2 | 6 | 4 |
| 80’s | 90 (26%) | 350 | 45 | 33 | 54 | 128 | 15 | 22 | 7 |
| 90’s | 35 (25%) | 138 | 38 | 6 | 20 | 44 | 9 | 16 | 5 |
| 100’s | 1(14%) | 7 | 3 | 0 | 0 | 2 | 2 | 1 |
Alzheimer’s disease (AD) “pure” is defined as AD without any of the other listed diagnoses. AD was defined as a clinically-documented dementia with NIA-Reagan intermediate or high AD pathology.
VaD = vascular dementia; DLB = dementia with Lewy bodies; AD/LB = AD with Lewy body disease insufficient for diagnosis of either PD or DLB; PSP = progressive supranuclear palsy; FTLD-TDP = frontotemporal lobar degeneration with TDP-43 proteinopathy.
Categories with mixed diagnoses are not mutually exclusive. Note that “pure” AD does not exclude comorbid minor neuropathological diagnoses.
Table 3.
Decadal changes in aging and disease, Banner Sun Health Research Institute Brain and Body Donation Program/Arizona Study of Aging and Neurodegenerative Disorders.
| Decade (n) | Mean Control Brain Weight F/M | Mean Control MMSE (SD)a | Mean Control UPDRS III (SD)b | Mean Control CERAD NP Density (SD) | Mean Control Braak NFT Stage (SD) | % with Infarcts/Mean Total Infarct Volume, cc (SD)c | Mean Number of Neuropathological Diagnoses |
|---|---|---|---|---|---|---|---|
| 50s & 60s (112) | 1235d/1326 | NA | NA | 0.2 | 1.3 | 19/0.5 (2.4) | 2.4 |
| 70s (311) | 1141/1282 | 28.5 (1.3) | 3.9 (2.5) | 0.9 (1.1) | 2.3 (1.2) | 39/7.8 (41) | 2.8 (1.3) |
| 80s (595) | 1130/1257 | 28.0 (2.0) | 7.8 (7.2) | 1.3 (1.2) | 3.0 (1.0) | 49/10.7 (62) | 3.4 (1.4) |
| 90s (259) | 1110/1216 | 27.6 (1.9) | 11.2 (6.6) | 1.7 (1.2) | 3.6 (0.7) | 57/12.9 (55) | 3.9 (1.6) |
| 100s | 1069/NA | 25.5 (4.4) | 13.5 (10.4) | 1.4 (1.5) | 3.75 (0.5) | 92/5.5 (12) | 4.6 (1.3) |
MMSE score available for 24 (70s), 110 (80s), 69 (90s) and 4 (100s)
UPDRS score available for 17 (70s), 101 (80s), 71 (90s) and 4(100s).
Any number of acute, subacute or old infarcts were each counted as one diagnosis.
Based on n = 3 or less
CERAD=Consortium to Establish a Registry for Alzheimer’s Disease; F/M=Female/Male; MMSE=Minimental Status Exam; NA=not available; NFT=Neurofibrillary Tangle; NP=Neuritic Plaque; SD=Standard Deviation; UPDRS III=Unified Parkinson’s Disease Rating Scale III
IV. Clinical Need
5.4 million Americans and 44 million people worldwide have AD, and with the aging population incidence and prevalence figures are expected to double by 2050.33 Compared to Caucasians, the relative risk of dementia is twice that in African Americans and 1.5 times that in Hispanic Americans34 mainly related to contributory factors such as cardiovascular disease and diabetes,35 as well as a higher prevalence of apolipoprotein E (APOE) ε4 in African Americans.36 Incidence rates for the age brackets 65–74, 75–84, and 85 and older are 2, 13, and 37 per 1000 respectively with a lifetime risk for those aged 65 years and older of 9% for men and 17% for women.33
To place recent challenges and developments in context, consider that the January 23, 2014 issue of the New England Journal of Medicine contained two back to back articles37,38 that disappointed a field that anticipated nothing less than a potential cure for AD based on a wealth of evidence that immunotherapy against Aβ-amyloid should halt disease progression, yet failed. For the past 25 years, the amyloid hypothesis, which simply stated posits the accumulation of Aβ amyloid in the brain as the inciting event that triggers neurodegeneration causing AD, has been the prevailing paradigm for AD pathogenesis, and has therefore guided the development of disease modifying treatments.39 Evidence supporting the amyloid hypothesis is strong. Amyloid accumulation begins early in the disease process, and dominantly inherited AD (DIAD) can be caused by highly pathogenic variants in any of three genes, all of which impact cerebral amyloid production or aggregation including the amyloid precursor protein (APP) gene,40 presenilin 1 (PSEN1)41 and presenilin 2 (PSEN2).42 The presenilins encode the active site of gamma secretase,43 a key enzyme that leads to the production of Aβ amyloid fragments that are prone to aggregation and plaque formation. Further, while most APP variants are pathogenic, one has been described that actually protects against AD by reducing beta secretase (BACE 1) cleavage and so Aβ production.44
V. Scientific Background
The normal roles of APP and amyloid in the brain are far from well understood but shed further light onto their potential role in AD pathogenesis. The APP gene is ancient. The ancestral gene is present in invertebrates, and the amyloidogenic sequence that predisposes to AD is found in all vertebrates.45 Knockouts of the APP gene and its homologues in mice are lethal.46 In a zebrafish model, knockdown of APP results in a deformed fish that is restored by wild type human APP but not the AD-related Swedish mutation APP.47
In post-natal humans, neurogenesis involves roughly a third of hippocampal neurons (including the majority of neurons in the dentate gyrus) at a rate of 700 new neurons in each hippocampus per day, corresponding to an annual turnover of 1.75% within the renewing fraction (.004% of dentate gyrus neurons daily). By contrast 51% of non-neuronal cells turn over annually at a rate of 3.5% per year. There is a decline with aging,48 and the degree of ongoing neurogenesis in the adult human brain is still debated. (A recent estimate indicates that the rates of adult neurogenesis in the subventricular and subgranular zones approach that of the surrounding parenchyma and reflect microglia rather than neurons49).
In the non-amyloidogenic (normal aging) pathway, APP is cleaved mostly within the plasma membrane by alpha-secretase within the Aβ region (destroying the Aβ sequence) releasing a soluble fragment, sAPPalpha (or Aβ1-40) to the extracellular space. Alpha secretase includes members of the ADAM (a disintegrin and metalloproteinase) family, ADAM10 and ADAM 17. In the brain sAPPalpha levels are particularly high in the subventricular zone, one of two areas of neurogenesis,and sAPPalpha is an essential proliferation factor for neural and non-neural adult stem cells.50
In the amyloidogenic pathway that leads to AD, APP is first cleaved instead by BACE1 releasing soluble sAPPbeta to the extracellular space. sAPPbeta drives stem cells toward neural differentiation.51 sAPPalpha and sAPPbeta are normally produced in a 9:1 ratio, and together stimulate neural stem cell proliferation and differentiation.52 The remaining membrane bound C terminal APP stub (C99) is subsequently cleaved by gamma secretase releasing the insoluble Aβ1-42 peptide (and the APP intracellular domain; figure 2). Synaptic activity results in the release of Aβ1-42 peptide into the extracellular space driving aggregation.53 Insoluble Aβ1-42 aggregates into plaques and is thought to trigger tau hyperphosphorylation although currently it is unknown how that occurs. The result, however, leads to the loss of cytoskeletal structure, dendritic spines, axonal degeneration, synaptic connectivity, and neuronal death.
Figure 2.
Metabolic pathways of amyloid precursor protein illustrating the nonamyloidogenic cleavage by alpha secretase and the amyloidogenic cleavage by beta secretase (BACE1) that results in the insoluble Aβ fragment. CTF=carboxyl terminal fragment; sAPP=soluble amyloid precursor protein.
More recently it has been shown that Aβ amyloid may have antimicrobial properties. In animal models, Aβ oligomers bind to microbial cell walls, inhibit their adhesion to host cells, and mediate agglutination and eventual entrapment of microbes. Aβ deposition is accelerated and colocalized with invading bacteria suggesting that Aβ deposition in AD may reflect dysregulation of the brain’s innate immune system responding to microbial or sterile inflammatory stimuli.54 TREM2 is a microglial surface receptor which has also been linked to AD susceptibility. TREM2 deficiency enhances Aβ accumulation as dysfunctional microglia fail to cluster around Aβ plaques and become apoptotic.55 Together, these findings raise the possibility of a role for innate immune dysregulation in AD pathogenesis.
Tau is a microtubule stabilizer that is dynamically phosphorylated and dephosphorylated to allow it to dissociate from microtubules during cellular mitosis. In 2009, Clavaguera et al showed, in a transgenic mouse model, that hyperphosphorylated filamentous tau can spread from neuron to neuron in prion–like fashion, taken up by neighboring neurons by bulk endocytosis.56 They subsequently showed that human tau from the brains of patients with progressive supranuclear palsy and related tauopathies, when injected into the brains of transgenic mice, also spread in a prion like fashion.57 It has since been shown that amyloid too may spread in a prion-like fashion in human brains,58 but there is currently no evidence of human to human transmission.59
VI. Challenges and Pitfalls
Immunomodulatory strategies for AD were introduced by Schenk and colleagues in 1999 through the demonstration that active immunization of transgenic mice (containing the human APP mutation) with Aβ amyloid prevented the formation of amyloid plaques if immunized when young, and reduced pathological burden if immunized when old (and pathology had already developed).60 This was considered a major discovery and development of human trials proceeded in an expedited fashion. The initial human active immunization trial (AN1792) resulted in the unexpected occurrence of autoimmune meningoencephalitis with associated cerebral edema in 6% of participants bringing the trial to a halt.61 Subsequent neuropathological examinations revealed evidence of patchy plaque removal (but equivocal impact on overall Aβ load62–64) generally supporting the concept of an immunomodulatory approach and leading ultimately to the launch of passive immunization strategies. Bapeneuzumab37 and solanezumab38 are monoclonal antibodies directed against epitopes of Aβ amyloid. Passive immunization strategies with these agents were shown to engage their molecular targets with improvement in surrogate biomarkers, yet neither reduced the rate of cognitive or functional decline in symptomatic cohorts.37,38 A subsequent secondary analysis of those patients in the solanezumab trial with only mild stage AD appeared to show a 34% reduction in the rate of progression over 18 months,65 but recently reported results from a phase 3 trial in mild stage AD failed to achieve a similar outcome. The relative lack of efficacy of this highly strategic approach has caused intense reconsideration of current disease models, trial design, diagnostic and therapeutic approaches.66 Progress in light of these setbacks has led to greater understanding of AD’s extended preclinical phases, the development of imaging and biofluid biomarkers for earlier detection and tracking of disease progression especially before the onset of symptoms, and the complex genomics of AD with the hope of identifying new therapeutic targets.
The conclusion reached by many in reaction to the failed immunotherapy trials was that interventions were “too little too late” but that success might yet be achieved if treatment was started earlier in the disease course. The earliest stages of AD are characterized by amyloid deposition and followed later in the disease course by tau pathology. By the time mild to moderate dementia stages are reached, amyloid levels have essentially plateaued and deficits are driven by the progressive tau pathology.67 Intervention at the dementia stage therefore could well be too late if amyloid is no longer driving the disease process.68 Tau pathology is primarily intracellular and so would seem to be a less viable immunotherapy target although passive immunization strategies in mouse models have shown encouraging effects in limiting AD spread.69 While cerebral amyloid levels build, patients remain largely asymptomatic. Designing a clinical trial for an asymptomatic cohort requires some understanding of the preclinical phase, and surrogate disease biomarkers that can be tracked so as to assess potential therapeutic efficacy.
VII. Possible Solutions
Evidence for a preclinical phase of AD, a time when AD pathology is building yet no symptoms are evident, originally came from autopsy studies of elderly patients who appeared clinically normal yet harbored moderate degrees of AD pathology in their brains.70,71 With the discovery that the APOEε4 allele is a prevalent and powerful genetic risk factor for AD,72,73 such preclinical pathology was found to correlate with APOEε4.74,75 Among APOEε4 carriers dying from unrelated causes in their 50’s, roughly 40% harbor AD relevant pathology in the form of either amyloid plaques or medial temporal NFT’s,74 and autopsy studies of elderly nondemented APOEε4 carriers has shown that they harbor higher amyloid levels in cerebral parenchyma and vasculature than e4 noncarriers.75
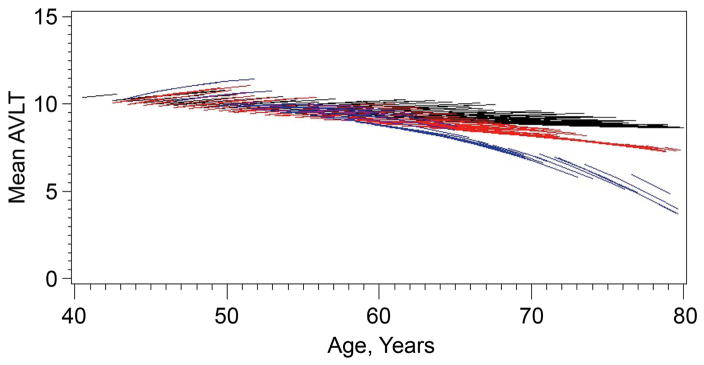
Fluorodeoxyglucose (FDG)-PET studies of asymptomatic APOE 3ε4 carriers in their 50’s reveal metabolic patterns resembling AD.76,77 Volumetric MRI studies show accelerated hippocampal atrophy in advance of MCI diagnosis,78,79 and longitudinal neuropsychological studies show accelerated memory decline beginning in the mid to late 50’s and early 60’s, an estimated 10 to 15 years in advance of MCI symptoms80,81 (figure 3). Evidence from the Nun study suggested that preclinical changes may exist even during young adulthood,82 supported by imaging83 and some neuropathological evidence as well.84 Possibly such early differences are developmental rather than early stage AD, a possibility indirectly supported by subtle neuroanatomical differences found in APOE e4 positive infants.85
Figure 3.
Porcupine plots of longitudinal memory performance on the Auditory Verbal Memory Test (AVLT) Long Term Memory Score in apolipoprotein E (APOE) ε4 homozygotes (blue), heterozygotes (red), and noncarriers (black) demonstrating ε4 gene dose-related preclinical decline in memory performance in 34 carriers.
With the advent of amyloid ligands suitable for PET, it has become possible to noninvasively show that cerebral amyloid increases with age and is accelerated in a gene-dose fashion by APOEε4.86,87 Such studies are now being incorporated into trial design to address another possible reason for prior therapeutic failures: up to a third of APOEε4 noncarriers with clinically diagnosed AD lack significant amounts of cerebral amyloid, thus implying they lack the target for an immunotherapeutic agent.88 Recently published results of a phase one trial of aducanumab, a human monocloncal antibody that selectively targets aggregated Aβ in which all patients enrolled were required to have demonstrated cerebral amyloid on PET scans showed the wisdom of this approach by achieving robust dose and time dependent amyloid clearing, and even a hint of possible cognitive benefit.89
These preclinical data were synthesized into operational research criteria for preclinical AD in 2011 that defined the stages of asymptomatic cerebral amyloidosis followed by neurodegeneration and finally preclinical cognitive decline.90 Further refinement of the preclinical AD concept came from Jack et al who employed a combination of amyloid and neurodegeneration biomarkers, specifically amyloid PET and volumetric MRI respectively to describe 4 possible stages for preclinical AD: stage 0 (amyloid negative, neurodegeneration negative), stage 1 (amyloid positive, neurodegeneration negative), stage 2 (amyloid positive, neurodegeneration positive), and suspected non-Alzheimer pathology (SNAP) (amyloid negative, neurodegeneration positive)91 the pathological basis of which is likely heterogeneous and largely comprised of age-associated tauopathy, cerebrovascular and Lewy body pathology.92
The Dominantly Inherited Alzheimer’s Disease Network (DIAN) is a multicenter study pooling members from kindreds with differing genetic backgrounds that share a disease causing autosomal dominant mutation. Individuals carrying one of the known DIAD mutations offer the advantage that the age window of expected symptomatic onset (within a kindred) is known, allowing investigators to time presymptomatic biomarker and cognitive change. DIAN has shown that biomarker changes follow a sequence with the earliest change being declining CSF Aβ amyloid levels occurring as much as 25 years in advance of predicted symptomatic onset, followed by increasing CSF tau, accelerated cortical atrophy on MRI, declining cerebral metabolic glucose rates (CMRglucose) on FDG-PET, falling memory scores, and finally falling mental status test scores 5 years before symptoms begin.93 Analogous results were demonstrated in a large Colombian kindred harboring a PSEN1 mutation.94 Both cohorts have become the focus of secondary prevention trials employing agents that target amyloid.
Biomarkers
Biomarkers reflect disease-specific pathology that may appear prior to the onset of clinically evident symptoms. Imaging and cerebrospinal fluid (CSF) biomarkers in particular have made possible the exposition of disease-specific pathology in real time, and while not currently recommended for most clinical purposes, have revolutionized preclinical diagnosis, disease tracking, and clinical trial design (figure 4).
Figure 4.
A 58 year old woman with a 3 year history of symptoms of visual dysfunction diagnosed as the visual variant of Alzheimer’s disease (posterior cortical atrophy). A. Pittsburgh Imaging Compound B (PIB) amyloid positron emission tomography (PET) scan showing diffuse increased tracer retention throughout cortex. B. AV1451 Tau PET scan showing marked tracer retention in the posterior cortical regions. C. Statistical Surface Reconstruction of fluorodeoxyglucose (FDG) PET showing asymmetric posterior parietal hypometabolism.
MRI. Medial temporal NFT pathology underlies the amnestic syndrome that characterizes AD.95 Hippocampal volume declines early in patients with AD and progresses in parallel with dementia severity, an observation that led Jack and colleagues to pioneer hippocampal volumetry as perhaps the first readily accessible AD biomarker.96 Since its initial descriptions it has been included in the multicenter Alzheimer’s Disease Neuroimaging Initiative (ADNI),97 and commercially available adaptations have been derived that are now available for clinical application.98 More recent recommendations from Knopman, Jack, and colleagues are that cortical/gray matter thickness may be a more consistent, age-independent AD biomarker in contrast to hippocampal volumes that must account for age-specific norms and head size.99 Many other MRI-based techniques are used including ventricular volume, white matter tract integrity (diffusion tensor imaging), resting state functional MRI (fMRI) as well as activation paradigm-based fMRI among others that all share the general principle of progressive decline in cerebral anatomy and functional integrity reflecting AD progression.
Molecular Imaging. A little over a decade ago Klunk et al described the first human trial of an amyloid ligand adapted for PET termed Pittsburgh Compound-B that imaged a key molecular component of AD in living patients, Aβ-amyloid.100 Its short half-life limited its general accessibility and spurred the development of amyloid ligands with longer half-lives that make them more accessible to medical centers lacking the ability to generate radiopharmaceuticals. All have been validated for the detection of moderate or frequent neuritic plaques by large antemortem-postmortem correlation studies.101–106 Clinical interpretations of amyloid scans are dichotomized into positive or negative depending on the relative cerebral amyloid burden compared with the cerebellum (an area typically spared by AD), but amyloid accumulation is detectable preclinically.86 Correlations between cerebral amyloid levels or its topographical distribution and cognitive deficits have been poor.107 More recently, tau-PET has entered into research applications108 and topographical patterns of ligand distribution correlate well with clinical deficits.109 Taken together, amyloid and tau imaging essentially recapitulate in living patients the major neuropathology of AD.
Metabolic Imaging. FDG-PET typically shows reduced CMRglucose in parietal, lateral temporal, and posterior cingulate cortices in patients with AD. Analyzing FDG-PET images from 146 patients with mild to moderate dementia who were subsequently followed for at least 2 years and 139 patients who had post-mortem neuropathological assessments an average of 3 years later, FDG-PET readings were associated with about 93% sensitivity and 75% specificity in predicting subsequent clinical decline and the neuropathological diagnosis of AD.110 Based on these and other findings, the United States Center for Medicare and Medicaid determined that “FDG-PET is reasonable and necessary in patients with documented cognitive decline and a recently established diagnosis of dementia who meet clinical criteria for both AD and frontotemporal dementia, who have been evaluated for specific alternate neurodegenerative diseases or causative factors, and for whom the cause of the clinical symptoms remains uncertain” (Decision Memo CAG-00088R, September 15, 2004).
CSF. With disease progression, soluble Aβ amyloid aggregates into insoluble amyloid plaques raising brain amyloid levels while reducing soluble CSF levels. As neurons die, total and phosphorylated tau is released into the CSF raising tau levels. CSF total tau, phosphotau, and Aβ reliably distinguish AD patients from controls.111 Serial sampling of CSF within individuals has shown that CSF Aβ levels fluctuate over the day in a sinusoidal fashion. Average maximum values are 200% those of minimum values.112 Differences between diagnostic laboratories have also resulted in large variability in CSF biomarker levels highlighting the need for standardization.113 Plasma levels of Aβ have been less reliable in distinguishing patients and controls possibly due to peripheral production of Aβ. Plasma levels of total tau distinguish the groups better, but significant inter-study variability makes this unreliable presently as a diagnostic test.111
Genomics
Genes can inform disease susceptibility, and in symptomatic patients confirm the specific genetic basis for a familial disease. Freer and colleagues recently showed that genomics can inform tissue vulnerability as well. They reported genomic profiles of brain regions related to Aβ and tau aggregation in a composite vulnerability score and found that brain regions with higher scores (greater vulnerability) closely matched Braak NFT staging.114
Mendelian forms. DIAD can be caused by rare autosomal dominant variants in the APP,40 PSEN141 and PSEN242 genes that together account for roughly 5–10% of young-onset AD. Of the 39 APP mutations reported to date in 93 families, all shift APP proteolysis toward Aβ1-42 production resulting in greater amyloid aggregation.115 Patients with Down’s syndrome (trisomy 21) have an extra copy of the APP gene resulting in greater Aβproduction and consequently fibrillar amyloid deposition.116 If a patient with Down's syndrome lives beyond the age of 40 years, there will be neuropathological evidence of Alzheimer’s disease at autopsy. Progressive dementia increases with age and peaks at roughly 40–75% over the age of 60. Analysis of the amyloid plaques has shown that trisomy 21 predisposes to larger plaques, presumably reflecting increased production of Aβ amyloid.117 The presenilins encode the active site of gamma secretase43 which leads to the production of insoluble Aβ amyloid that is prone to aggregation and plaque formation. PSEN1 is the most prevalent of the autosomal dominant mutations and PSEN2 mutations are the least prevalent. In PSEN1 DIAD, mutant gamma secretase results in longer aggregation-prone peptides.118
Apolipoprotein E. The APOEε4 allele located on chromosome 19 accounts for more cases of AD than any other. It is associated with late onset familial and "sporadic" AD affecting both overall disease risk as well as age of onset with a generally deleterious gene-dose effect of ε4 and protective effect of ε2.72,73 Prevalence varies worldwide from roughly 5–10% in Mediterranean and Asian regions to nearly 30% in parts of Northern Europe (and in North America is approximately 23%).119,120 Overall, AD risk is increased three to four fold in APOEε4 heterozygotes and 14 fold in e4 homozygotes with some variance depending on age, sex, and race.121 Whether there is a single key effect of APOE is unclear but both amyloid (e.g., enhanced aggregation, reduced clearance) and non-amyloid (e.g., neurotoxic carboxyl fragments, enhanced tau phosphorylation) effects have been described.122 APOE does not follow typical Mendelian patterns of disease causation, but has been proposed to represent a low penetrant autosomal semi-dominant form based upon absolute disease risk estimates.123 An additional controversy is the potential contribution of the gene for translocase of the outer mitochondrial membrane (TOMM40) to AD risk. TOMM40 is adjacent to APOE on chromosome 17 and has been proposed by Roses to contribute to AD risk, and possibly may account for some of the risk attributed to APOE itself.124
Non-Mendelian forms. With the advent of large scale genome wide association studies (GWAS), the number of genetic associations with AD risk has accelerated. Unlike Mendelian inheritance, or even APOE, the impact of each such variant is low. If a single copy of the APOE ε4 allele increases AD risk by 300–400%, the impact of the “GWAS” variants averages 10–25%, though is higher for some.116 Population surveys show that these variants occur in clinically unaffected individuals as well as those with AD. Some affected genes lend themselves to pathway categorization including cholesterol metabolism (e.g., APOE, ABCA7), intracellular vesicle trafficking (e.g., SORL1, ABCA7), synaptic/membrane function (e.g., PICALM, BIN1, EPHA1), innate immunity (e.g., TREM2, CR1, CD33) and of course Aβ metabolism (APP, PSEN1, PSEN2). It is an attractive concept but fraught with challenges. Not all genes lend themselves to simple functional categorization. A gene’s known major function may be disturbed yet the resulting disease may result from an unrelated and unknown effect. A single variant might be classified as benign due to its prevalence in the normal population, but a single gene can harbor multiple benign variants whose cumulative impact is unknown. Nonetheless, given the heritability of AD, it seems likely that some combination of variants explain disease susceptibility, age of onset, progression rate, and other phenotypic differences.
VIII. Unresolved Clinical Questions: Diagnosis
The overriding principle of diagnosis remains the exclusion of reversible mimics. To that end, a standard clinical evaluation includes a thorough history and physical examination, structural brain imaging, basic laboratory studies, and cognitive assessment which may be an office-based brief mental status exam or detailed neuropsychological assessment to establish the quality and severity of the cognitive syndrome. Spinal fluid examination, electroencephalography, and many other tests are possible, depending on individual circumstances. The main controversies in diagnosis are the utility of genetic and biomarker tests.
Genetic counseling and testing (for APP, PSEN1, and PSEN2) should be offered to any patient or family with suspected DIAD. Typically the age of onset in these cases is under 60 years, and there is usually a strong family history of dementia on one parent’s side. Identification of the causative mutation in the patient offers the chance to test first degree relatives, and particularly children who may alter their life plans depending on their understanding of their own disease risk and expected age of onset. APOE testing is another story. The negative predictive value of APOE ε4 is poor, so its absence does not rule out AD, but the presence of an APOE ε4 allele in a patient with dementia has high positive predictive value for a diagnosis of AD.125 APOE status does not affect the therapeutic alternatives for a patient with dementia or their family members in the way that DIAD genes do. Therefore, while APOE testing is possible, it is not currently recommended for routine clinical purposes.126
Biomarker tests are not generally useful for identifying potentially reversible mimics, but they may enhance diagnostic certainty,106 especially in patients with clinically atypical presentations and there are a few scenarios in which they can be particularly helpful. For example, a young patient still working becomes disabled by memory loss and needs positive proof of AD, not simply tests that “rule out” other causes. Another example is that of a patient being considered for ventriculoperitoneal shunting for suspected normal pressure hydrocephalus. AD is the most frequent reason for therapeutic failure127 so a biomarker test indicating the presence of AD pathology may help avoid unnecessary surgery. Whether or not an older patient with clinically probable AD should also undergo biomarker testing to further enhance diagnostic confidence is less clear. Biomarker tests add substantially to the cost of the evaluation and have only a minor impact on therapeutic decisions.128
More controversial is the utility of presymptomatic testing. Many people express a desire for such testing even in the absence of an effective therapy. The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) study has examined the impact of APOE genotype disclosure on healthy volunteers. REVEAL’s main message has been interpreted to be that disclosure can be done safely,129 but the careful screening and followup employed by the REVEAL study is unlikely to accompany widespread clinical practice and does not now accompany direct to consumer marketing. In the absence of such a best practice, respondents to an internet-based survey generally endorsed positively adaptive reactions (such as leading a healthier lifestyle and obtaining long term care insurance) to “bad news”, but over 18% endorsed spending all their money, and roughly 11% endorsed consideration of suicide. Responses were similar for biomarker testing.130 With the advent of presymptomatic trials as well as the commercial availability of genetic tests, such testing is happening, despite these caveats.
IX. Unresolved Clinical Questions: Therapy
In the absence of disease modifying therapy, the overriding principle of treatment is maximizing quality of life through symptom management. A systematic approach that considers prevention (or mitigation of symptom progression), intellectual impairment, behavioral problems, sleep disorders, commonly associated problems (for example, parkinsonism), abrupt or unexpected clinical decline, and lifestyle issues (particularly driving, weapons, advanced directives, and assisted living/extended care) are all part of good clinical practice. Medications that are specifically identified as AD therapy are limited to the cholinesterase inhibitors131-133 and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine.134 All cholinesterase inhibitors are FDA approved for the treatment of mild to moderate stages of dementia caused by AD, and memantine is FDA approved for the moderate to late stages of AD. An unresolved question is when to stop them, but the decision is typically individualized according to a family’s wishes.
With regard to AD prevention and the mitigation of symptom progression, there is no level 1 evidence for a neuroprotective effect for anything despite television ads claiming such and numerous news stories generally extrapolated from animal model or epidemiological findings. There is, in fact, level 1 evidence against protective effects for nonsteroidal anti-inflammatory drugs,135 estrogen replacement therapy in women.136 vitamin E,137 B vitamin mediated homocysteine lowering,138 ginkgo biloba,139 statins,140 and other agents as well. Epidemiological evidence has supported a modestly protective effect for Mediterranean diet,141 physical exercise,142 and recreational cognitive activity143 against dementia in general although it is less clear to what extent the effect is specific to AD or cerebrovascular contributions to dementia. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was a randomized trial of a multidomain intervention combining diet, exercise, cognitive training, and vascular risk monitoring, and showed a modestly protective effect against cognitive decline in this elderly cohort.144 Similarly, higher educational background, intellectually oriented occupations, and related characteristics have been linked to the phenomenon of cognitive reserve in which greater intellectual attainment seems to mitigate the adverse impact of dementia pathology.145 However, CR proxies have been shown to primarily affect cerebrovascular components of dementia,146 and when a developmental proxy (sex-based memory advantage) is used instead, there does not appear to be any mitigation of preclinical memory decline.147
Of the various behavioral problems experienced by patients with dementia, the most problematic are agitation and psychosis. There are no agents that have received FDA approval for the specific treatment of agitation or psychosis in patients with AD (although very recently pimavanserin was approved for treatment of Parkinson’s disease-related psychosis148). In the absence of level 1 evidence, anecdotal experience and meta-analysis suggest that atypical antipsychotic agents may be the preferred agents for AD related agitation,149 but because they lack FDA approval for such an indication, clinical judgment is essential. Common patterns of usage in a community based setting150 as well as more aggressive regimens aimed at higher minimum doses151 often fail to achieve therapeutic benefit. Further, the FDA has required a black box warning label on the use of atypical antipsychotics in treating patients with dementia because of data suggesting an increased risk of mortality (http://www.fda.gov/cder/drug/advisory/antipsychotics.htm). The "typical" antipsychotic drugs such as haloperidol have a high likelihood of causing or exacerbating parkinsonism, tardive dyskinesia (if used chronically), and have an even higher mortality risk compared to the newer atypical neuroleptics.152
Perhaps the greatest controversy however is one that has not yet materialized, but may be looming: balancing the cost of therapy with efficacy. Immunotherapy trials involving monoclonal antibodies have failed to stop AD progression, but with earlier intervention, agents targeting different epitopes, and other factors, it seems quite possible that one day such an agent will achieve statistical significance in its primary endpoint. If we may infer the potential cost of these agents from their counterparts in the oncological realm, they are going to be expensive, ranging in the tens of thousands of dollars annually, and quite possibly resulting in a national cost that exceeds the current total dementia care cost of more than 200 billion dollars annually. If such an agent fails to halt disease progression but only slows it by10–30%, would that justify the cost to society? Should patients with late stage dementia receive such treatment, or should treatment be limited to those with mild stage disease? Should taxes be raised to provide the needed supplemental dollars to entitlement programs that may not otherwise be funded to shoulder such a cost given the huge number of patients affected by AD? And if such treatment were preventive, should the 2% of the US population that is an APOE ε4 homozygote all receive treatment, and starting at what age? What about ε4 heterozygotes? What about everyone with Down’s syndrome and the less common DIAD gene carriers?
Conclusion
The challenges raised by AD may be unlike any other disease. When it strikes younger people it is undeniably a disease. When we find traces of it in the brains of nondemented nonagenarians we confront its association with normal aging. Our ability to diagnose it is increasing in precision, and our therapies may be getting closer to disease modification, but at a cost. The greatest challenges ahead may lie not in the lab or the clinic, but in our social conscience and government.
Abbreviations
- AD
Alzheimer’s disease
- APP
Amyloid Precursor Protein
- APOE
Apolipoprotein E
- CMR
Cerebral Metabolic Rate
- CSF
Cerebrospinal fluid
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- DIAD
Early Onset Familial Alzheimer’s Disease
- FDG
Fluorodeoxyglucose
- FDA
Food and Drug Administration
- MRI
Magnetic Resonance Imaging
- MCI
Mild Cognitive Impairment
- NFT
Neurofibrillary tangles
- PET
Positron Emission Tomography
- PSEN
Presenilin
Footnotes
Disclosures Dr. Caselli is an investigator in clinical trials sponsored by Merck and Novartis; and receives research support from NIA P30AG19610, NIA R01AG031581, and the Arizona Alzheimer’s Consortium.
Dr. Beach is an investigator in clinical trials sponsored by Avid Radiopharmaceuticals and Navidea Biopharmaceuticals, has served as a consultant for Avid Radiopharmaceuticals and GE Healthcare; and receives or has received research support from the NIH, grants U24NS072026, P30 AG19610, R01 AG044372, R21NS093222 and R21AG044723, the Michael J. Fox Foundation for Parkinson’s Research and the Arizona Alzheimer’s Consortium.
Dr. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH, grants P50 AG16574, U01 AG06786 and, R01 AG41851.
Dr. Graff Radford is an investigator in clinical trials sponsored by Biogen, Lilly, TauRX and Axovant; and he has served as a consultant for Cytox.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer A. Uber eine eigenartige Erkrankung der Himrinde. Allemagne Zeitschrift fur Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;64:148–148. [Google Scholar]
- 2.Katzman R. The prevalence and malignancy of Alzheimer disease. A Major Killer. Arch Neurol. 1976;33(4):217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 3.Terry RD. My own experience in early research on Alzheimer disease. J Alzheimers Dis. 2006;9(3 suppl):117–119. doi: 10.3233/jad-2006-9s313. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganguli M, Dodge HH, Shen C, et al. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63(1):115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 11.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to dementia. Arch Neurol. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 12.National Alzheimer’s Coordinating Committee. The Uniform Data Set, Version 3.0. 2015. [Google Scholar]
- 13.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workshops on diagnostic guidelines for Alzheimer’s disease. Alz Dem. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caselli RJ, Stelmach GE, Caviness JN, et al. A kinematic study of progressive apraxia with and without dementia. Mov Disord. 1999;14(2):276–287. doi: 10.1002/1531-8257(199903)14:2<276::aid-mds1013>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53(4):795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 19.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 20.Mirra SS, Heyman A, McKeel D, et al. The Consortium to establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 23.NIA and Reagan Institute Working Group. Consensus Recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(suppl 4):S1–S2. [PubMed] [Google Scholar]
- 24.Thal DR, Rub U, Orantes M, Braak H. Phases of AB-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 25.Thal DR, Arendt T, Waldmann G, Holzer M, Zedlick D, Rub U, Schober R. Progression of neurofibrillary changes and PHF-tau in end-stage Alzheimer’s disease is different from plaque and cortical microglial pathology. Neurobiol Aging. 1998;19(6):517–525. doi: 10.1016/s0197-4580(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 26.Dugger BN, Davis K, Malek-Ahmadi M, et al. Neuropathological comparisons of amnestic and nonamnestic mild cognitive impairment. [Accessed December 20, 2016];BMC Neurol. 2015 Aug 20;15:146. doi: 10.1186/s12883-015-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ study. Neurology. 2015;85(6):535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng L, Vinters HV, Mack WJ, et al. Differential effects of ischemic vascular disease and Alzheimer’s disease on brain atrophy and cognition. J Cereb Blood Flow Metab. 2016;36(1):204–215. doi: 10.1038/jcbfm.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86(11):1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ringman JM, Monsell S, Ng DW, et al. Neuropathology of autosomal dominant Alzheimer Disease in the National Alzheimer Coordinating Center Database. J Neuropathol Exp Neurol. 2016;75(3):284–290. doi: 10.1093/jnen/nlv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross sectional study. Lancet Neurol. 2016;15(9):934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35(4):354–389. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer’s disease in the United States (2010–2050) estimated using the 2010 Census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 35.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 36.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alz Dem. 2016;12(6):669–677. doi: 10.1016/j.jalz.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. New Engl J Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. New Engl J Med. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 39.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St George-Hyslop PH, Tanzi RE, Polinsky RJ, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235(4791):885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- 41.Schellenberg GD, Bird TD, Wijsman EM, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258(5028):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 42.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 43.DeStrooper B, Saftog B, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 45.Tharp WG, Sarkar IN. Origins of amyloid-B. [Accessed December 30, 2016];BMC Genomics. 2013 Apr 30;14:290. doi: 10.1186/1471-2164-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Kock CS, Zheng H, Chen H, et al. Generation of APLP2 KO mice and early postnatal lethality in APLP2/APP double KO mice. Neurobiol Aging. 1997;18(6):661–669. doi: 10.1016/s0197-4580(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 47.Joshi P, Liang JO, DiMonte K, Sullivan J, Pimplikar SW. Amyloid precursor protein is required for convergent-extension movements during zebrafish development. Dev Biol. 2009;335(1):1–11. doi: 10.1016/j.ydbio.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 48.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: an immunohistochemical study. [Accessed December 30, 2016];Neuropathol Appl Neurobiol. 2016 Dec;42(7):621–638. doi: 10.1111/nan.12337. Epub 2016 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demars MP, Batholomew A, Strakova Z, Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. [Accessed January 3, 2017];Stem Cell Res Ther. 2011 Aug 30;2(4):36. doi: 10.1186/scrt77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. J Biol Chem. 286(27):24264–24274. doi: 10.1074/jbc.M111.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pimplikar SW, Ghosal K. Amyloid precursor protein: more than just neurodegeneration. Stem Cell Res Ther. Oct 14;2(5):39. doi: 10.1186/scrt80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid- β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Kumar DKV, Choi SH, Washicosky KJ, et al. Amyloid-B peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Trans Med. 2016;8(340):1–15. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Cella M, Mallinson K, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clavaguera F, Akatsu H, Fraser G, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA. 2013;110(23):9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaunmuktane Z, Mead S, Ellis M, et al. Evidence for human transmission of amyloid-B pathology and cerebral amyloid angiopathy. Nature. 2015;525(7568):247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- 59.Edgren G, Hjalgrim H, Rostgaard K, et al. Transmission of neurodegenerative disorders through blood transfusion: a cohort study. Ann Intern Med. 2016;165(5):316–24. doi: 10.7326/M15-2421. [DOI] [PubMed] [Google Scholar]
- 60.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 61.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after AB42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 62.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 63.Holmes C, Boche D, Wikinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomized, placebo-controlled phase 1 trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 64.Patton RL, Kalback WM, Esh CL, et al. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer's disease patients: a biochemical analysis. Am J Pathol. 2006;169(3):1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siemers ER, Sundell KL, Carlson C, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alz Dem. 2016;12(2):110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 66.Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014;76(2):185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyman BT, Marzloff K, Arriagada PV. The lack of accumulation of senile plaques or amyloid burden in Alzheimer’s disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993;52(6):594–600. doi: 10.1097/00005072-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68(8):1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 69.Sankaranarayanan S, Barten DM, Vana L, et al. Passive immunization with phosphor-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One. 2015 May 1;10(5):e0125614. doi: 10.1371/journal.pone.0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dayan AD. Quantitative histological studies on the aged human brain. I. Senile plaques and neurofibrillary tangles in “normal” patients. Acta Neuropathol. 1970;16(2):85–94. doi: 10.1007/BF00687663. [DOI] [PubMed] [Google Scholar]
- 71.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 72.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 73.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 74.Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65(6):650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 75.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett. 2010;473(3):168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. New Engl J Med. 1996;334(12):752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 77.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–9. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiman EM, Uecker A, Caselli RJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44(2):288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 79.Den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59(5):746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- 80.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. New Engl J Med. 2009;361(3):255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caselli RJ, Locke DE, Dueck AC, et al. The neuropsychology of normal aging and preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(1):84–92. doi: 10.1016/j.jalz.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275(7):528–532. [PubMed] [Google Scholar]
- 83.Reiman EM, Chen KW, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer's susceptibility gene. J Alzheimers Dis. 2010;22(1):307–13. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dean DC, 3rd, Jerskey BA, Chen K, et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross sectional imaging study. JAMA Neurol. 2014;71(1):11–22. doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fleisher AS, Chen K, Liu X, et al. Apolipoprotein E e4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34(1):1–2. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Monsell SE, Kukull WA, Roher A, et al. Characterizing apolipoprotein E e4 carriers and noncarriers with the clinical diagnosis of mild to moderate Alzheimer dementia and minimal B-amyloid plaques. JAMA Neurol. 2015;72(10):1124–31. doi: 10.1001/jamaneurol.2015.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces AB plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–58. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 90.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alz Dem. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;12(5):571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 96.Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42(1):183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 97.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu P, Sun J, Wolz R, et al. Operationalizing hippocampal volume as an enrichment biomarker for amnestic mild cognitive impairment trials: effect of algorithm, test-retest variability, and cut point on trial cost, duration, and sample size. Neurobiol Aging. 2014;35(4):808–818. doi: 10.1016/j.neurobiolaging.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Age and neurodegeneration imaging biomarkers in persons with Alzheimer disease dementia. Neurology. 2016;87:691–698. doi: 10.1212/WNL.0000000000002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound–B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 101.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir F18) J Nucl Med. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 103.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 104.Curtis C, Gamez JE, Singh U, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72:287–294. doi: 10.1001/jamaneurol.2014.4144. [DOI] [PubMed] [Google Scholar]
- 105.Sabri O, Sabbagh MN, Seibyl J, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer's disease: phase 3 study. Alzheimers Dement. 2015;11:964–974. doi: 10.1016/j.jalz.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Beach TG, Schneider JA, Sue LI, et al. Theoretical impact of Florbetapir (18F) amyloid imaging on diagnosis of alzheimer dementia and detection of preclinical cortical amyloid. J Neuropathol Exp Neurol. 2014;73:948–953. doi: 10.1097/NEN.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(Pt5):1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG-PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- 111.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurology. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 112.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68(9):666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 113.Mattson N, Andreasson U, Persson S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alz Dem. 2011;7(4):386–395. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Freer R, Sormanni P, Vecchi G, Ciryam P, Dobson CM, Vendruscolo M. A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. [Accessed August 23, 2016];Sci Adv. 2016 Aug 10;2(8):e1600947. doi: 10.1126/sciadv.1600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 116.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Head E, Lott IT. Down syndrome and beta-amyloid deposition. Curr Opin Neurol. 2004;17(2):95–100. doi: 10.1097/00019052-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 118.Szaruga M, Veugelen S, Benurwar M, et al. Qualitative changes in human gamm-secretase underlie familial Alzheimer’s disease. J Exp Med. 2015;212(12):2003–2013. doi: 10.1084/jem.20150892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gerdes LU, Klausen IC, Sihm I, et al. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genet Epidemiol. 1992;9(3):155–167. doi: 10.1002/gepi.1370090302. [DOI] [PubMed] [Google Scholar]
- 120.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a 'thrifty' allele? Ann Hum Genet. 1999;63(Pt. 4):301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 121.Farrer LA, Cupples A, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 122.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014;72(Pt. A):3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16(9):903–907. doi: 10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roses AD, Lutz MW, Amrine-Madsen H, et al. A TOMM40 variable length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics Journal. 2009 Oct;10(5):375–84. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nalbantoglu J, Gilfix BM, Bertrand P, et al. Predictive value of apolipoprotein E genotyping in Alzheimer's disease: results of an autopsy series and an analysis of several combined studies. Ann Neurol. 1994;36(6):889–895. doi: 10.1002/ana.410360614. [DOI] [PubMed] [Google Scholar]
- 126.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcomittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 127.Pomeraniec IJ, Bond AE, Lopes MB, Jane JA., Sr Concurrent Alzheimer’s pathology in patients with clinical normal pressure hydrocephalus: correlation with high-volume lumbar puncture results, cortical brain biopsies, and outcomes. J Neurosurg. 2016;124(2):382–388. doi: 10.3171/2015.2.JNS142318. [DOI] [PubMed] [Google Scholar]
- 128.Caselli RJ, Woodruff BK. The clinical impact of amyloid PET: is it worth the cost? JAMA Neurol. 2016;73(12):1396–1398. doi: 10.1001/jamaneurol.2016.3792. [DOI] [PubMed] [Google Scholar]
- 129.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. New Engl J Med. 2009;361(3):245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caselli RJ, Langbaum J, Marchant GE, et al. Public perceptions of presymptomatic testing for Alzheimer disease. Mayo Clin Proc. 2014;89(10):1389–96. doi: 10.1016/j.mayocp.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50(1):136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 132.Rosler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ. 1999;318(7184):633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raskind MA, Peskind ER, Wessel T, et al. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 134.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. New Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 135.Aisen PS, Schaefer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 136.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 137.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. New Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 138.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 141.Scarmeas N, Stern Y, Tang MX, et al. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67(1):80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sajeev G, Weuve J, Jackson JW, et al. Late-life cognitive activity and dementia: a systematic review and bias analysis. Epidemiology. 2016;27(5):732–742. doi: 10.1097/EDE.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;383(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 145.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53(9):1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 146.Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links to neuropathology and cognition in later life. Acta Neuropathol. 2014;127(1):137–150. doi: 10.1007/s00401-013-1226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Caselli RJ, Dueck AC, Locke DE, Baxter LC, Woodruff BK, Geda YE. Sex-based memory advantages and cognitive aging: a challenge to the cognitive reserve construct? J Int Neuropsychol Soc. 2015;21(2):95–104. doi: 10.1017/S1355617715000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 149.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 150.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 151.Kurlan R, Cummings J, Raman R, Thal L for the ADCS. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356–1363. doi: 10.1212/01.wnl.0000260060.60870.89. [DOI] [PubMed] [Google Scholar]
- 152.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. New Engl J Med. 2005;353(22):2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]