Abstract
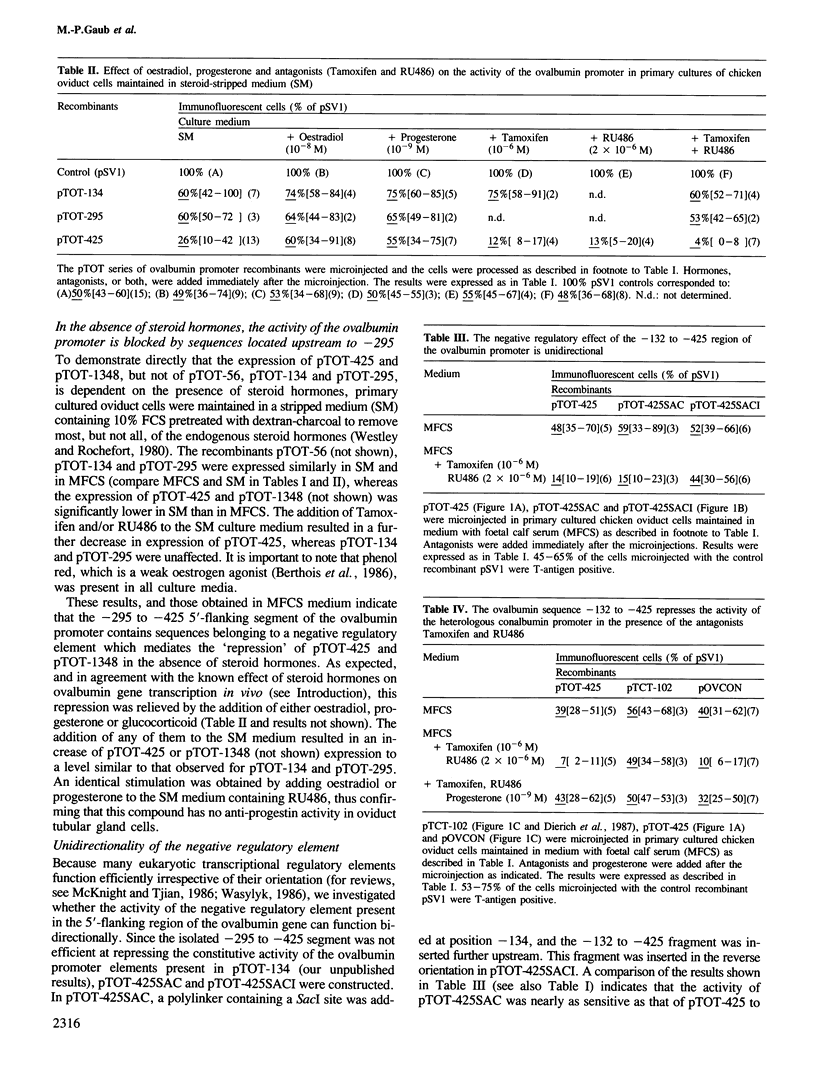
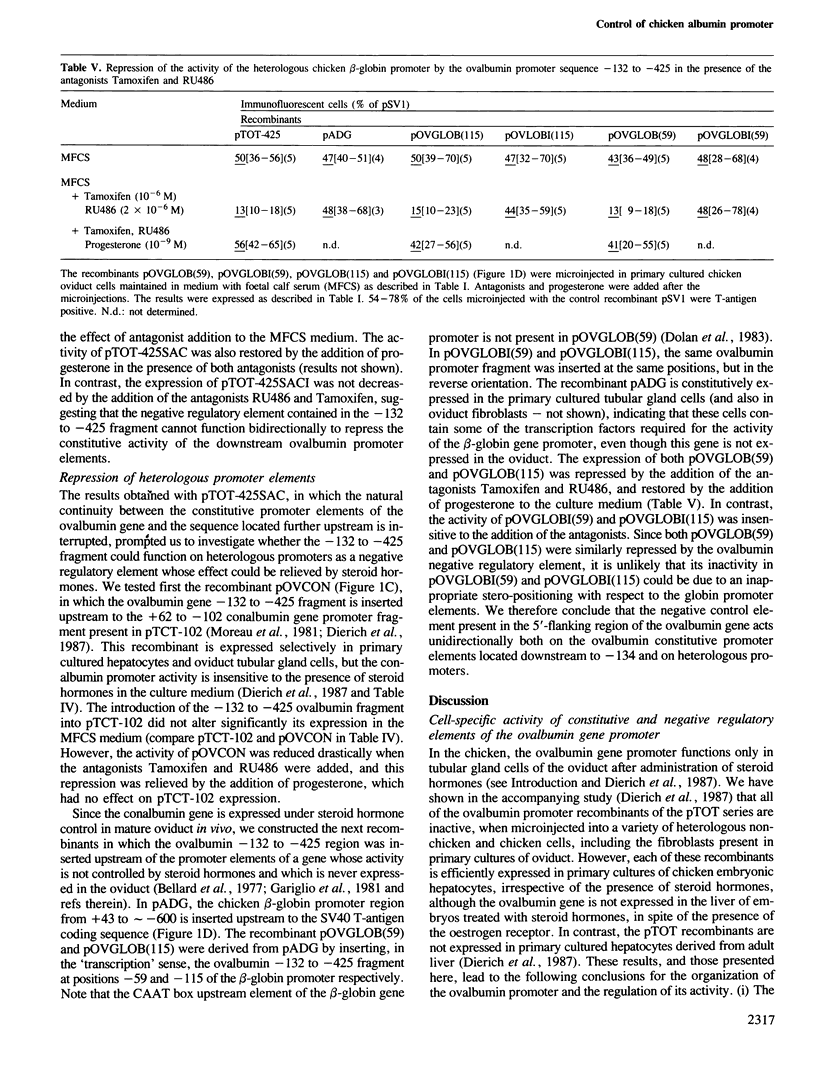
Steroid hormone regulation of activity of the chicken ovalbumin promoter was studied by microinjection of chimeric genes into the nuclei of primary cultured oviduct tubular gland cells. The chimeric genes contained increasing lengths of ovalbumin gene 5'-flanking sequences fused to the sequence coding for the SV40 T-antigen. Promoter activity was estimated by monitoring synthesis of T-antigen. The activity of the ovalbumin promoter is cell-specifically repressed in these oviduct cells and the repression is relieved upon addition of steroid hormones. The -132 to -425 region of the ovalbumin promoter which is responsible for this negative regulation behaves as an independent functional unit containing the regulatory elements necessary for both repression (in the presence of steroid hormone antagonists) and induced derepression (in the presence of steroid hormones) of linked heterologous promoters.
Full text
PDF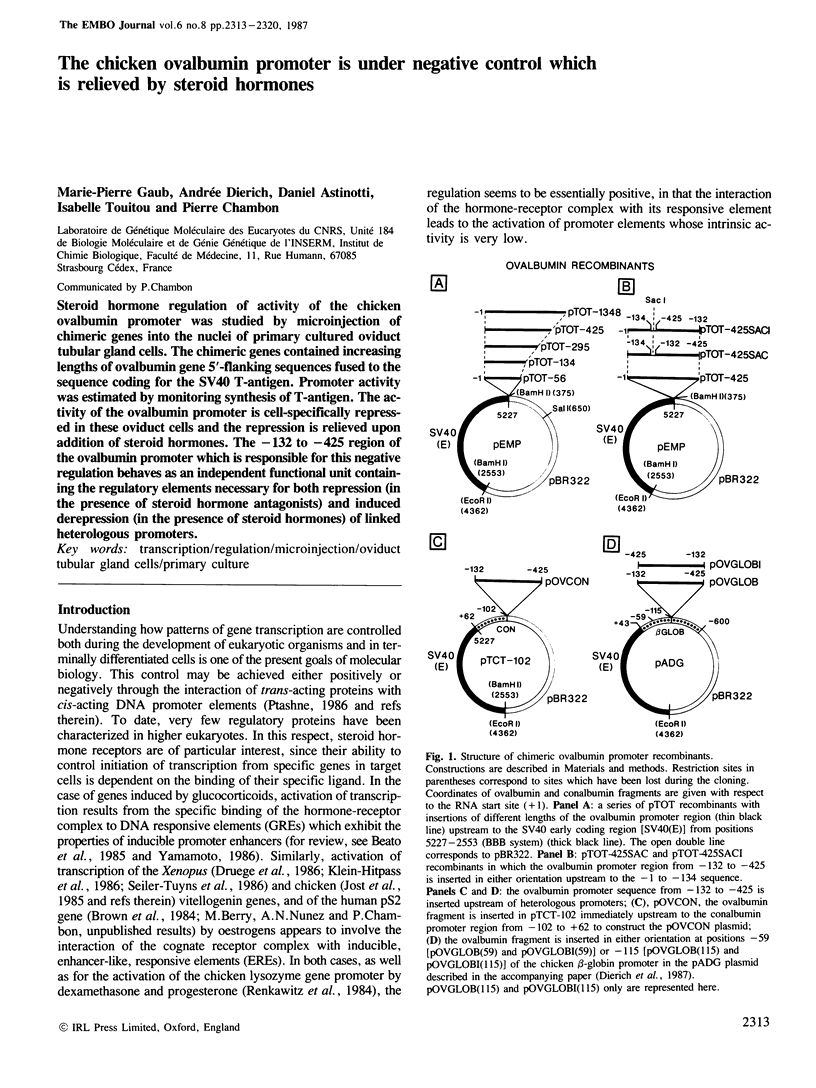



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss J. M., Strominger J. L. Regulation of a transfected human class II major histocompatibility complex gene in human fibroblasts. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9139–9143. doi: 10.1073/pnas.83.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mantei N., Chambon P. Corrected splicing of a chicken ovalbumin gene transcript in mouse L cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):740–744. doi: 10.1073/pnas.77.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Gope R., Knoll B. J., Riser M. E., O'Malley B. W. A similar 5'-flanking region is required for estrogen and progesterone induction of ovalbumin gene expression. J Biol Chem. 1984 Aug 25;259(16):9967–9970. [PubMed] [Google Scholar]
- Dean D. C., Knoll B. J., Riser M. E., O'Malley B. W. A 5'-flanking sequence essential for progesterone regulation of an ovalbumin fusion gene. Nature. 1983 Oct 6;305(5934):551–554. doi: 10.1038/305551a0. [DOI] [PubMed] [Google Scholar]
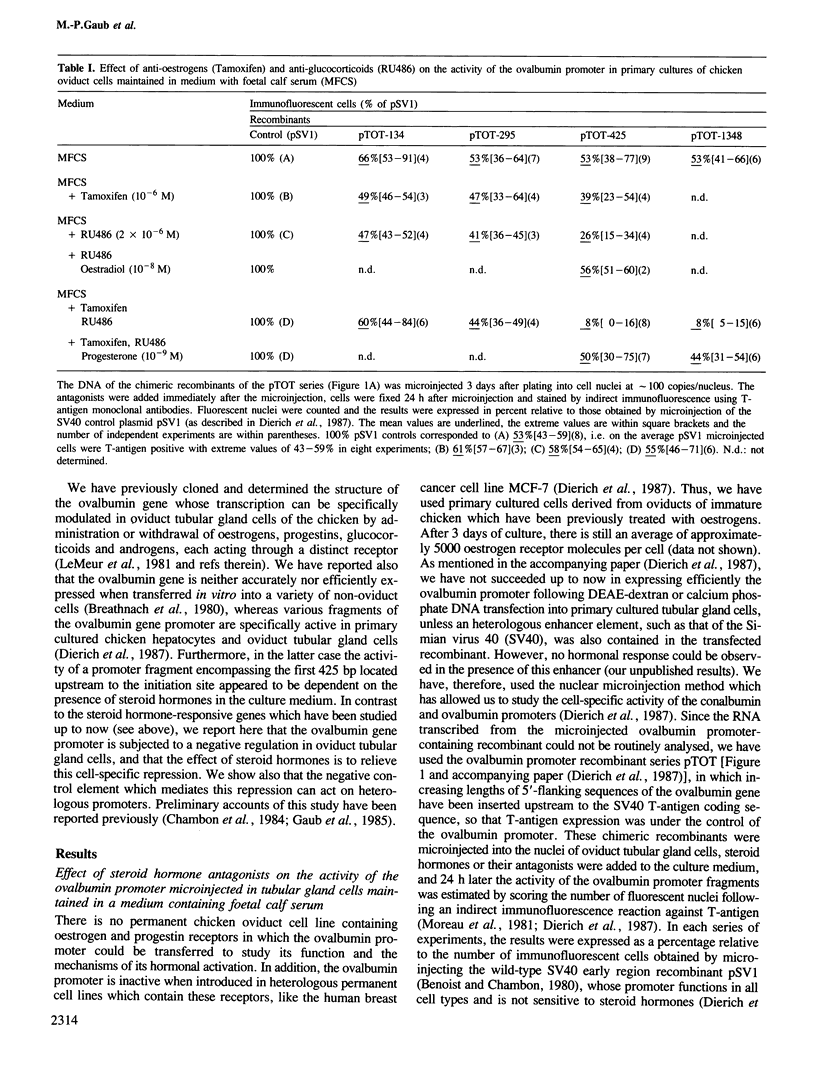
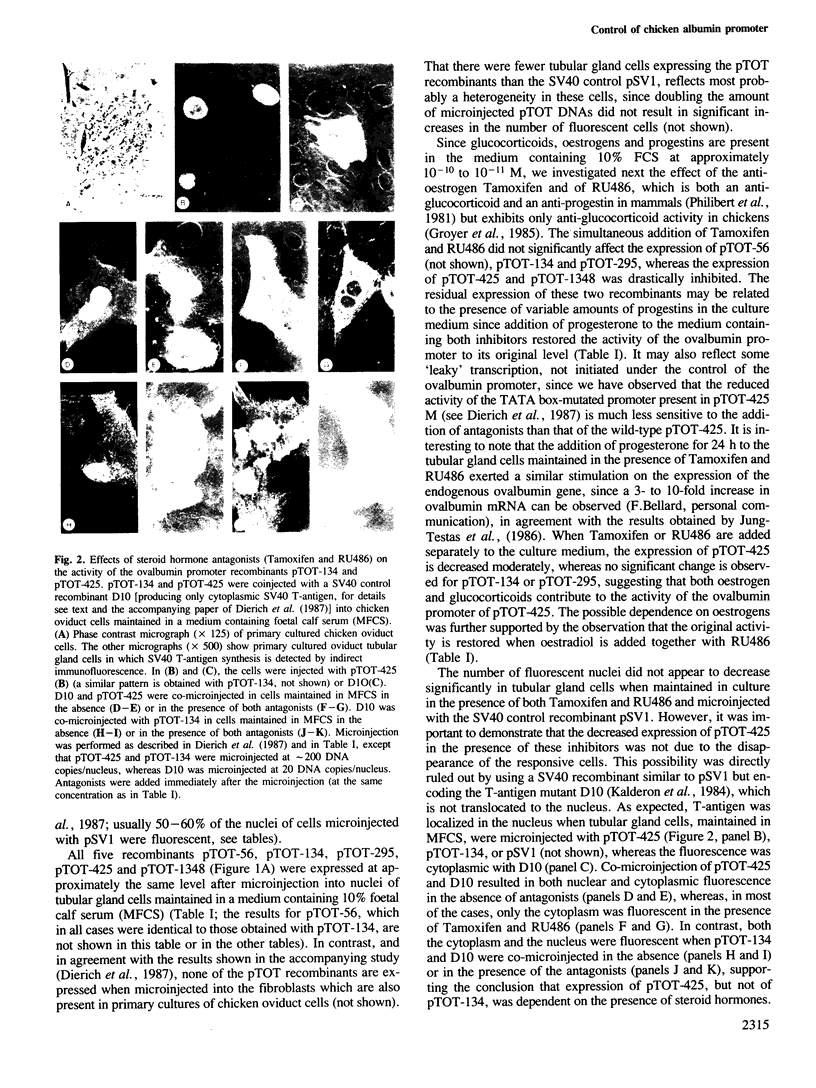
- Dierich A., Gaub M. P., LePennec J. P., Astinotti D., Chambon P. Cell-specificity of the chicken ovalbumin and conalbumin promoters. EMBO J. 1987 Aug;6(8):2305–2312. doi: 10.1002/j.1460-2075.1987.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Druege P. M., Klein-Hitpass L., Green S., Stack G., Chambon P., Ryffel G. U. Introduction of estrogen-responsiveness into mammalian cell lines. Nucleic Acids Res. 1986 Dec 9;14(23):9329–9337. doi: 10.1093/nar/14.23.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Groyer A., Le Bouc Y., Joab I., Radanyi C., Renoir J. M., Robel P., Baulieu E. E. Chick oviduct glucocorticosteroid receptor. Specific binding of the synthetic steroid RU 486 and immunological studies with antibodies to chick oviduct progesterone receptor. Eur J Biochem. 1985 Jun 3;149(2):445–451. doi: 10.1111/j.1432-1033.1985.tb08945.x. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Sassone-Corsi P., Chambon P. An enhancer element is located 340 base pairs upstream from the adenovirus-2 E1A capsite. Nucleic Acids Res. 1983 Dec 20;11(24):8747–8760. doi: 10.1093/nar/11.24.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Kédinger C. Negative regulatory sequences in the EIa-inducible enhancer of the adenovirus-2 early EIIa promoter. Nucleic Acids Res. 1986 Mar 25;14(6):2651–2669. doi: 10.1093/nar/14.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Geiser M., Seldran M. Specific modulation of the transcription of cloned avian vitellogenin II gene by estradiol-receptor complex in vitro. Proc Natl Acad Sci U S A. 1985 Feb;82(4):988–991. doi: 10.1073/pnas.82.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Testas I., Garcia T., Baulieu E. E. Steroid hormones induce cell proliferation and specific protein synthesis in primary chick oviduct cultures. J Steroid Biochem. 1986 Jan;24(1):273–279. doi: 10.1016/0022-4731(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kaye J. S., Pratt-Kaye S., Bellard M., Dretzen G., Bellard F., Chambon P. Steroid hormone dependence of four DNase I-hypersensitive regions located within the 7000-bp 5'-flanking segment of the ovalbumin gene. EMBO J. 1986 Feb;5(2):277–285. doi: 10.1002/j.1460-2075.1986.tb04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Lazier C. B. Ontogeny of the vitellogenic response to oestradiol and of the soluble nuclear oestrogen receptor in embryonic-chick liver. Biochem J. 1978 Jul 15;174(1):143–152. doi: 10.1042/bj1740143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorcic M., Wang H., Elbrecht A., Tsai S. Y., Tsai M. J., O'Malley B. W. Control of transcription initiation in vitro requires binding of a transcription factor to the distal promoter of the ovalbumin gene. Mol Cell Biol. 1986 Aug;6(8):2784–2791. doi: 10.1128/mcb.6.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol. 1986 Dec;6(12):4259–4267. doi: 10.1128/mcb.6.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Walker P., Martinez E., Mérillat A. M., Givel F., Wahli W. Identification of estrogen-responsive DNA sequences by transient expression experiments in a human breast cancer cell line. Nucleic Acids Res. 1986 Nov 25;14(22):8755–8770. doi: 10.1093/nar/14.22.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P. J., Buchanan J. M., Anselmo V. C., Martin P. M. Equilibrium: the intracellular distribution of steroid receptors. Nature. 1979 Dec 6;282(5739):579–582. doi: 10.1038/282579a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Vigneron M., Matthes H., Wildeman A., Zenke M., Chambon P. Requirement of stereospecific alignments for initiation from the simian virus 40 early promoter. Nature. 1986 Jan 9;319(6049):121–126. doi: 10.1038/319121a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Chambon P. Short and long range activation by the SV40 enhancer. Nucleic Acids Res. 1984 Jul 25;12(14):5589–5608. doi: 10.1093/nar/12.14.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. The immunoglobulin heavy-chain B-lymphocyte enhancer efficiently stimulates transcription in non-lymphoid cells. EMBO J. 1986 Mar;5(3):553–560. doi: 10.1002/j.1460-2075.1986.tb04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz A., Coppola L., Bresciani F. Specific binding of estrogen receptor to sites upstream and within the transcribed region of the chicken ovalbumin gene. Biochem Biophys Res Commun. 1986 Sep 14;139(2):396–402. doi: 10.1016/s0006-291x(86)80004-3. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]