Abstract
Recent epidemiological data suggest the relation between hearing difficulty and depression is more evident in younger and middle-aged populations than in older adults. There are also suggestions that the relation may be more evident in specific subgroups; that is, other factors may influence a relationship between hearing and depression in different subgroups. Using cross-sectional data from the UK Biobank on 134,357 community-dwelling people and structural equation modelling, this study examined the potential mediating influence of social isolation and unemployment and the confounding influence of physical illness and cardiovascular conditions on the relation between a latent hearing variable and both a latent depressive episodes variable and a latent depressive symptoms variable. The models were stratified by age (40s, 50s, and 60s) and gender and further controlled for physical illness and professional support in associations involving social isolation and unemployment. The latent hearing variable was primarily defined by reported hearing difficulty in noise. For all subgroups, poor hearing was significantly related to both more depressive episodes and more depressive symptoms. In all models, the direct and generally small association exceeded the indirect associations via physical health and social interaction. Significant (depressive episodes) and near significant (depressive symptoms) higher direct associations were estimated for males in their 40s and 50s than for males in their 60s. There was at each age-group no significant difference in estimated associations across gender. Irrespective of the temporal order of variables, findings suggest that audiological services should facilitate psychosocial counselling.
Keywords: hearing, depression, epidemiology, UK Biobank, social isolation, cardiovascular disease, unemployment, physical health
A large body of research has demonstrated that poor functional hearing is associated with poor mental health when considering conditions such as depression and emotional distress (e.g., Carabellese et al., 1993; Dalton et al., 2003; Gopinath et al., 2012; Kramer, Kapteyn, Kuik, & Deeg, 2002; Saito et al., 2010, Strawbridge, Wallhagen, Shema, & Kaplan, 2000). The mechanism underpinning this association is generally believed to be via social isolation. That is, the hearing problem causes communication difficulties, which make social interaction challenging, resulting in withdrawal from social engagements and hence a feeling of loneliness and depression (Strawbridge et al., 2000, Weinstein & Ventry, 1982). Some recent studies have observed that the association between poor hearing and poor mental health is greater for younger and middle-aged persons than for older populations. For example, in Tambs’s (2004) study, the association between measured hearing loss and self-reported symptoms on mental health, including anxiety, depression, self-esteem, and well-being (satisfaction with life), was investigated separately for men and women aged between 20 and 44 years, between 45 and 64 years, and over 65 years. Overall, the study found that a severe low-frequency hearing loss was more strongly associated with the mental health variables among the young and middle-aged participants than among the elderly. Keidser, Seeto, Rudner, Hygge, and Rönnberg (2015) reported greater associations between functional hearing and two multifactorial variables related to depression among younger participants of a large cohort of 40–70 years old. Nachtegaal et al. (2009) further reported differences in associations between functional hearing and psychosocial health factors in each age-group. While poor hearing was associated with loneliness among the youngest participants (18–29 years) and there was no significant association between hearing and various psychosocial health factors among the oldest participants (60–70 years), poor hearing predicted higher levels of distress, depression, anxiety, and self-efficacy among participants in their 40s.
In an elderly cohort (63–93 years), Pronk et al. (2011) found that the relation between hearing and loneliness varied within specific subgroups. For example, self-reported hearing difficulty related significantly to social loneliness for those with medium/high income, those living with a partner in the household, and those not using hearing aids. Significant adverse relations between hearing difficulty and emotional loneliness were observed for men, those without cardiovascular conditions, those living with a partner in the household, and non-hearing aid users. Consistent with previous studies, no significant association was seen between hearing and depression in this elderly population. The variation in associations across such subgroups would also suggest that different factors may mediate (carry) or confound (distort) an association between hearing difficulty and mental health variables between age and gender groups. To our knowledge, this has not previously been directly investigated.
The onset of hearing loss can occur at any time during a life span but is more common in older adults. Epidemiological studies demonstrate a notable increase in the prevalence of hearing problems from about 55 years of age (Agrawal, Platz, & Niparko, 2008; Cruickshanks et al., 1998; Dawes et al., 2014). Although hearing problems are less prevalent in younger and middle-aged people, communication difficulties could have more severe effects in this population, as they could affect the ability to find and maintain meaningful work (e.g., Hogan, O’Loughlin, Davis, & Kendig, 2009) and to interact effectively with family members and friends (e.g., Hallberg, 1996; Kerr & Cowie, 1997), emphasizing the sense of loneliness and social isolation. Even in a sample of the general population, Hawthorne (2008) found a higher proportion of socially isolated cases among younger (15–30 years) as compared with older (>60 years) participants. Social interactions are also important for the general well-being of older hearing-impaired adults, but this population may be more accepting of their hearing loss by associating it with being a natural part of the aging process (Nachtegaal, Festen, & Kramer, 2012; Tambs, 2004) and hence of the consequences of experiencing communication difficulties. With hearing impairment being more prevalent in the older population, the associated problems are possibly also better recognized and accepted among peers. Therefore, social isolation and related factors such as unemployment could be speculated to be factors that more likely mediate the association between hearing problems and depression among younger and middle-aged than older people.
Keidser et al. (2015) showed that people who reported higher levels of depression were more likely to have sought professional help for their condition. It is uncertain whether those who are being professionally treated for their condition are better or worse equipped to socialize and stay in employment than those who have not sought help. In either case, professional support with depression may confound an association between social isolation/unemployment and depression and ideally should be controlled for.
The etiology of hearing loss generally varies between younger and older populations. While congenital hearing loss is mostly due to genetic problems, acquired hearing loss is mainly the result of an age-related degeneration of the auditory system (presbycusis), a disease of the ear, or noise exposure (Hogan, Phillips, Brumby, Williams, & Mercer-Grant, 2015). Presbycusis may be accelerated by such factors as life style (e.g., smoking) and physical disorders (e.g., cardiovascular diseases and diabetes; Cruickshanks et al., 1998; Fransen et al., 2008; Gates, Cobb, D'Agostino, & Wolf, 1993; Kakarlapudi, Sawyer, & Staecker, 2003). When the hearing loss is related to other chronic disorders, the associated health problems may affect the person’s mental health more than the hearing problems. As comorbidity is more common in old age, physical health problems could be speculated to be a factor that more likely confounds an association between hearing problems and depression in the older population.
A hypothesis that poor hearing is associated with cardiovascular diseases is supported by data from various epidemiological studies (e.g., Helzner et al., 2011; Liew et al., 2007; Torre et al., 2005). The mechanism behind the association is suggested to be via micro- or macrovascular pathology that reduces the blood supply to the cochlea. In brief, poor blood supply to the cochlea disrupts the chemical balance of the inner ear fluid, which affects the electrical activity of the hair cells, resulting in poor hearing sensitivity. The association has been reported both in a middle-aged (40- to 59-year old) population (Rosen & Olin, 1965) and in older adults (>65 years; Rubinstein, Hildesheimer, Zohar, & Chilarovitz, 1977), and thus cardiovascular diseases could be a confounding factor across a wide age-range.
Using data from the UK Biobank and structural equation modelling (SEM, a technique for analyzing theoretical models of how different variables are related to each other), this study explores the potential mediating and confounding influences of such factors as social isolation, unemployment, cardiovascular conditions, and physical illness on the direct association between a latent hearing difficulty variable and latent depressive episodes and latent depressive symptoms variables among people in their 40s, 50s, and 60s. The study further compares the strength of the associations between females and males in each age-group.
Method
Sample
The UK Biobank offers epidemiology data related to health and life style on over 500,000 volunteers between 40 and 70 years of age. People in this age bracket living in the UK were invited to attend 1 of the 22 assessment centers. At these centers, self-reported data were obtained through computerized, self-administered questionnaires and tests, using a touch screen as the response platform. An automated behavioral hearing test was included at a later stage, so only a subset of 134,357 participants aged between 40 and 69 years had provided complete data on hearing and depression. The female-to-male ratio in this sample was 53.5:46.5, which is representative of the sample as a whole and of the 2001 UK census (see Dawes et al., 2014). Table 1 shows the number of female and male participants falling within each of three age-groups; 40–49 years, 50–59 years, and 60–69 years, and the average age of each group.
Table 1.
Descriptive Data by Gender and Age-Group. Mean Values, With the Standard Deviation in Brackets, Are Shown for Continuous and Ordinal Variables.
| Variable | Female, 40s | Female, 50s | Female, 60s | Male, 40s | Male, 50s | Male, 60s |
|---|---|---|---|---|---|---|
| N | 16,438 | 23,689 | 31,773 | 13,440 | 18,811 | 30,206 |
| Age (years) | 44.9 | 54.8 | 64.0 | 44.8 | 54.9 | 64.2 |
| Hearing difficulty in noise (% yes) | 23 | 29 | 35 | 29 | 40 | 50 |
| BESRTn (dB SNR) | −7.9 (1.36) | −7.6 (1.45) | −7.1 (1.68) | −8.0 (1.34) | −7.7 (1.53) | −7.0 (1.87) |
| Depression, longest episode (weeks) | 9.2 (29.46) | 9.3 (31.34) | 8.4 (30.70) | 6.2 (25.54) | 7.4 (32.75) | 5.9 (27.62) |
| Depression, no of episodes | 3.1 (17.99) | 3.0 (20.28) | 2.5 (16.45) | 2.8 (17.85) | 3.0 (20.60) | 2.3 (18.27) |
| Unenthusiastic, longest episode (weeks) | 6.0 (25.70) | 6.2 (27.71) | 5.0 (25.26) | 4.0 (21.78) | 4.6 (25.48) | 3.7 (25.06) |
| Unenthusiastic, no of episodes | 2.6 (22.10) | 2.4 (17.93) | 2.0 (17.46) | 2.1 (16.05) | 2.4 (21.00) | 1.7 (16.66) |
| Number of depressive symptoms | 2.4 (1.84) | 2.2 (1.86) | 1.8 (1.78) | 2.0 (1.81) | 1.8 (1.79) | 1.5 (1.65) |
| Number of anxiety symptoms | 2.6 (1.77) | 2.5 (1.75) | 2.4 (1.70) | 2.1 (1.81) | 2.0 (1.77) | 1.8 (1.69) |
| Feeling down (score) | 1.5 (0.59) | 1.5 (0.57) | 1.3 (0.47) | 1.5 (0.58) | 1.4 (0.54) | 1.3 (0.45) |
| Unhappiness (score) | 2.6 (0.59) | 2.5 (0.56) | 2.4 (0.51) | 2.7 (0.60) | 2.6 (0.60) | 2.4 (0.55) |
| Unemployment (% yes) | 2 | 2 | 0 | 4 | 5 | 2 |
| Social isolation (score) | 4.2 (1.41) | 4.3 (1.44) | 4.7 (1.44) | 3.9 (1.43) | 3.9 (1.49) | 4.2 (1.49) |
| Heart attack (% yes) | 0 | 1 | 2 | 1 | 3 | 6 |
| Angina (% yes) | 0 | 1 | 3 | 1 | 3 | 7 |
| Stroke (% yes) | 0 | 1 | 2 | 1 | 1 | 3 |
| High blood pressure (% yes) | 11 | 20 | 32 | 15 | 28 | 39 |
| Number of physical Illnesses | 0.5 (0.71) | 0.6 (0.73) | 0.6 (0.74) | 0.5 (0.58) | 0.5 (0.70) | 0.5 (0.71) |
| Professional support (% yes) | 44 | 44 | 39 | 27 | 29 | 26 |
Note. BESRTn = best ear speech reception threshold in noise; SNR = signal-to-noise ratio.
Functional Hearing Latent Variable
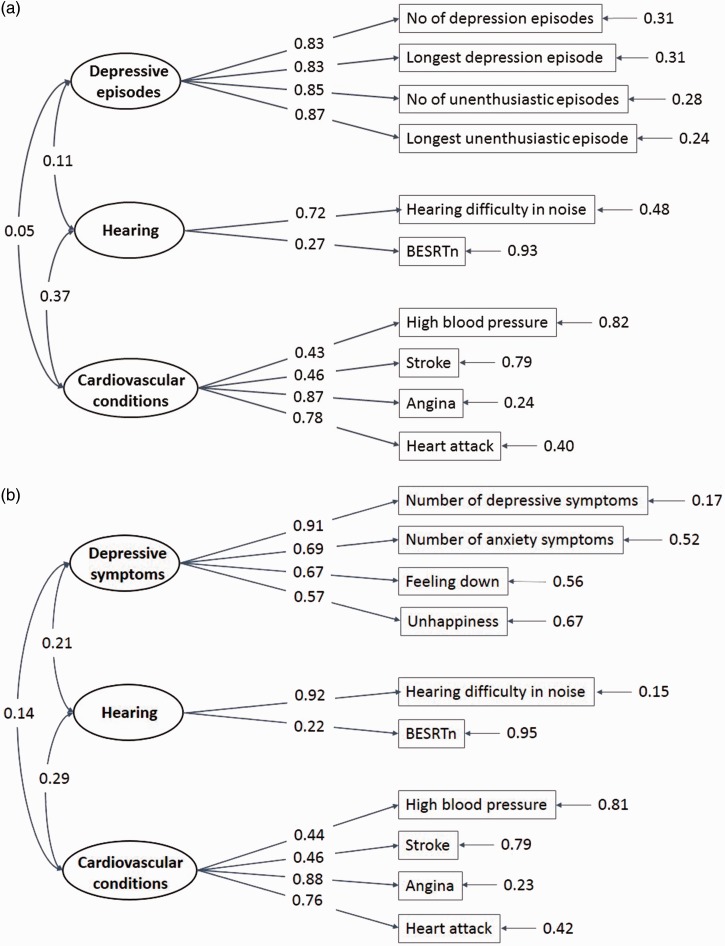
Two measures of functional hearing in noise, one self-reported and one behavioral, were used to create a latent functional hearing variable (hearing). The self-report produced a binary yes/no answer to the question: “Do you find it difficult to follow a conversation if there is background noise (such as TV, radio, children playing)?” The behavioral measure was based on an English version of the digit triplets test (DTT) proposed by Smits, Kapteyn, and Houtgast (2004). During testing, sets of three random digits were presented in speech-shaped noise under headphones to one ear at a time, and participants were asked to enter the three digits heard on a simulated numerical keyboard on the touch screen (forced choice). If the triplet was correctly identified, the noise level was increased; otherwise, the noise level was decreased. The resulting speech reception threshold in noise was the signal-to-noise ratio (SNR) arrived at after 15 presentations. The SNR could vary between −12 and +8 dB. Prior to testing, participants adjusted the volume of the speech to their most comfortable level for each ear. All participants were tested unaided, and the best ear speech reception threshold in noise (BESRTn) was obtained for each participant and used as a continuous variable. For those who completed the test on only one ear, it was assumed that this was the better ear. A third available measure of functional hearing (a binary yes/no answer to the question: “Do you have any difficulty hearing?”) was not included in the latent functional hearing variable. This was partly because this question is less specific and appeared open to interpretation (e.g., not everyone who agreed to having difficulty hearing in noise thought they had any difficulty hearing) and partly because when including this measure, the structural equation models produced a negative error variance estimate. Table 1 shows the distribution and mean values for the hearing measures by gender and age-group. As expected, both measures showed that functional hearing difficulty increased with age.
Depression Latent Variables
Based on a factor analysis reported in Keidser et al.’s (2015) study, two latent variables were created for depression: one on depressive episodes and one on depressive symptoms. These latent variables are not directly related to any psychiatric conceptualization of depression. However, given that people having sought professional help for depression, relative to those who had not, show much higher levels of depressive episodes than depressive symptoms, the latent depressive episodes variable would seem to constitute a more debilitating form of depression than does the latent depressive symptoms variable (see Keidser et al., 2015). The measures associated with the latent depressive episodes variable were reported longest period of feeling depressed (in weeks), number of depression episodes (in numbers), longest period of feeling unenthusiastic/disinterested (in weeks), and number of unenthusiastic/disinterested episodes (in numbers). In Keidser et al.’s (2015) study, these four measures loaded highly on one factor, all with weights above .80. The mean and standard deviation values of the four measures are listed in Table 1 by gender and age-group. Within each age-group, females reported longer periods of feeling depressed and unenthusiastic than males, but a similar frequency of episodes. The lowest mean values were generally reported by older males. As all four variables showed high positive skew, they were log transformed (natural logarithm of [x + 1]) for further analyses.
The measures associated with the latent depressive symptoms variable included number of depressive symptoms experienced (out of six), number of anxiety symptoms experienced (out of six), frequency of feeling down (average rating to four questions on frequency of feeling depressed, unenthusiastic/disinterested, tense/restless, and tired), and satisfaction with life (average rating of happiness with each of job, health, family situation, friendship situation, finances, and generally). The response options for frequency of feeling down were not at all, several days, more than half the days, and nearly every day. Scores from 1 to 4 were assigned to these responses. For the happiness ratings, the response options were extremely happy, very happy, moderately happy, moderately unhappy, very unhappy, and extremely unhappy. Scores from 1 to 6 were assigned to these responses. These four variables (depressive symptoms, anxiety symptoms, frequency of feeling down, and satisfaction with life), which included measures of mood and emotionality, loaded more highly on a second factor with weights of .78, .63, .69, and .56, respectively (see Keidser et al., 2015, for more details). Table 1 shows the mean and standard deviation values of the four measures. These values tend to decrease (i.e., less depressed) with increasing age, with females showing slightly higher values than males on some of the measures.
Mediating and Confounding Variables
Two mediating and two confounding variables were considered in this investigation. The mediating variables were unemployment and social isolation. The binary yes/no measure of unemployment was extracted from a question on employment for which participants could select more than one answer: “Which of the following describes your current situation?” Response options were in paid employment or self-employed, retired, looking after home and family, unable to work because of sickness or disability, unemployed, doing unpaid or voluntary work, and full- or part-time student. Participants who gave contradictive answers, for example, responded in the affirmative to both being in paid employment and being unemployed were removed from this investigation.
Two measures of frequency of visits and engagement in social activities were consolidated to provide the social isolation variable. This is because it was assumed that depending on life style, some participants mainly socialized through interaction with family and friends and others mainly through group activities. This assumption was confirmed by the two variables being only weakly correlated. Participants indicated if they had visits from family or friends: daily, 2–4 times a week, once a week, once a month, every few months, or never or almost never—resulting in a score from 0 (daily) to 5 (never or almost never). Participants further indicated if they were engaged in sports club, pub/social club, religious group, adult education, or other group activity to produce a score from 0 (engaged in all activities) to 5 (engaged in none of the activities). The two scores were added to provide a total social isolation score of between 0 (highly engaged socially) and 10 (socially isolated). Less than 0.01% in the test sample reached the maximum score of 10 for social isolation, with only 0.1% and 0.8% of the total test sample producing scores of 9 and 8, respectively.
The two confounding variables included cardiovascular conditions and physical illness. Four measures of cardiovascular conditions were used to create a latent cardiovascular conditions variable. The measures consisted of binary (yes/no) answers to questions about having been diagnosed by a medical practitioner with heart attack, angina, stroke, and high blood pressure. The measure of physical illness was the number of the following illnesses diagnosed by a medical practitioner: blood clot in the leg (deep vein thrombosis), blood clot in the lung, emphysema/chronic bronchitis, asthma, hay fever/allergic rhinitis/eczema, diabetes, and cancer. As few confirmed to be diagnosed with more than three of these illnesses, the score of this measure varied from 0 to 3, with a score of 3 indicating three or more illnesses. Of the total test sample, 60% reported no illnesses and only 1.5% reported being diagnosed with three or more illnesses. Hay fever and asthma were the most commonly reported illnesses by those in their 40s and 50s (11–27%), with diabetes and cancer being the most prominent illnesses (9–25%) alongside hay fever and asthma (9–22%) in the 60s cohort.
Finally, a binary yes/no measure of professional support was included, where an affirmative response indicated that a participant had at some point seen a general practitioner or a psychiatrist “for nerves, anxiety, tension, or depression.”
Table 1 shows the distribution and mean values for these measurements by gender and age-group. Of note is that while the prevalence of cardiovascular conditions increased with age and was more prevalent in males, the number of physical illnesses was relatively consistent across age-groups and gender. In comparison to the national unemployment rates, reported by the UK Office of National Statistics for similar age-groups during the time period the UK Biobank data were collected (3.5–5.5% for 35–49 years, 3–5% for 50–64 years, and 1.5–3.5% for 65+ years), measured unemployment rates for the female participants were lower, while the rates for the male participants fell within the national ranges.
Structural Equation Modeling
SEM is a multivariate technique that tests hypotheses about relations among observed (directly measured) and latent (inferred from several measures) variables using linear statistical models. The hypotheses tested usually serve to further our understanding of the processes through which independent variables affect dependent variables. The technique is often used in mediation analyses aiming to examine to what extent different variables intervene with the relationship between a dependent and independent variable of interest, such as depression and functional hearing in this study. The intervening variable may be a confounder; that is, the direct relationship is explained by the intervening variable having an association with both the independent and dependent variables, or may be a mediator; that is, the independent variable has an association with the mediating variable that has an association with the dependent variable. The hypothesized model can be expressed as a set of equations, including several unknown parameters, and fitting the model involves using the observed data to estimate the values of those parameters.
In this study, structural equation models were fitted to data using the diagonally weighted least squares method with robust standard errors and a mean- and variance-adjusted test statistic, often called WLSMV (Kline, 2011; Rosseel, 2015b). Ordered categorical variables with fewer than eight categories were treated as ordinal rather than continuous, and missing data were handled using pairwise deletion. The fit of a structural equation model can be evaluated in several ways. A χ2 test is available with the null hypothesis being that the model is correct, so that a significant p value indicates rejection of the model. However, the χ2 test has the characteristic (similarly to any statistical test) that if the sample size is very large then the null hypothesis is likely to be rejected even if there is only a very slight discrepancy between the model and the data. Since the sample size here was very large, we relied more heavily on other measures of model fit known as fit indices. The fit indices we considered were the root mean square error of approximation (RMSEA), which compares the fit of the model to a model that fits perfectly, taking model complexity into account, and the comparative fit index (CFI), which measures improvement in fit relative to the simplest possible model in which all variables are independent. According to Hu and Bentler (1999), an acceptable model fit is indicated by RMSEA ≤ .06 and CFI ≥ .95. The associations between variables are reported as standardized parameter estimates (b). When the absolute value of the estimates, or path coefficients, is less than .10, the association between variables is considered small. Absolute values around .30 suggest a medium association, and absolute values greater than .5 suggest a large association. The models presented here were fitted using the lavaan package (Rosseel, 2015a) in R (R Core Team, 2015).
Results
Figure 1 shows for each latent depression variable how the measured variables on functional hearing, depression, and cardiovascular conditions relate to the latent variables across age and gender. For both measurement models, the fit index RMSEA was less than .05 and the fit index CFI was greater than .96, suggesting that the models are acceptable fit to data. It should be noted that the latent depressive episodes variable is generally highly associated with all its measures (all correlation coefficients exceed .8), whereas the latent depressive symptoms variable shows particularly high association with the measure of depressive symptoms (correlation coefficient = .91). In both models, the latent hearing variable is more highly associated with the self-reported than the behavioral measure. The correlations between the different measures are listed in Table 2.
Figure 1.
The measurement models for (a) depressive episodes and (b) depressive symptoms. The numbers indicate the correlations between latent variables (circled), and between each latent variable and the measurements (boxed) that define it, and measurement error variances (shown to the right of the measurements).
Table 2.
The Correlations Among the Observed Variables in the Measurement Models.
| Measurements | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Depression, longest episode | 0.78 | 0.76 | 0.63 | 0.30 | 0.23 | 0.27 | 0.22 | 0.09 | −0.01 | 0.00 | 0.01 | 0.03 | 0.01 |
| 2. Depression, no of episodes | 0.61 | 0.74 | 0.33 | 0.25 | 0.31 | 0.25 | 0.09 | −0.01 | 0.00 | 0.02 | 0.03 | 0.01 | |
| 3. Unenthusiastic, longest episode | 0.82 | 0.29 | 0.21 | 0.29 | 0.22 | 0.07 | 0.00 | 0.03 | 0.04 | 0.06 | 0.02 | ||
| 4. Unenthusiastic, no of episodes | 0.33 | 0.23 | 0.35 | 0.25 | 0.08 | 0.00 | 0.04 | 0.05 | 0.06 | 0.02 | |||
| 5. Depressive symptoms | 0.66 | 0.58 | 0.47 | 0.16 | 0.01 | 0.03 | 0.07 | 0.06 | 0.04 | ||||
| 6. Anxiety symptoms | 0.41 | 0.31 | 0.15 | 0.01 | 0.00 | 0.04 | 0.01 | 0.04 | |||||
| 7. Feeling down | 0.51 | 0.14 | 0.03 | 0.08 | 0.11 | 0.09 | 0.06 | ||||||
| 8. Unhapiness | 0.14 | 0.01 | 0.09 | 0.12 | 0.10 | 0.07 | |||||||
| 9. Hearing difficulty in noise | 0.20 | 0.15 | 0.19 | 0.11 | 0.11 | ||||||||
| 10. BESRTn | 0.09 | 0.12 | 0.11 | 0.09 | |||||||||
| 11. Heart attack | 0.71 | 0.30 | 0.29 | ||||||||||
| 12. Angina | 0.32 | 0.35 | |||||||||||
| 13. Stroke | 0.27 | ||||||||||||
| 14. High blood pressure |
Note. Within-latent variable correlations are shown in bold. BESRTn = best ear speech reception threshold in noise.
The model investigated in this study explores the possible confounding influences of physical illness and cardiovascular conditions, as well as mediating influences of unemployment and social isolation, on the direct association between functional hearing and depression (see Figures 2 and 3). An increasing number of chronic health conditions have been significantly associated with increasing likelihood of feeling socially isolated and lonely (Havens, Hall, Sylvestre, & Jivan, 2004; Hawthorne, 2008). As poor health could also be the underlying reason for unemployment (Hogan et al., 2009), leading to depression, the model controls for physical illness in the associations involving the two social interaction variables. Further, as suggested in the introduction, the model controls for professional support in the direct associations between social isolation/unemployment and depression.
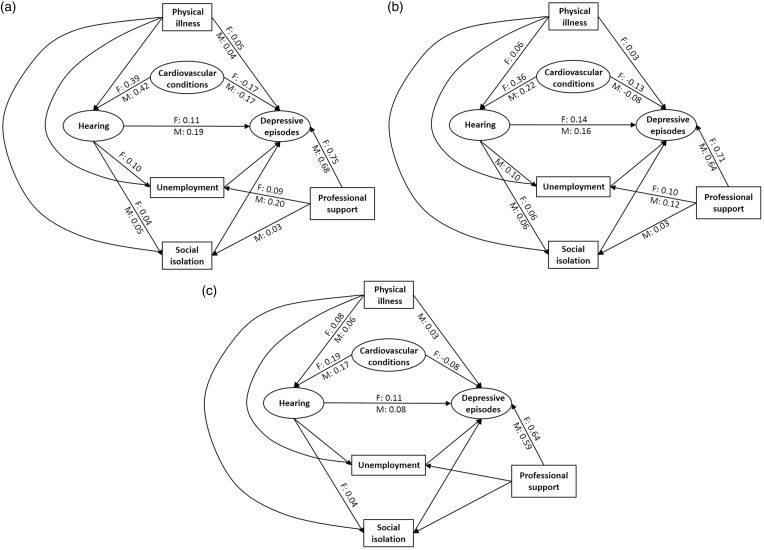
Figure 2.
Models examining confounding and mediating effects on the direct association between functional hearing and depressive episodes by age and gender (F: female, M: male). Variables in ovals and boxes represent latent variables and single measurements, respectively. Only significant path coefficients are shown. (a) 40–49 years, (b) 50–59 years and (c) 60–69 years.
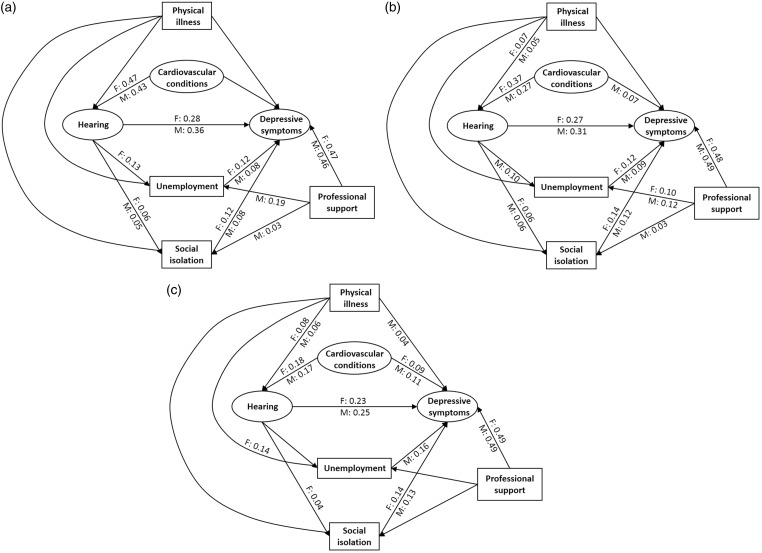
Figure 3.
Models examining confounding and mediating effects on the direct association between functional hearing and depressive symptoms by age and gender (F: female, M: male). Variables in ovals and boxes represent latent variables and single measurements, respectively. Only significant path coefficients are shown. (a) 40–49 years, (b) 50–59 years and (c) 60–69 years.
Depressive Episodes
Figure 2 shows the model for each age-group when considering the latent depressive episodes variable. All models were rejected (p < .001), which was expected because of the large sample size, but the goodness-of-fit statistics showed a reasonable fit to data (RMSEA < .05 and CFI > .95). In each model, significant (p < .01), standardized path coefficients (b) are shown separately for females (F) and males (M). In all age-groups and for both genders, poor hearing was significantly associated with more depressive episodes when all other variables in the model were held constant, although in all cases was the strength of the association somewhat weak (b < .2). The direct association exceeded all indirect associations in strength and was generally greater, on average, for the two younger cohorts (b = .15) than for the oldest cohort (b = .10). For the males, the path coefficient estimated for each of the two youngest age-groups (b = .19 and b = .16) was significantly greater than that estimated for the oldest age-group (b = .08; p < .02; see Table 3). In the youngest cohort, the association was greater for males than females (b = .19 vs. b = .11), but this difference was not significant (p = .14; see Table 3). Of the mediating and confounding factors under investigation, only cardiovascular conditions showed a small significant partial confounding influence. Cardiovascular conditions had a medium to large association with poor hearing in the youngest cohort (b > .39), while small to medium associations were seen in the other age-groups (b = .17–.36). The association between cardiovascular conditions and depressive episodes was weaker (mostly small) and was negative for all groups. The negative association is a result of controlling for professional support, which has a large association with depressive episodes (b = .67, on average). This means that for equivalent levels of professional support, people with cardiovascular conditions in this test sample were less depressed. Some other general patterns to note are that poor hearing is only sporadically and weakly associated with unemployment (b = .10 for females in their 40s and for males in their 50s) and weakly associated with social isolation (b = .05, on average). Neither of these variables have a significant association with depressive episodes within equivalent levels of professional support.
Table 3.
The Path Coefficients (in Bold) for the Direct Asssociations Between Functional Hearing and Depression by Gender and Age-Group, and the p Levels When Testing for Equality Between Coefficients Estimated for Each Gender (Column 4), Depression Variable (Column 5), and Age-Groups by Depression Variable and Gender (Bottom Rows).
| Age-groups (years) | Depressive episodes |
Depressive symptoms |
p levels (gender) |
p levels (depression) |
||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Depressive episodes | Depressive symptoms | Females | Males | |
| 40–40 | 0.11 | 0.19 | 0.28 | 0.36 | 0.14 | 0.09 | .007 | <.001 |
| 50–59 | 0.14 | 0.16 | 0.27 | 0.31 | 0.62 | 0.10 | <.001 | <.001 |
| 60–69 | 0.11 | 0.08 | 0.23 | 0.25 | 0.06 | 0.15 | <.001 | <.001 |
| p Levels (age) | ||||||||
| 40s vs. 50s | 0.38 | 0.47 | 0.88 | 0.35 | ||||
| 40s vs. 60s | 0.61 | 0.02 | 0.27 | 0.06 | ||||
| 50s vs. 60s | 0.14 | 0.002 | 0.16 | 0.06 | ||||
Depressive Symptoms
Figure 3 shows the models when the latent depressive episodes variable has been exchanged with the latent depressive symptoms variable. Again, all models were rejected (p < .001), but the fit indices suggested an acceptable fit of data to all models, with RMSEA being less than .05 and CFI being greater than .96. A significant direct association between poor hearing and depressive symptoms is seen for both genders in all age-groups when holding all other variables in the model constant. Relative to the direct associations between hearing and depressive episodes, the direct associations between hearing and depressive symptoms are significantly greater (p < .01; see Table 3). In all cases, the estimated path coefficients suggest a medium association between hearing and depressive symptoms, with the greatest association estimated for males in their 40s (b = .36). The path coefficient for the youngest males is in this case not significantly different from older peers (b = .25; p = .06) or their female contemporaries (b = .28, p = .09; see Table 3). Significant, but weak, partial indirect associations are evident in all models, including a mediating influence of unemployment for females in their 40s and males in their 50s, a mediating influence of social isolation in all groups except males in their 60s, a confounding influence of cardiovascular conditions in the oldest cohort and in males in their 50s, and a confounding influence of physical illness in males in their 60s. Note that in the models for which cardiovascular conditions has a significant association with depressive symptoms, the relation is positive, suggesting that cardiovascular conditions are associated with more depressive symptoms for equivalent levels of professional support. The association between professional support and depressive symptoms is also rather strong (b ≥ .46), although not as strong as for the same association with depressive episodes. Another difference between the models in Figures 2 and 3 is that unemployment and social isolation generally have independent significant associations with depressive symptoms, but not depressive episodes, after controlling for professional support. For both the social interaction variables, the positive path coefficients suggest that people who are unemployed and socially isolated are more likely to report more depressive symptoms, irrespective of whether professional support was sought or not. The strength of these associations are mostly weak (b < .14) and similar across gender and age-groups.
Subjective Versus Behavioral Measures of Hearing
The latent hearing variable used in this study loaded heavily on the subjective measure of hearing difficulty (see Figure 1). In Keidser et al. (2015), it was established that reported hearing difficulty had a stronger association with depressive episodes than did the behavioral measure, while both measures showed medium associations with depressive symptoms. The SEM was repeated, replacing the latent hearing variable with the behavioral measure of functional hearing (BESRTn). In these models, no significant direct or indirect associations between BESRTn and depressive episodes were seen. Significant direct associations between BESRTn and depressive symptoms were observed for all subgroups. The direct associations were very small, and similar across subgroups (b = 0.03, on average), but were greater than the indirect associations to suggest an independent relation between hearing and depression (as seen for the latent hearing variable). As data in this investigation are cross-sectional, the temporal order of the hearing and depression variables is uncertain. While it seems unlikely that depression would cause people to perform more poorly on a behavioral hearing test, it is possible that depressed people may have a more negative view on their ability to hear. To examine this further, the original models were repeated with the arrow between the hearing and depression variables reversed. These models showed similar path coefficients for the association between hearing and depression, with little variation to other path coefficients. This observation confirms the uncertainty about the temporal order of hearing difficulty and depression when the hearing variable is dominated by the subjective measure.
Discussion
Using the UK Biobank data and linear regression modelling, a previous study found a significant association between poor hearing and depression that was generally greater for depressive symptoms than depressive episodes, for younger (in their 40s) than older (in their 60s) participants, and for females than males (Keidser et al., 2015). The findings applied whether behavioral or reported measures of hearing difficulty in noise were considered. Based on the same data and SEM, this study explored whether a significant association between poor hearing and depression could be influenced by such factors as physical illness, cardiovascular conditions, unemployment, or social isolation and whether any influence differed with age and gender. Creating a latent hearing variable from the behavioral and reported measures of hearing difficulty, which loaded heavily on the subjective measure, SEM revealed that none of these potential confounding and mediating factors eliminated the independent and significant association between poor hearing and more depressive episodes or between poor hearing and more depressive symptoms for any one of three age groups or gender. As observed in Keidser et al.’s (2015) study, the association between poor hearing and depression was greater for depressive symptoms than depressive episodes, that is, with a less debilitating form for depression. This observation holds when considering only the association between a behavioral measure of hearing difficulty and the two depression variables and is consistent with findings by Lupsakko, Mantyjarvi, Kautiainen, and Sulkava (2002). However, while the linear analyses in Keidser et al.’s (2015) study for both depression variables suggested a steady increase in the strength of the association as participants got younger and a greater association among females than males, the structural equation models using the latent hearing variable, stratified by age and gender, showed a more variegated pattern. There was still a trend for the association between hearing and depression to be greater among the youngest (in their 40s) than the oldest (in their 60s) cohort, which is in agreement with Tambs’s (2004) and Nachtegaal et al.’s (2009) studies, but the difference in estimates was only significant, or near significant, among males (see Table 3). Contrary to the analyses in Keidser et al.’s (2015) study, the models showed a trend for the youngest males to report notably higher levels of both depressive episodes and depressive symptoms than their female counterparts, but the difference in estimates did not reach significance (p > .09). The difference in pattern across the two studies could suggest that the indirect associations investigated in this article reduce the direct association between hearing and depression in younger females the most. No age or gender effect was observed when considering only the behavioral measure of hearing difficulty in the models. In an older population (>63 years), Pronk et al. (2011) found no significant association between hearing status and depression, but males with poor hearing (whether measured subjectively or behaviorally) reported significantly higher levels of emotional loneliness than did females, lending support to a potential gender effect.
For participants in their 40s and 50s, the association between poor hearing and depressive symptoms was medium. Whether the association is driven by people experiencing more hearing difficulty when listening in noise being more depressed, or vice versa, the observation calls for further investigation into why the association is particular strong in the middle-aged population. In the UK Biobank sample, Keidser et al. (2015) found no remediating influence of hearing aid usage on the association between functional hearing and depression. If, in the future, other factors cannot be identified that may explain the association, then the observation suggests that additional services to hearing aid rehabilitation, for example, counselling and communication strategies, are required to properly address the functional hearing problems experienced by the middle-aged population. The finding corroborates calls from other countries for audiologists to pay more attention to working age clients (Danermark & Gellerstedt, 2004) and to be more active in identifying anxiety and depression in their clients and to assist them by working more closely with mental health professionals (Lindsey, 2016). There is also evidence that an offer of counselling is missed by clients. Examining the effects of acquiring a severe or profound hearing loss in middle age, Hallam, Ashton, Sherbourne, and Gailey (2006) found that counselling/psychological therapy was the second most wanted service that was not received in the dealings with their hearing problems among this population. Providing psychosocial counselling to those who may need it the most could be challenging however, as males are typically less likely than females to reach out for help with emotional problems and depression (e.g., Addis and Mahalik, 2003; Wadsworth & Möller-Leimkühler, 2002). The UK Biobank data support this notion with a higher proportion of females than males reporting to have seen a professional about depression (see Table 1).
Of the influencing factors under investigation, only cardiovascular conditions seemed to have a nondiscriminating and partial confounding influence of note on the association between hearing and depressive episodes. More factors showed partial influences in the models concerning depressive symptoms, with a trend for social interaction being a slightly greater issue among the younger cohorts and physical illness a greater issue for the over 60s as hypothesized in the introduction. The small indirect influences of other variables on the association between hearing and depressive episodes and depressive symptoms seen in this study may be influenced by the measurements largely being made up of simple binary variables and ordinal variables with a small number of categories. Each of the influencing factors and their relation to hearing and depression, which largely remained consistent with changes to the hearing variable and direction between hearing and depression, are further discussed below.
Social Interaction
It is generally believed that hearing loss leads to communication difficulties that further leads to withdrawal from social interactions. While our models in agreement with other studies showed a significant association between hearing difficulty and social isolation (e.g., Gopinath et al., 2012; Hawthorne, 2008; Strawbridge et al., 2000), the estimate of the association was surprisingly small (b < .07). The small association may be explained by the social isolation factor in this study being related to quantity (frequency of contacts) rather than quality (satisfaction of contacts) of social interactions (Routasalo, Savikko, Tilvis, Strandberg, & Pitkala, 2006), which further suggests that hearing-impaired people are not so much physically withdrawing from social activities, as they are finding their interactions with other people to be of low value. This issue may be worth exploring further. Social isolation has frequently been linked to depression (Adams, Sanders, & Auth, 2004; Hawthorne, 2008). In the UK Biobank sample, there was, after accounting for help seeking, a significant association between social isolation and depressive symptoms across all three age-groups and gender but not between social isolation and the more debilitating form for depression (depressive episodes). Again, the relation between social isolation and depressive symptoms was not particularly strong (b < .15) and was generally less than the direct relation between poor hearing and depressive symptoms. We note that Hawthorne (2008), who examined what factors led to perceived social isolation in a community sample, found that depression was a confounder for the association between hearing difficulty and social isolation. This observation supports our finding of an independent association between hearing difficulty and depression that is not greatly mediated by social isolation.
Employment brings opportunities for daily social interactions. As several studies have reported slightly higher unemployment rates among people experiencing hearing problems relative to the population at large (Gellerstedt & Danermark, 2004; Hogan et al., 2009), unemployment was investigated separately as a potential mediator of the association between hearing difficulty and depression in this study. Our models did not show a consistent significant association between hearing difficulty and unemployment. It is possible that data in this study were biased by the test sample that appears to represent a population with higher socioeconomic status than the general UK population (Dawes et al., 2015). It is likely that a measure comparing occupation with educational attainment would have been a better candidate for a mediating variable, as it is further evident that hearing-impaired people in employment are overrepresented in less demanding jobs and among low-income earners (Gellerstedt & Danermark, 2004; Hogan et al., 2009). That unemployment, which can lead to financial problems, is linked to higher levels of depression seems uncontroversial and has been demonstrated in many studies (e.g., Dooley, Catalano, & Wilson, 1994; Montgomery, Cook, & Bartley, 1999). Results from our model support such findings by showing a significant relation between unemployment and depressive symptoms, but not depressive episodes, although the estimates of the relation are less than half of the direct association between hearing difficulty and depressive symptoms for the two youngest cohorts.
Physical Health
Comorbidity is more common in older people, and in agreement with Gopinath et al. (2012), who found an association between reported hearing handicap and poor health in a community sample of over 55-year olds, our data showed a significant association between number of physical conditions and hearing difficulty in the two older cohorts, although the estimate of the relation was small (b < .09). Generally, in this test sample, physical illness had no significant association with unemployment or social isolation. The response rate to the call for participation among candidates for the UK Biobank resource was low at 5.4%, and it is possible that people with more and significant health problems declined the invitation to attend an assessment centre to provide data for the resource. As pointed out in the methodology, 60% of the test sample reported no illness and among the remaining participants, asthma and hayfever were the most frequently reported illnesses, especially in the two youngest cohorts. This, and the fact that participants were under 70 years of age, may also be the reason why the models showed only small (b < .05) and sporadic associations between physical illness and depression, whereas other studies have found that number of chronic diseases was a significant predictor of particularly depressive symptoms in aging (>55–60 years) populations (e.g., Adams et al., 2004; Beekman et al., 1997). Seriously ill people may have identified themselves with the response options being “unable to work because of sickness or disability” or “retired” rather than “unemployed,” and this may specifically have affected the measured relation with unemployment.
With a growing body of data showing that cardiovascular conditions are significantly associated with hearing loss (e.g., Helzner et al., 2011; Liew et al., 2007; Torre et al., 2005), the confounding influence of this disease was examined independently. Consistent with other recent epidemiological studies, our models showed significant associations between cardiovascular conditions and poor functional hearing, with particularly large estimates of the association seen in the two youngest cohorts (b > .39). Because the blood supply to the cochlea is most distal at the apex, the insufficient blood supply following from vascular diseases is expected to affect low-frequency sound transduction the most. Low-frequency hearing loss is reportedly more prominent in women (Pearson et al., 1995), and at least two studies have found that the association between cardiovascular conditions and hearing loss is stronger in women than in men (Cruickshanks, Nondahl, Klein, & Klein, 1996; Gates et al., 1993). Our models lend further support to this notion as the association was generally greater in the female (b = .36–.47) than male (b = .22–.43) population of the two younger cohorts. Despite the strong link between cardiovascular conditions and hearing difficulty, this variable did not fully confound the direct association between hearing difficulty and depression, as our models did not produce strong relations between cardiovascular conditions and the depressive variables. While many of the models in Figures 2 and 3 showed a significant association between cardiovascular conditions and depression when holding other variables constant, the picture was inconsistent, with the association interacting with professional support in the Figure 2 models. In models investigating only the association between cardiovascular conditions and depression, the significant, but very small, path coefficients were positive. Previous findings suggest a bidirectional association between poor cardiovascular conditions and depression (Testuz, 2009), with the effect of cardiovascular disease onset on depression being much greater than vice versa (Kendler, Gardner, Fiske, & Gatz, 2009), and the increased risk toward cardiovascular conditions in people suffering from depression being due to some common pathophysiology (e.g., Maes, Ruckoanich, Chang, Mahanonda, & Berk, 2010). The contradicting and mixed picture seen in this study may be the result of the relatively small proportion of test participants, particularly in their 40s and 50s, reporting diagnosis with the more serious conditions of heart attack, angina, and stroke (see Table 1), and the lack of control of professional support in previous studies.
Study Limitations
A strength of this study is the number of observations included in the modelling. However, as pointed out earlier, the response rate to the UK Biobank resource was low and the resource may have attracted a healthier and more affluent sample of the general population. The large-scale data collection structure further precluded the use of lengthy, but standardized and more robust measures, including those used to form the latent hearing and latent depression variables. Using SEM and data from the UK Biobank, Dawes et al. (2015) investigated the mediating influence of social isolation and depression on any association between hearing aid usage and cognition. In their models, hearing was based only on the performance on the DTT, and the social isolation and depression variables were based on single, binary measures of feeling lonely (Do you often feel lonely?) and depressed or down (Over the past 2 weeks, how often have you felt down, depressed, or hopeless?). Their models suggest a slightly stronger direct association between hearing and social isolation than between hearing and depression, which is opposite to the observations reported in this study. This discrepancy between findings is likely explained by the actual measures used and their interaction with the factors controlled for in each model. Dawes et al. (2015) further found a relatively strong association between social isolation and depression, which in relation to our data suggest that their selected depression measure is a predictor of a less debilitating form for depression (depressive symptoms). Overall, the differences in observations between the two studies highlight the sensitivity of statistical modelling to the measures selected for inclusion.
Future Studies
Data from this study warrant further investigations into the pathway from hearing to depression among middle-aged people. Particularly, findings from this study suggest that future studies should consider the value of social interactions and educational attainment as mediating factors. There is also a scope for investigating how psychosocial counselling is best integrated into current hearing health-care models. Longitudinal studies are required to make a firm conclusion about the temporal order of all the modelled associations, particularly of the association between perceived hearing difficulty in noise and depressive symptoms.
Conclusion
Hearing difficulty had an independent association with depression, especially depressive symptoms, that was neither fully confounded by chronic illness nor mediated by reduced social interaction, in a large community-based population in the UK. Irrespective of the temporal order of the variables (which is unknown), findings suggest that audiologists should be more aware of psychological issues. The younger males (40–59 years) showed the strongest association when the hearing variable was largely driven by subjective reports. This might reveal a need for urgent reviews on how to best encourage this population to seek intervention, whether for depression, a hearing problem, or both. Possible caveats of these findings include the use of a sample that may be healthier and more affluent than the general population, nonstandardized measures, and insensitive measures of interacting factors of interest.
Authors’ Notes
This research has been conducted using the UK Biobank Resource. Data were obtained under a grant held by Professor Jerker Rönnberg from the Linnaeus Centre HEAD, Swedish Institute for Disability Research, Linköping University, Sweden. The National Acoustic Laboratories is part of the Australian Hearing Hub, an initiative of Macquarie University, that brings together Australia’s leading hearing and health-care organizations to collaborate on research projects.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding acknowledgment
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Australian Hearing Hub, an initiative of Macquarie University and the Australian Government, provided financial support for the publication of this research article. The authors also acknowledges the financial support of the Swedish Research Council, the HEARing Cooperative Research Centre, established and supported under the Business Cooperative Research Centres Programme, and the Commonwealth Department of Health and Aging.
References
- Adams K. B., Sanders S., Auth E. A. (2004) Loneliness and depression in independent living retirement communities: Risk and resilience factors. Aging and Mental Health 8: 475–485. [DOI] [PubMed] [Google Scholar]
- Addis M. E., Mahalik J. R. (2003) Men, masculinity, and the contexts of help seeking. American Psychologist 58(1): 5–14. [DOI] [PubMed] [Google Scholar]
- Agrawal Y., Platz E. A., Niparko J. K. (2008) Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999–2004. Archives of Internal Medicine 168(14): 1522–1530. [DOI] [PubMed] [Google Scholar]
- Beekman A. T. F., Penninx B. W. J. H., Deeg D. J. H., Ormel J., Draam A. W., van Tilburg W. (1997) Depression and physical health in later life: Results from the Longitudinal Aging Study Amsterdam (LASA). Journal of Affective Disorders 46: 219–231. [DOI] [PubMed] [Google Scholar]
- Carabellese C., Appollonio I., Rozzini R., Bianchetti A., Frisoni G. B., Frattola L., Trabucchi M. (1993) Sensory impairment and quality of life in a community elderly population. Journal of the American Geriatrics Society 41: 401–407. [DOI] [PubMed] [Google Scholar]
- Cruickshanks K. J., Nondahl D. M., Klein R., Klein B. E. K. (1996) Sex, cardiovascular disease, and hearing loss. American Journal of Epidemiology 143: S65. [Google Scholar]
- Cruickshanks K. J., Wiley T. L., Tweed T. S., Klein B. E. K., Klein R., Mares-Perlman J. A., Nondahl D. M. (1998) Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The epidemiology of hearing loss study. American Journal of Epidemiology 148: 879–886. [DOI] [PubMed] [Google Scholar]
- Dalton D. S., Cruickshanks K. J., Klein B. E., Klein R., Wiley T. L., Nondahl D. M. (2003) The impact of hearing loss on quality of life in older adults. Gerontologist 43: 661–668. [DOI] [PubMed] [Google Scholar]
- Danermark B., Gellerstedt L. C. (2004) Psychosocial work environment, hearing impairment and health. International Journal of Audiology 43: 383–389. [DOI] [PubMed] [Google Scholar]
- Dawes P., Emsley R., Cruickshanks K. J., Moore D. R., Fortnum H., Edmondson-Jones M., McCormack A., Munro K. J. (2015) Hearing loss and cognition: The role of hearing aids, social isolation and depression. PLoS One 10(3): e0119616 doi:10.1371/journal.pone.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes P., Fortnum H., Moore D. R., Emsley R., Norman P., Cruickshanks K., Munro K. (2014) Hearing in middle age: A population snapshot of 40- to 69-year olds in the United Kingdom. Ear and Hearing 35(3): e44–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D., Catalano R., Wilson G. (1994) Depression and unemployment: Panel findings from the epidemiologic catchment area study. American Journal of Community Psychology 22(6): 745–765. [DOI] [PubMed] [Google Scholar]
- Fransen E., Topsakal V., Hendrickx J. J., Van Laer L., Huyghe J. R., Van Eyken E., Van Camp G. (2008) Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: A European population-based multicenter study. Journal of the Association of Research in Otolaryngology 9(3): 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates G. A., Cobb J. L., D'Agostino R. B., Wolf P. A. (1993) The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Archives of Otolaryngology—Head and Neck Surgery 119(2): 156–161. [DOI] [PubMed] [Google Scholar]
- Gellerstedt L. C., Danermark B. (2004) Hearing impairment, working life conditions, and gender. Scandinavian Journal of Disability Research 6(3): 225–245. [Google Scholar]
- Gopinath B., Hickson L., Schneider J., McMahon C. M., Burlutsky G., Leeder S. R., Mitchell P. (2012) Hearing-impaired adults are at increased risk of experiencing emotional distress and social engagement restrictions five years later. Age and Ageing 41: 618–623. [DOI] [PubMed] [Google Scholar]
- Hallam R., Ashton P., Sherbourne K., Gailey L. (2006) Acquired profound hearing loss: Mental health and other characteristics of a large sample. International Journal of Audiology 45: 715–723. [DOI] [PubMed] [Google Scholar]
- Hallberg R.-M. (1996) Occupational hearing loss: Coping and family life. Scandinavian Audiology Supplementum 43: 25–33. [PubMed] [Google Scholar]
- Havens B., Hall M., Sylvestre G., Jivan T. (2004) Social isolation and loneliness: Differences between older rural and urban Manitobans. Canadian Journal on Aging 23: 129–140. [DOI] [PubMed] [Google Scholar]
- Hawthorne G. (2008) Perceived social isolation in a community sample: Its prevalence and correlates with aspects of people’s lives. Social Psychiatry and Psychiatric Epidemiology 43: 140–150. [DOI] [PubMed] [Google Scholar]
- Helzner E. P., Patel A. S., Pratt S., Sutton-Tyrrell K., Cauley J. A., Talbott E., Newman A. B. (2011) Hearing sensitivity in older adults: Associations with cardiovascular risk factors in the health, aging and body composition study. Journal of the American Geriatrics Society 59(6): 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan A., O’Loughlin K., Davis A., Kendig H. (2009) Hearing loss and paid employment: Australian population survey findings. International Journal of Audiology 48: 117–122. [DOI] [PubMed] [Google Scholar]
- Hogan A., Phillips R. L., Brumby S. A., Williams W., Mercer-Grant C. (2015) Higher social distress and lower psycho-social wellbeing: Examining the coping capacity and health of people with hearing impairment. Disability and Rehabilitation 37(22): 2070–2075. [DOI] [PubMed] [Google Scholar]
- Hu L., Bentler P. M. (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 6: 1–55. [Google Scholar]
- Kakarlapudi V., Sawyer R., Staecker H. (2003) The effect of diabetes on sensorineural hearing loss. Otology & Neurotology 24(3): 382–386. [DOI] [PubMed] [Google Scholar]
- Keidser G., Seeto M., Rudner M., Hygge S., Rönnberg J. (2015) On the relationship between functional hearing and depression. International Journal of Audiology 54(10): 653–664. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Gardner C. O., Fiske A., Gatz M. (2009) Major depression and coronary artery disease in the Swedish twin registry: Phenotypic, genetic, and environmental sources of comorbidity. Archives of General Psychiatry 66(8): 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. C., Cowie R. I. D. (1997) Acquired deafness: A multi-dimensional experience. British Journal of Audiology 31: 177–188. [DOI] [PubMed] [Google Scholar]
- Kline R. B. (2011) Principles and practice of structural equation modeling, 3rd ed New York, NY: Guilford. [Google Scholar]
- Kramer S. E., Kapteyn T. S., Kuik D. J., Deeg D. J. (2002) The association of hearing impairment and chronic diseases with psychosocial health status in older age. Journal of Aging and Health 14: 122–137. [DOI] [PubMed] [Google Scholar]
- Liew G., Wong T. Y., Mitchell P., Newall P., Smith W., Wang J. J. (2007) Retinal microvascular abnormalities and age-related hearing loss: The blue mountains hearing study. Ear and Hearing 28: 394–401. [DOI] [PubMed] [Google Scholar]
- Lindsey H. (2016) Mental well-being tightly linked to hearing health. The Hearing Journal 69(3): 14–18. [Google Scholar]
- Lupsakko T., Mantyjarvi M., Kautiainen H., Sulkava R. (2002) Combined hearing and visual impairment and depression in a population aged 75 years and older. International Journal of Geriatric Psychiatry 17: 808–813. [DOI] [PubMed] [Google Scholar]
- Maes M., Ruckoanich P., Chang Y. S., Mahanonda N., Berk M. (2011) Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry 35: 769–783. [DOI] [PubMed] [Google Scholar]
- Montgomery S. M., Cook D. G., Bartley M. J. (1999) Unemployment pre-dates symptoms of depression and anxiety resulting in medical consultation in young men. International Journal of Epidemiology 28(1): 95–100. [DOI] [PubMed] [Google Scholar]
- Nachtegaal J., Festen J. M., Kramer S. E. (2012) Hearing ability in working life and its relationship with sick leave and self-reported work productivity. Ear and Hearing 33(1): 94–103. [DOI] [PubMed] [Google Scholar]
- Nachtegaal J., Smit J. H., Smits C., Bezemer P. D., Van Beek J. H., Festen J. M., Kramer S. E. (2009) The association between hearing status and psychosocial health before the age of 70 years: Results from an internet-based national survey on hearing. Ear and Hearing 30: 302–312. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Morrell C. H., Gordon-Salant S., Brant L. J., Metter E. J., Klein L. L., Fozard J. L. (1995) Gender differences in a longitudinal study of age-associated hearing loss. Journal of the Acoustical Society of America 97(2): 1196–1205. [DOI] [PubMed] [Google Scholar]
- Pronk M., Deeg D. J., Smits C., van Tilburg T. G., Kuik D. J., Festen J. M., Fozard J. L. (2011) Prospective effects of hearing status on loneliness and depression in older persons: Identification of subgroups. International Journal of Audiology 50(12): 887–896. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2015). R: A language and environment for statistical computing (version 3.1.3) [Software]. Retrieved from http://cran.r-project.org.
- Rosen S., Olin P. (1965) Hearing loss and coronary heart disease. Archives of Otolaryngology 82: 236–243. [DOI] [PubMed] [Google Scholar]
- Rosseel, Y. (2015a). lavaan: Latent variable analysis (version 0.5-18) [Software]. Retrieved from http://cran.r-project.org/package=lavaan.
- Rosseel, Y. (2015b). The lavaan tutorial. Retrieved from http://lavaan.ugent.be/tutorial/tutorial.pdf.
- Routasalo P. E., Savikko N., Tilvis R. S., Strandberg T. E., Pitkala K. H. (2006) Social contacts and their relationship to loneliness among aged people—A population-based study. Gerontology 52: 181–187. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Hildesheimer M., Zohar S., Chilarovitz T. (1977) Chronic cardiovascular pathology and hearing loss in the aged. Gerontology 23: 4–9. [DOI] [PubMed] [Google Scholar]
- Saito H., Nishiwaki Y., Michikawa T., Kikuchi Y., Mizutari K., Takebayashi T., Ogawa K. (2010) Hearing handicap predicts the development of depressive symptoms after 3 years in older community-dwelling Japanese. Journal of the American Geriatrics Society 58: 93–97. [DOI] [PubMed] [Google Scholar]
- Smits C., Kapteyn T. S., Houtgast T. (2004) Development and validation of an automatic speech-in-noise screening test by telephone. International Journal of Audiology 43: 15–28. [DOI] [PubMed] [Google Scholar]
- Strawbridge W. J., Wallhagen M. I., Shema S. J., Kaplan G. A. (2000) Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist 40: 320–326. [DOI] [PubMed] [Google Scholar]
- Tambs K. (2004) Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trondelag hearing loss study. Psychosomatic Medicine 66: 776–782. [DOI] [PubMed] [Google Scholar]
- Testuz A. (2009) Depression and myocardial infarction. Revue Médicale Suisse 5(193): 515–519. [PubMed] [Google Scholar]
- Torre P., III, Cruickshanks K. J., Klein B. E. K., Klein R., Nondahl D. M. (2005) The association between cardiovascular disease and cochlear function in older adults. Journal of Speech Language and Hearing Research 48: 473–481. [DOI] [PubMed] [Google Scholar]
- Wadsworth M. E., Möller-Leimkühler A. M. (2002) Barriers to help-seeking by men: A review of sociocultural and clinical literature with particular reference to depression. Journal of Affective Disorders 71: 1–9. [DOI] [PubMed] [Google Scholar]
- Weinstein B. E., Ventry I. M. (1982) Hearing impairment and social isolation in the elderly. Journal of Speech and Hearing Research 25(4): 593–599. [DOI] [PubMed] [Google Scholar]