Abstract
Background
Leishmaniasis is a major public health problem worldwide. The aim of the present study was to investigate medicinal plants with anti-Leishmania activity which used in Iran.
Methods
Data were systematically gathered from five English databases including Ebsco, Science Direct, PubMed, Google Scholar and Scopus, four Persian databases including Magiran, Iran doc, Iran medex and the Scientific Information Database (SID) from 1999 to April 2015. Information obtained included plant family, extraction method, concentrations of extracts, animal models and parasite strains.
Results
A total of 68 articles including 188 experiments (140 in vitro and 48 in vivo) between 1999 and 2015, met our eligibility criteria. Thoroughly, 98 types of plants were examined against three genera of Leishmania spp. For the heterogeneity study conducted, it was showed that there was a great deal of variation among studies. Based on random effect, meta-analysis pooled mean of IC50 was obtained 456.64 (95% CI: 396.15, 517.12).
Conclusion
The most Iranian plants used as anti-leishmanial activity were Artemisia species, Allium sativum, Achilleamille folium, Peganum harmala and Thymus vulgaris. The present systematic and meta-analysis review provide valuable information about natural products with anti-Leishmania activity, which would be examined in the future experimental and clinical trials and herbal combination therapy.
Keywords: Leishmania, medicinal plants, natural products, herbal extracts, systematic review
Highlights
-
•
We systematically reviewed all published papers regarding herbal medicine with antileishmanial activity in Iran among nine databases from 1999 to April 2015.
-
•
Overall 68 articles including 140 in vitro and 48 in vivo, met our eligibility criteria. Also, 98 types of plants were examined against three genera of Leishmania spp.
-
•
Our study shows, the most Iranian plants with anti-leishmanial activity were Artemisia species, Allium sativum, Achilleamille folium, Peganum harmala and Thymus vulgaris.
1. Introduction
Leishmaniasis is a parasitic disease caused by an obligate intracellular parasite of genus Leishmania, which is transmitted to human by the bite of a female sand fly [1]. The disease has wide clinical spectrums from self-limiting cutaneous to fatal visceral form which depends on both host immune response and the species of Leishmania parasite. The World Health Organization (WHO) emphasizes on leishmaniasis as one of the seven important infections [2]. Approximately, 350 million people in 98 countries are at the risk of infection. It is estimated that 12 million people are affected with the disease and about 1.5 million new cases of cutaneous leishmaniasis (CL) are reported annually. Approximately, 90% of the CL cases occur in eight countries of Afghanistan, Saudi Arabia, Syria, Iran, Algeria, Iraq, Brazil and Peru [3], [4]. Pentavalent antimony is conventionally used from 1959 for leishmaniasis but it is toxic with side effects, which requires prolong injections. The efficacy of pentavalents has been decreased and the emergence of resistance limits their usage [5], [6]. The first line drugs in leishmaniasis including meglumine antimoniate (Glucantime), pentamidine (Pentacarinat), and sodium stibogluconate (Pentostam) are not effective orally and require prolonged injections. The second line drugs such as amphotricine B and pentamidine are very toxic [5]. In the absence of an effective vaccine, there is an urgent need for new and more effective drugs to replace or supplement those in current use. Plant derivatives or plant extracts are likely to provide a valuable source of new medicinal agents. The urgent need for substituting treatments has led to a program for screening natural products in leishmaniasis. Actually, the WHO recommended the use of traditional medicine in societies with poor health services. Moreover, the data obtained from reviewing would lead to the emergence of natural products with anti-leishmanial activity and would be the way for the production of new effective synthetic compounds. It has been estimated that there are about 250,000 medicinal plant species in the world. Nevertheless, the biological activities of only about 6% of them have been evaluated. Besides, only around less than 1% of medicinal plant compounds have been assessed in clinical trials [6], [7].
About 35% of approved drugs belong to natural products or semi synthetic derivatives, while 30% are synthetic molecules based on natural products or pharmacophore developed from natural compounds. It is noteworthy, out of 15 antiparasitic medications that have been approved by health authorities between January 1981 and June 2006, 65% are natural products or derivatives [8].
Medicinal plants are an effective source of pharmaceutical products in Iran [9], [10]. A critical evaluation of the clinical data due to the adverse effects has shown that herbal medicine is generally accepted better than synthetic medications. However, potentially, serious adverse events including herbal drug interactions have been described. This suggests the need to be attentive when using herbal therapies, mainly in specific situations such as throughout pregnancy and in the children age group [11]. About 820 forms of herbal drugs are produced in Iran [12], [13]. However, in different cultures and countries, indigenous medicinal plants are used to treat parasitic diseases such as leishmaniasis. Hence, clinical trials and empirical studies have been carried out about medicinal plants in different parts of the world especially in Asian countries including Iran [9], [14]. Our study attempts to provide an overview on the native medicinal plants, which was investigated against Leishmania parasite in Iran.
2. Methods
2.1. Search method
An exclusive search was performed through all scientific databases from April 1999 to August 2015 including five English databases (Science Direct, Scopus, Ebsco, Pub Med and Google Scholar), four Persian databases (Iran medex, Magiran, Iran doc) and the Scientific Information Database (SID). All articles which related to the medicinal plants and leishmaniasis were chosen (Fig. 1). Additionally, reference lists of all articles were reviewed for prevention of missing relevant data. The search terms were: “ Leishmania,” “plant extract,” “herbal extract,” “medicinal plants,” “traditional medicine,” and “ herbal medicine“ alone or in combination together. Furthermore, the synonyms of herbal medicines were considered as follow: herbal preparations, herbal medications, herbal products, herbal remedies, medicinal herbs and phytopharmaceuticals. Other relevant topics such as Leishmania parasite were also reviewed and included if the appropriate outcomes were retrieved. The search was performed both in English and Persian languages.
Figure 1.
Flowchart describing the study design process.
2.2. Paper selection
Papers selected for inclusion were studied carefully; repetitive papers, studies out of Iran and papers with poor methodology were excluded. (See Fig. 1). The following information was extracted: the year of publication, the first author, parasite species, herbal plant, type of extract, part of plant used for extraction, concentrations, exposure time, animal models, diameter of lesions and outcomes. Two reviewers independently screened studies identified for inclusion and determined study eligibility (Kapp index showed an agreement 89% between two reviewers). Disagreements were resolved by the third opinion.
2.3. Statistical analysis
In this meta-analysis, the mean and 95% confidence intervals of the half-maximal inhibitory concentration (IC50) values were calculated for each individual study in order to estimate the pooled mean of herbal extract effect on Leishmania spp. in Iran. The results were reported using a random-effect model with 95% confidence interval (CI). Heterogeneity among studies was evaluated by the Q-Cochran test (p < 0.1 indicate heterogeneity) and I-square statistic [low (25%–49%), moderate (50%–74%) and high (≥75%) [12]. Subgroup analysis was performed to investigate potential sources of heterogeneity [14]. Publication bias was evaluated using the funnel plots and Egger test [15]. Statistical software Stata 11 (Stata Corp, College Station, TX, USA) was used to data analysis.
3. Results
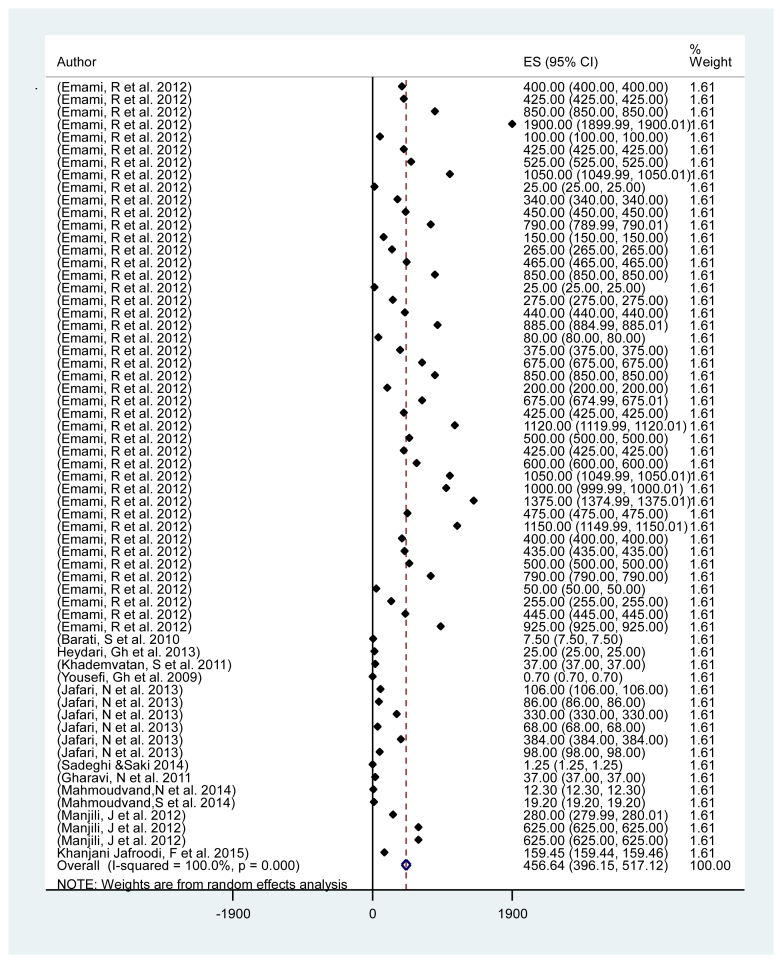
Out of 7500 articles of literature searched from 1999 to 2015, 68 articles with 188 experiments (140 in vitro and 48 in vivo), met our eligibility criteria and included the current systematic review and meta-analysis. Unpublished data, duplicated papers, congresses proceeding abstracts were excluded from our systematic review and meta-analysis. Totally, data extracted comprised of 98 types of plants, their families, extraction methods, animal models IC50 and Leishmania species. In the in vitro studies, all of plant extracts were tested on three genera of Iranian strains of Leishmania spp including L. tropica, L. major and L. infantom. In the in vivo studies, most of L. major strains were including (MHOM/64/IR/ER75), (MRHO/IR/75/ER), (MRHO/SU/59/P), (MRHO/IR/76/ER) and also most L. tropica strains were (MHOM/IR/2002/Mash2) and (MHOM/TN/80/IPI1), which were maintained in Balb/c mice and human. Therefore, we attempt to summarize a list of various studies in the list of herbs and natural products with antileishmanial activity (Table 1, Table 2). Briefly, most leishmanicidal agents studied from natural sources in Iran were Artemisia species, Allium sativum, Achillea millefolium (Yarrow), Peganum harmala and Thymus vulgaris. Heterogeneity test was conducted and Q statistic was very large (Q = 1945, df = 61, I-square = 100%, p < 0.001), showing that there was a great variation among studies. Based on random effect model, the pooled mean of IC 50 was obtained 456.64 (95% CI: 396.15, 517.12). Begg's test showed that there was no publication bias among all studies (t = 1.25, p = 0.215). Subgroup meta-analysis of characters such as stem bark, preparation, family and botanical name was carried out. The results showed that IC50 was significantly different among the parts used (or stem bark) (p < 0.001) and the “Aerial” and “Leaves or twigs” parts were most parts used. Also subgroup analysis revealed that there was a significant difference in preparing characters including: hexane, dichloromethane, hydroalcoholic, ethyl acetate with higher IC50 values and aqueous or methanolic with lower IC50 values (p < 0.001) (Table 3). The IC50 values for Allium spp, Alkanna spp and Artemisia spp showed the significant difference (p < 0.001), so that it could be with the highest value for Allium spp. For details on the models or mechanism-based bioassays utilized for selecting crude plant extracts, fractions and pure compounds against the Leishmania parasite, the original references should be consulted (Fig. 2).
Table 1.
Included publications of survey on the efficacy and activity of herbal medicines used against leishmaniasis in vitro in Iran.
| Family and botanical name | Preparation | Organism (strain) tested | Stem bark | Concentration | Exposure time | Result | Reference |
|---|---|---|---|---|---|---|---|
| Allium hirtifolium | Hydro alcoholic | L. infantum | Fruit | 0.01, 0.05, 0.1 and 0.2 mg/mL | For 7 days | Parasite growth at all concentrations was stopped after 3 days but at concentrations of 0.2 the first day was inhibited | [16] |
| A. aucheri | Methanolic | L. major | Aerial parts | 150, 300, 450, 600, and 750 μg/mL | 24, 48, and 72 h | 750 μg/mL methanolic extract of A. aucheri was able to kill about 25% of both developmental stages of the parasite after 72 h | [17] |
| Camellia sinensis | Methanolic | L. major | Green leaves | 150, 300, 450, 600, and 750 μg/mL hours | 24, 48 and 72 h | Methanolic extract of C. sinensis inhibited the parasite multiplication | |
| Mimosa tenuiflora | Methanolic | L. tropica | NR | 20,200,1000 and 2000 μg/mL | 72 h | Concentration of 1000 and 500 μg/mL suppressed multiplication of promastigotes but at a concentration of 100 μg/mL it accelerated growth of promastigotes. | [18] |
| Perovskia abrotanoides Karel | Methanolic | L. major (MRHO/IR/75/ER) | Root | 0.06, 0.12, 0.25, 0.5 and 1 mg/mL | 2, 4 and 6 days incubation | IC50 = 926, 723 and 550 μg/mL after 2, 4 and 6 days Of incubation |
[19] |
| P. abrotanoides Karel | Ethanolic | L. major (MRHO/IR/75/ER) | Root | 0.06, 0.12, 0.25 0.5 and 1 mg/mL | 2, 4 and 6 days incubation | IC50 = 213, 652 and 343 μg/mL after 2, 4 and 6 days of incubation, |
|
| Peganum harmala | Unknown | L. major (MRHO/SU/59/P) | Seed | 5000-20000 μg/mL and 62.5–500 μg/mL | 72 h | IC50° = 1832.65 ± 89.72 μg/mL | [20] |
| Ferula szowitsiana | Unknown | L. major | Root | 10,100, 500 and 1000 μg/mL | 48 h | IC50 = 4.9 μg/mL | [21] |
| Peganum harmala | Aqueous | L. major (MRHO/IR/75/ER) | Seeds | 20,40,100 and 200 μg/mL |
24, 48 and 60 h | IC50 P. harmala after 60 h = 0.7 μg/mL IC50 A. tinctoria after 60 h = 0.7 μg/mL IC50 combination of two extracts after 60 h = 0.6 μg/mL |
[22] |
| Alkanna tincturia | Chloroformic | L. major (MRHO/IR/75/ER) | Stems and roots | 20,40,100and 200 μg/mL |
24, 48 and 60 h | ||
| A. aucheri | Methanolic | L. major (MRHO/IR/76/ER) | Aerial parts | 31.25, 62.5, 125, 250, 500 and 5000 μg/mL | NR | IC50 = 7.5 μg/mL | [23] |
| F. asafoetid | Methanolic | L. major (MRHO/IR/76/ER) | Gum | 31.25, 62.5, 125, 250, 500 and 5000 μg/mL | NR | IC50 = 5.9 μg/mL | |
| Gossypium hirsutum | Methanolic | L. major (MRHO/IR/76/ER) | Boll | 31.25, 62.5, 125, 250, 500 and 5000 μg/mL | NR | IC50 = 3.6 μg/mL (better effect) | |
| Echinacea purpurea | Ethanolic | L. major (MRHO/IR/75/ER) | Root | 0.5, 2.5, 50 and 125 mg/mL | 8, 16, 24, 48 and 72 h | LD50 for the promastigotes were determined as 22,300, 16,700, 36,600, 19,800 and 1230 μg/mL at 8, 16, 24, 48 and 72 h respectively. 125,000 and 50,000 μg/mL concentrations of this extract were able to kill 100% of the parasite after 48 h |
[24] |
| Calendula officinalis | Aqueous | L. major (MRHO/IR/75/ER) | Flowers | 500, 250, 125 and 62.5 μg/ml | 24,48,72 h | IC50 was calculated for ethanolic & watery C. officinalis; 170 μg/mL, 215μg/mL after 24 h respectively. The extract at concentration of 500 μg/ml was found to kill all the parasites. |
[25] |
| C. officinalis | Ethanolic | L. major (MRHO/IR/75/ER) | Flowers | 500, 250, 125 and 62.5 μg/ml | 24,48,72 h | ||
| A. sativum | Aqueous | L. major (MRHO/IR/75/ER) | Small pieces | 0, 10, 20, 40, 60, 80, 100 μg/mL | 72 h | IC50 = 37 μg/mL. Cytotoxic effect in L. major with almost 100% death at a concentration of 93 μg/mL |
[26] |
| Satureja khuzestanica | Ethanolic | L. major (MRHO/IR/75/ER) | Aerial parts | 0.07–19.9 mg/ml | after 24 h | Ethanolic extract IC100 = 2400 μg/mL IC50 = 300 μg/mL. |
[27] |
| S. khuzestanica | Methanolic | L. major (MRHO/IR/75/ER) | Aerial parts | 0.07–19.9 mg/mL | after 24 h | Methanoic extract IC100 = 4800 μg/mL IC50 = 600 μg/mL. |
|
|
A. sativum |
Aqueous | L. major (MRHO/IR/75/ER) | Bulbs | (9.25, 18.5, 37, 74, 148 mg/mL | 18, 24 and 48 h | IC50 = 37,000 μg/mL. | [28] |
| Arnebia euchroma | Alcoholic | L. major (MRHO/IR/75/ER) | Root | 0.78, 1.5, 3.2, 6.5 and 12.5 mg/mL | 0, 24, 48, 72 and 96 h | The results showed a significant decrease in the number of Leishmania parasites over time was due to the effect of the extract. | [29] |
| Achillea millefolium | Alcoholic | L. major (MRHO/IR/75/ER) | Root | 0.78, 1.5, 3.2, 6.5 and 12.5 mg/mL | 0, 24, 48, 72 and 96 h | ||
| Green tea | Ethanolic | L. major (MRHO/IR/75/ER) | Leaves | 3, 6, 12, 24, 48 and 96 mg/mL | 24,48,72 h | The effect of different concentrations of the plant extract on Leishmania revealed that all the concentrations of this extract can reduce the number of Leishmania parasites | [30] |
| A. millefolium | Alcoholic | L. major (MRHO/IR/75/ER) | Leaves | 3, 6, 12, 24, 48 and 96 mg/mL | 0,24,48,72 h | ||
| Wormwood | Alcoholic | L. major (MRHO/IR/75/ER) | Flowers | 25 mg/mL | 0,24,48,72 h | The effect of different concentrations of the plant extract on Leishmania revealed that all the concentrations of This extract can reduce the number of Leishmania parasites. Plant extracts increased immobility parasite that this lack of mobility is directly related to time. |
[31] |
| Walnut leaves | Alcoholic | L. major (MRHO/IR/75/ER) | Leaves | 25 mg/mL | 0,24,48,72 h | ||
| Verbascum thapsus | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 452 ± 4.47 μg/mL | [32] |
| Caparis spinosa | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 375 ± 2.96 μg/mL | |
| Amarusthus rutroflena | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 275 ± 7.45 μg/mL | |
| Sesamum indium | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 245 ± 3.78 μg/mL | |
| A. absintin | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 280 ± 5.96 μg/mL | |
| Tribulus terresttis | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 ≥ 625 μg/mL | |
| Ficus bengalensis | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 200 ± 23.14 μg/mL | |
| Prosropis juliflorea | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 312 ± 14.625 μg/mL | |
| A. dracunculus | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 ≥ 625 μg/mL | |
| Paliurus spina Christi | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 ≥ 625 μg/mL, | |
| Rhamnus persica boiss | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 75 ± 13.44 μg/mL | |
| Caesalpinia gilliesii | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 9.76 ± 1.27 μg/mL | |
| Acacia faresiana | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 625 ± 12.75 μg/mL | |
| Satureia hortensis | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 15.625 ± 3.76 μg/mL | |
| Carum copticum heirm | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 15.625 ± 3.76 μg/mL | |
| Thymus migricus | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50 = 31.25 ± 15.44 μg/mL | |
| A. vulgari | Hydro alcoholic | L. major (MRHO/IR/76/ER) | Leaves or twigs | 5 to 500 μg/mL | After 28–30 h | IC50= >625 μg/mL | |
| Stachys lavandulifolia Vahl | Hydro alcoholic | L. major (MRHO/75/IR) | Aerial part | 50, 100, 250, 500 and 1000 μg/mL | NR | With increasing concentrations of S. lavandulifolia and leaves M. germanica extract reduced the number promastigotes. Efficacy of the two extract were not significant difference and almost have same effect on the average number of Leishmania promastigotes | [33] |
| Mespilus germanica | Hydro alcoholic | L. major (MRHO/75/IR) | Leaves | 50, 100, 250, 500 and 1000 μg/mL | NR | ||
| Calotropis gigantea | Methanolic | L. major | Aerial parts | 0.12, 0.25, 0.50 and 1.0 mg/mL | 24, 48, 72 h | IC50 = 96.3 μg/mL | [34] |
| C. gigantea | Hexane | L. major | Aerial parts | 0.12, 0.25, 0.50 and 1.0 mg/mL | 24, 48, 72 h | IC50 = 92.5 μg/mL | |
| C. gigantea | Aqueous | L. major | Aerial parts | 0.12, 0.25, 0.50 and 1.0 mg/mL | 24, 48, 72 h | IC50 = 11.3 μg/mL | |
| C. gigantea | Butanolic | L. major | Aerial parts | 0.12, 0.25, 0.50 and 1.0 mg/mL | 24, 48, 72 h | IC50 = 58.7 μg/mL | |
| Artimisinin | Ethanolic | L. major (MR HO/HR/75/ER) | Leaves | 10, 25, 50 and 100 μg/mL | incubated for 72 h | IC50 = 68.16 μg/mL | [35] |
| Artemisia annua | Ethanolic | L. major | Aerial parts | NR | NR | IC50 = 400 ± 0.8 μg/mL | [36] |
| A. annua | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 425 ± 1.5 μg/mL | ||
| A. annua | Dichloromethane | L. major | Aerial parts | NR | IC50 = 850 ± 0.9 μg/mL | ||
| A. annua | Hexane | L. major | Aerial parts | NR | IC50 = 1900 ± 2.4 μg/mL | ||
| A. biennis | Ethanolic | L. major | Aerial parts | NR | IC50 = 100 ± 0.9 μg/mL | ||
| A. biennis | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 425 ± 0.5 μg/mL | ||
| A. biennis | Dichloromethane | L. major | Aerial parts | NR | IC50 = 525 ± 1.1 μg/mL | ||
| A. biennis | Hexane | L. major | Aerial parts | NR | IC50 = 1050 ± 2.0 μg/mL | ||
| A. ciniformis | Ethanolic | L. major | Aerial parts | NR | IC50 = 25 ± 0.4 μg/mL The ethanol extracts of A. ciniformis has one of the most potent leishmanicidal activity. |
||
| A. ciniformis | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 340 ± 1.2 μg/mL | ||
| A. ciniformis | Dichloromethane | L. major | Aerial parts | NR | IC50 = 450 ± 1.0 μg/mL | ||
| A ciniformis | Hexane | L. major | Aerial parts | NR | IC50 = 790 ± 1.7 μg/mL | ||
| A. sieberi | Ethanolic | L. major | Aerial parts | NR | IC50 = 150 ± 1.0 μg/mL | ||
| A. sieberi | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 265 ± 0.7 μg/mL | ||
| A. sieberi | Dichloromethane | L. major | Aerial parts | NR | IC50 = 465 ± 0.8 μg/mL | ||
| A. sieberi | Hexane | L. major | Aerial parts | NR | IC50 = 850 ± 1.5 μg/mL | ||
| A. kulbadica | Ethanolic | L. major | Aerial parts | NR | IC50 = 25 ± 0.5 μg/mL The ethanol extracts of A. kulbadica has one of the most potent leishmanicidal activity. |
||
| A. kulbadica | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 275 ± 1.4 μg/mL | ||
| A. kulbadica | Dichloromethane | L. major | Aerial parts | NR | IC50 = 440 ± 0.7 μg/mL | ||
| A. kulbadica | Hexane | L. major | Aerial parts | NR | IC50 = 885 ± 1.8 μg/mL | ||
| A. santolina | Ethanolic | L. major | Aerial parts | NR | IC50 = 80 ± 0.8 μg/mL The ethanol extracts of A. santolina has one of the most potent leishmanicidal activity. |
||
| A. santolina | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 375 ± 1.1 μg/mL | ||
| A. santolina | Dichloromethane | L. major | Aerial parts | NR | IC50 = 675 ± 1.4 μg/mL | ||
| A. santolina | Hexane | L. major | Aerial parts | NR | IC50 = 850 ± 1.4 μg/mL | ||
| A. turanica | Ethanolic | L. major | Aerial parts | NR | IC50 = 200 ± 1.3 μg/mL | ||
| A. turanica | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 675 ± 2.1 μg/mL | ||
| A. turanica | Dichloromethane | L. major | Aerial parts | NR | IC50 = 425 ± 0.9 μg/mL | ||
| A turanica | Hexane | L. major | Aerial parts | NR | IC50 = 1120 ± 2.5 μg/mL | ||
| A. absinthium. | Ethanolic | L. major | Aerial parts | NR | IC50 = 500 ± 0.6 μg/mL | ||
| A. absinthium. | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 425 ± 1.3 μg/mL | ||
| A absinthium | Dichloromethane | L. major | Aerial parts | NR | IC50 = 600 ± 0.8 μg/mL | ||
| A. absinthium. | Hexane | L. major | Aerial parts | NR | IC50 = 1050 ± 2.5 μg/mL | ||
| A. fragrans | Ethanolic | L. major | Aerial parts | NR | IC50 = 1000 ± 2.0 μg/mL | ||
| A. fragrans | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 1375 ± 2.2 μg/mL | ||
| A. fragrans | Dichloromethane | L. major | Aerial parts | NR | IC50 = 475 ± 1.0 μg/mL | ||
| A. fragrans | Hexane | L. major | Aerial parts | NR | IC50 = 1150 ± 2.2 μg/mL | ||
| A. khorassanica | Ethanolic | L. major | Aerial parts | NR | IC50 = 400 ± 1.1 μg/mL | ||
| A. khorassanica | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 435 ± 0.7 μg/mL | ||
| A. khorassanica | Dichloromethane | L. major | Aerial parts | NR | IC50 = 500 ± 1.2 μg/mL | ||
| A. khorassanica | Hexane | L. major | Aerial parts | NR | IC50 = 790 ± 1.5 μg/mL | ||
| A. kopedaghensis | Ethanolic | L. major | Aerial parts | NR | IC50 = 50 ± 0.7 μg/mL | ||
| A. kopedaghensis | Ethyl acetate | L. major | Aerial parts | NR | IC50 = 255 ± 0.8 μg/mL | ||
| A. kopedaghensis | Dichloromethane | L. major | Aerial parts | NR | IC50 = 445 ± 0.5 μg/mL | ||
| A. kopedaghensis | Hexane | L. major | Aerial parts | NR | IC50 = 925 ± 1.6 μg/mL | ||
| A. seiberi | Aqueous | L. major (MRHO/IR/75/ER) | Aerial parts and roots | 5, 10, 25, 50 and 100 μg/mL | 24,48 and 72 h | IC50 = 25 μg/mL A. sieberi has a higher growth inhibitory effect on promastigotes but The cytotoxic effect of seven concentrations of Artemisia sieberi on uninfected splenic macrophages of Balb/c mice has h very low cytotoxic effect on uninfected and healthy macrophages |
[37] |
| A. sieberi | Aqueous | L. major (MRHO/IR/75/ER) | Aerial parts & root | 1, 5, 10,20 and 25% | 24, 48 and 72 h | That promastigotes in RPMI culture were killed completely under concentrations of 20% and 25% of Artemisia in the first day. Concentrations of 20% of Artemisia in the second day led to the complete elimination of amastigote of L. major in macrophages |
[38] |
| Scrophularia striata | Aqueous | L. major (MRHO/IR/75/ER) | Aerial parts & root | 1, 5, 10,20 and 25% | 24, 48 and 72 h | The parasites were killed by Scrophularia at the concentration of 25% within three days. Concentrations of 25% of Scrophularia in the third day led to the complete elimination of amastigote of L. major in macrophages |
|
| Indium curcumin | Unknown | L. major (MRHO/IR/75/ER) | Turmeric plant extracts | NR | NR | IC50 = 26 μg/mL IC50 standard (Amphotericine B) = 20 μg/mL I. curcumin with IC50 values of 26 μg/mL was more effective than other three test agents against Leishmania. |
[39] |
| Diacethyle curcumin | Unknown | L. major (MRHO/IR/75/ER) | Turmeric plant extracts | NR | NR | IC50 = 52 μg/mL | |
|
Gallium curcumin |
Unknown | L. major (MRHO/IR/75/ER) | Turmeric plant extracts | NR | NR | IC50 = 32 μg/mL | |
|
G. curcumin |
Unknown | L. major (MRHO/IR/75/ER) | Turmeric plant extracts | NR | NR | IC50 = 38 μg/mL | |
| Alkanna frigida | Ethyl acetate | L. major | Root limb | 62.5, 125, 250 and 500 μg/mL. | 24, 48, and 72 h | The inhibitory effects = 46% IC50 = 106 μg/mL |
[40] |
| A. frigida | Ethanolic | L. major | Root limb | 62.5, 125, 250 and 500 μg/mL | 24, 48, and 72 h | The inhibitory effects = 45% IC50 = 86 μg/mL |
|
| A.frigida | Chloroformic | L. major | Root limb | 62.5, 125, 250 and 500 μg/mL | 24, 48, and 72 h | The inhibitory effects = 13% IC50 value after 48 and 72 h = 330 and 68 μg/mL |
|
| A. frigida | Hexane | L. major | Root limb | 62.5, 125, 250 and 500 μg/mL | 24, 48, and 72 h | The inhibitory effects = 15% IC50 value= (384 μg/mL for 48 h) and (98 μg/mL 72 h). |
|
| Nerium oleander, ricinus communis, capsicum, almond powder | Unknown | L. major | Leaves and stems | 1/10, 1/100, 1/1000 and 1/10,000 | For 7 weeks | In terms of quantity, the number of promastigotes of Leishmania in the face of the herbal combination reduced compared with the control group | [41] |
| Artemether | Unknown | L. infantum (MHOM/TN/80/IPI1) | Ointment and injection | 0, 10, 25, 50, and 100 μg/mL. |
72 h | IC 50 = 25 μg/mL after 24 h | [42] |
| aloe-emodin | Unknown | L. major | Powder | 40, 80, 120 and 160 μg/mL | 24, 48 and 72 h | IC50 = 52.79 μg/mL. | [43] |
| Scrophularia striata | Aqueous | L. major | Aerial parts and root | 1, 5, 10,20 and 25% | 72 h | In treatment of promastigotes of L. major with S. striata extract at the concentration of 25%, the parasites were killed at the day three | [44] |
| Nigella sativa | Essential oil | L. major (MRHO/IR/75/ER) | Aerial parts | 0.1, 0.2, 0.4, 0.8, 1.2, 1.6 and 2% | 24, 48 and 72 h | There was a significant difference in reducing parasites on groups receiving Satureia hortensis and N. sativa with Glucantime | [45] |
| S. hortensis | Essential oil | L. major (MRHO/IR/75/ER) | Aerial parts | 0.1, 0.2, 0.4, 0.8, 1.2, 1.6, and 2% | 24, 48 and 72 h | ||
| A. cepa | Aqueous | L. major | Root | 0.312, 2.5 and 5 mg/mL | 24 and 48 h | The viability of the L. major promastigotes in the concentration of 312 μg/mL aqueous onion extracts was 80% and in the same concentration, 20% of the L. major promastigotes were unmovable. At the concentration of 2500 μg/mL aqueous A. cepa extracts, 70% of the L. major promastigotes were unmovable and the viability of promastigotes was 30%. Moreover, in the concentration of 5000 μg/mL of aqueous A. cepa extracts, 100% of the L. major promastigotes were unmovable and the viability of parasites in this concen-tration was 0%. IC50 = 1250 μg/mL IC100 = 5000 μg/mL |
[46] |
| Ixora brachiata | Ethanolic | L. major | Root | 0.312, 2.5 and 5 mg/mL | 24 and 48 h | In addition, at the concentra-tion of 2500 μg/mL ethanolic and methanolic extracts of I. brachiata root, 100% of the L. major promastigotes were unmovable and the viability of the L. major promasti-gotes in the same concentration was 0%.in the concentration of 5000 μg/mL of aqueous A. cepa extracts, 100% of the L. major promastigotes were unmovable and the viability of parasites in this concen-tration was 0%. Ethanolic extract of Ixora brachiata root IC50 = 78 μg/mL IC100 = 2500 μg/mL Aqueous A. cepa = IC50 = 1250 μg/mL IC100 = 5000 μg/mL |
|
| Hyssopus officinalis | Alcoholic | L. major (MRHO/IR/75/ER) | Leaves | 0, 05, 0.1, 0.2, 0.4 and 1 μg/mL | 24, 48 and 72 h | That extract was effective | [47] |
| Tussilago farfara | Alcoholic | L. major (MRHO/IR/75/ER) | Leaves | 0, 05, 0.1, 0.2, 0.4 and 1 μg/mL | 24, 48 and 72 h | That extract was effective | |
| Carum copticum | Alcoholic | L. major (MRHO/IR/75/ER) | Seed | 0, 05, 0.1, 0.2, 0.4 and 1 μg/mL | 24, 48 and 72 h | That extract was effective | |
| B. vulgaris | Methanolic | L. tropica (MHOM/IR-/2002/Mash2) | Aerial parts | Between 5 and 100 μg/mL and 1–10 μg/mL | for 48 h at 37 °C | Inhibitory effects against promastigote forms IC50 = 16.1 μg/mL Inhibitory effects against amastigote forms IC50 = 39.4 μg/mL |
[48] |
| B. vulgaris | Aqueous | L. tropica (MHOM/IR-/2002/Mash2) | Aerial parts | Between 5 and 100 μg/mL and 1–10 μg/mL | for 48 h at 37 °C | Inhibitory effects against promastigote forms IC50 = 26.6 μg/mL Inhibitory effects against amastigote forms IC50 = 59.2 μg/mL |
|
| B. vulgaris | Methanolic | L. infantum (MCAN/IR/07/Moheb-gh) | Aerial parts | Between 5 and 100 μg/mL and 1–10 μg/mL | for 48 h at 37 °C | Inhibitory effects against promastigote forms IC50 = 13.2 μg/mL Inhibitory effects against amastigote forms IC50 = 52.8 μg μg/mL |
|
| B. vulgaris | Aqueous | L. infantum (MCAN/IR/07/Moheb-gh) | Aerial parts | Between 5 and 100 μg/mL and 1–10 μg/mL | for 48 h at 37 °C | Inhibitory effects against promastigote forms IC50 = 21.6 μg/mL Inhibitory effects against amastigote forms IC50 = 3.9 μg/mL |
|
| Vinca major | Chloroformic | L. major | Leaves and stems | 0,150, 300, 450, 600, 750 μg/mL | 0,24,48,72 and 96 h | At a concentration of 750 mg/mL after 96 h led to destroy all parasites | [49] |
| A. sativum | Methanolic | L. tropica (MHOM/IR/2002/Mash2) | Bulbs | 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL | 72 h incubation | The IC50 values methanolic extracts of garlic IC50 = 12.3 μg/mL The IC50 values aqueous extracts of garlic IC50 = 19.2 l μg/mL The IC50 values methanolic extracts of garlic CC50 = 291.4 μg/mL The IC50 values aqueous extracts of garlic CC50 = 348.2 μg/mL |
[50] [51] |
| A. sativum | Aqueous | L. tropica (MHOM/IR/2002/Mash2) | Bulbs | 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL | 72 h incubation | ||
| Myrtus communis | Essential oil | L. tropica (MHOM/IR/2002/Mash2)(pro&ama) | Leaves | 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL | 72 h incubation | The IC50 values for essential oil and methanolic extract was 8.4 and 28.9 μg/mL against promastigotes, respectively. These values were 11.6 and 40.8 μg/mL against amastigote forms, |
|
| M.communis | Methanolic | L. tropica (MHOM/IR/2002/Mash2) | Leaves | 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL | 72 h incubation | ||
| N. sativa | Essential oil | L. tropica | Aerial parts | 0–200 μg/mL | 72 h incubation | Inhibitory effects against promastigote forms IC50 = 9.3 μg/mL Inhibitory effects against amastigote forms IC50 = 21.4 μg/mL |
[52] |
| N. sativa | Methanolic | L. tropica | Aerial parts | 0–200 μg/mL | 72 h incubation | Inhibitory effects against promastigote forms IC50 = 14.8 μg/mL Inhibitory effects against amastigote forms IC50 = 30.8 μg/mL |
|
| N. sativa | Essential oil | L. infantum | Aerial parts | 0–200 μg/mL | 72 h incubation | Inhibitory effects against promastigote forms IC50 = 11.7 μg/mL Inhibitory effects against amastigote forms IC50 = 26.3 μg μg/mL |
|
| N. sativa | Methanolic | L. infantum | Aerial parts | 0–200 μg/mL | 72 h incubation | Inhibitory effects against promastigote forms IC50 = 15.7 μg/mL Inhibitory effects against amastigote forms IC50 = 34.6 μg/mL |
|
| Kelussia odoratissim | Essential oil | L. major (MRHO/IR/75/ER) | Aerial parts | 7.5, 15, 25, 35.25 and 50 μl | 24, 48 and 72 h | Higher concentrations (35.25 and 50 μl/mL had a stronger effect on promastigotes, causing total mortality. | [53] |
| Cordia myxa | Mucilage | L. major | Fruits | 0.6,1.2,2.4,4.8,9.6,19.5,39,78 and 176 mg/mL | for 72 h | IC50 = 26,000 μg/mL | [54] |
| C. myxa | Mucilage | L. infantum | Fruits | 0.6,1.2,2.4,4.8,9.6,19.5,39,78 and 176 mg/mL | for 72 h | IC50 = 35,000 μg/mL | |
| Caparis spinosa | Methanolic | L. major (MRHO/IR/75/ER) | Root | 0.1, 0.3, 0.5, 0.7 and 0.9 mg/mL | 24, 48 and 72 h | It was determined that anti-protozoal activity of Caparis extract (900 μg/mL) was able to kill 97.8% of promastigotes after 72 h | [55] |
| Pistacia khinjuk | Alcoholic | L. tropica (MHOM/IR/2002/Mash2) & L. major (MRHO/IR/75/ER) | Fruits | 0–100 μ/mL | for 48 h | Promastigote: IC50 = 58.6 ± 3.15 μg/mL Amastigote: IC50 = 37.3 ± 2.51 μg/mL |
[56] |
| Eucalyptus camaldulensis | Aqueous | L. major (MRHO/SU/59/P) | Leaves | 25, 50, 312.5, 625 and 1,250 | 72 h | IC50 = 1108.6 ± 51.9 μ/mL , | [57] |
| E. camaldulensis | Methanolic | L. major (MRHO/SU/59/P) | Leaves | 25, 50, 312.5, 625 and 1,250 | 72 h | IC50 = 586.2 ± 47.6 μ/mL Methanolic extract was more effective than aqueous extract. This extract were less effective as compared to the control drμg |
|
| A. absinthium, | Methanolic | L. major (MROH/IR/75/IR) | Leaves | 31.25–1000 μg/mL. | 0-30 days | IC50 = 159.45 μ/mL Toxicity for macrophage cell line=10% |
[58] |
| Vitex agnuscastus | Methanolic | L. major (MROH/IR/75/IR) | Leaves | 31.25 to 1000 μg/mL. | 0-30 days | IC50 = 234.15 μ/mL Toxicity for macrophage cell line = 8% |
|
| Phytolaca americana | Methanolic | L. major (MROH/IR/75/IR) | Fruits | 31.25 to 1000 μg/mL. | 0-30 days | IC50 = 171.1 μ/mL Toxicity for macrophage cell line = 12% |
IC50: concentration of drμg that causes 50% growth inhibition of amastigote or promastigote forms of Leishmania.
IC100: concentration of drμg that causes 100% growth inhibition of amastigote or promastigote forms of Leishmania.
CC50: as the Cytotoxic concentration of the extracts to cause death to 50% of viable cells in the host.
LD50: (Lethal Dose, 50%) It is the amount of the substance required (usually per body weight) to kill 50% of the test population.
NR: Not reported.
Table 2.
Included publications of survey on the efficacy and activity of herbal medicines used against leishmaniasis in vivo in Iran.
| Family and botanical name | Preparation | Organism tested | Stem bark | Animals kind | Concentration | Result | Reference |
|---|---|---|---|---|---|---|---|
| Z-HEa | Crude extract | L. major | - | Human | Topical | In the group treated with Z-HE (group A), complete cure was observed in 74.4% (Figure 1, Figure 2), partial cure in 11.6%, and failure in 14.0%. In the group treated with meglumine antimoniate (Glucantime) (group B), complete cure was observed in 24.1%, partial cure in 14.1%, and failure in 58.8% | [59] |
| A. sativum | Aqueous | L. major | Bulbs | Mice (Balb/c) | Mices were subjected to 300,000 promastigotes. lesion was measured on days 1, 10, 20, 30 and 45 |
The diameter of lesion was reduced by aqueous extract of garlic within 30 days of treatment. However, the maximum reduction was induced when mice were subjected to vitamin A for 10 days before the administration of the aqueous extract for 30 days. A significant correlation between healing and the amount of NO release was also found. | [60] |
| Berberis vulgaris | Alcoholic | L. major | Leaves, stems and roots | Mice (Balb/c) | 2.5, 4.0, 5.5 and 7.0% | The results showed that after 2 weeks, a statistically significant decrease of ulcer size of treated mice observed, while in the control group the lesion growth continued. The examinations showed that using higher concentration of the extract caused more decrease in surface area of CL lesions on day 15 and negative direct smear on day 20. Alcoholic extract of B.vulgaris root was more effective than leaves and stem extract. | [61] |
| Eucalyptus globulus | Essence | L.major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Essence 10% | The essences reduced the diameter of lesions or caused small lesions to disappear completely. | [62] |
| Myrtus communis | Essence | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Essence 10%, 20% | No change was noticed in the size of the lesions or the number of parasites. | |
| Ferula gumosa | Essence | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Essence 10%, 20% | No change was noticed in the size of the lesions or the number of parasites. | |
| A. herbaalba | Essence | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Essence 10%, 20% | No change was noticed in the size of the lesions or the number of parasites. | |
| A. sativum | Tincture | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Tentor 50&100% | No change was noticed in the size of the lesions or the number of parasites. | |
| Urtica dioica | Crude extract | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Pure extract | No change was noticed in the size of the lesions or the number of parasites. | |
| A. dracunculus | Essence | L. major (MRHO/IR/76/ER) | NR | Mice (Balb/c) | Essence 10% | The essences of A. dracunculus reduced the diameter of lesions or caused small lesions to disappear completely. | |
| Cassia Fistula | Concentrated boiled | L. major | Fruits | Human | – | Mean healing time was 4.6 ± 3.7 weeks | [63] |
| C. Fistula | Hydro alcoholic | L. major | Fruits | Human | – | Mean healing time was 4.9 ± 3.8 weeks. There was no significant difference between the efficacy of concentrated boiled extract and that of the hydro alcoholic extract of the Cassia fistula | |
| Berberis vulgaris | Alcoholic | L. major | Stem skin | Mice (Balb/c) | 20,40,80% for 30 days | With the 20% preparation: by the end of the treatment period, the mean diameter of the lesions had decreased, with complete healing in 5 mice (27.7%), (p < 0.001). by the time of the decrease in diameter, the mean weight of the animals had increased and the number of parasites in the lesions had declined (80%). Total elimination of the parasites was observed in 12 animals (p < 0.001). At a concentration of 40%: mean ulcer diameter decreased, with complete healing in 2 mice (11.1%, p < 0.001). By the time of the decrease in diameter, the mean weight of the mice had increased (p < 0.05). The mean number of parasites in lesions decreased (64.3%), with total elimination in 9 animals (p< 0.05). |
[64] |
| Thymus vulgaris | Hydro alcoholic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | Mean of ulcer size reduction = 36.09% that T. vulgaris, hydro alcoholic extracts were significantly more effective in reduction of ulcer size as compared with Glucantim | [65] |
| Achillea millefolium | Hydro alcoholic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | Mean of ulcer size reduction = 43.29% that A. millefolium hydro alcoholic extracts were significantly more effective in reduction of ulcer size as compared with Glucantim | |
| propolis | Hydro alcoholic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | Mean of ulcer size reduction = 43.77% that propolis hydro alcoholic extracts were significantly more effective in reduction of ulcer size as compared with Glucantim | |
| Rubia Tinctorium | Dry extract | L.major (MRHO/IR/76/ER) | Roots | Mice (Balb/c) | 40, 60 and 80% | The mean weight of the mice that received 40, 60 and 80% concentrations of R. tinctorum extracts showed a statistically significant difference compared to the control group the mean of lesion size of the mice extracts concentrations showed no statistically significant difference compared to the control | [66] |
| A.sieberi | Hydro alcoholic | L. major (MHOM/64/IR/ER75) | NR | Mice (Balb/c) | 1,3,5% after 30 days | At the end of the 30 day treatment period with concentrations of Artemisia, any of the mice treated with complete remission were observed. And microscopic examination of samples taken from the animals tested were positive. | [67] |
| Thyme | Hydro alcoholic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | Observed significant difference between mean of lesion diameter before and after treatment in control, Yarrow and Thyme groups Paired t-test showed no significant difference between mean of lesion diameter after treatment between treatment and Glucantime groups | [68] |
| Thyme | Hydro alcoholic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | ||
| A. millefolium | Hydro alcoholic | L. major (MHOM/64/IR/ER75) | NR | Mice (Balb/c) | NR | Ulcer dimeter Before treatment = 4/35 ± 0/5 mm Ulcer dimeter after treatment = 3/4 ± 0/67 mm The mean ulcer size in the group receiving thyme has been good impact on preventing The process of development of wound |
[69] |
| T. vulgaris | Hydro alcoholic | L. major (MHOM/64/IR/ER75) | NR | Mice (Balb/c) | NR | Ulcer dimeter Before treatment = 5/39 ± 0/41 mm Ulcer dimeter after treatment = 4 ± 0/34 mm The mean ulcer size in the group receiving yarrow has been good Impact on preventing The process of development of wound |
|
| Henna | Hydro alcoholic | L. major (MHOM/64/IR/ER75) | NR | Mice (Balb/c) | NR | Ulcer dimeter Before treatment = 5.77 ± 0/30 mm Ulcer dimeter after treatment = 7/11 ± 0/56 mm Were did not showen statistical significant difference between mean diameter of lesions after treatment with the treated group with Plant extracts and treated with Glucantime |
|
| A. sativum | Hydro alcoholic | L. major (MHOM/64/IR/ER75) | NR | Mice (Balb/c) | NR | Ulcer dimeter Before treatment = 5.18 ± 0/47 mm Ulcer dimeter after treatment = 7/06 ± 1/09 mm Were did not showen statistical significant difference between mean diameter of lesions after treatment with the treated group with Plant extracts and treated with Glucantime |
|
| Pistacia Atlantica | Unknown | L. major (MHROM/IR/75/ER) | Gum obtioned of trunk and branches | Mice (Balb/c) | 0,4,8 week | Gum daily for 28 days decreased skin lesion size in the mice infected with L.major compared with that in the control. Treatment Balb/c mice with gum obtained P. atlantica var. kurdica and Glucantime causes decrease number of parasitologically positive mice | [70] |
| E. camaldulensis | Methanolic | L. major (MRHO/IR/75/ER) | NR | Mice (Balb/c) | NR | Amastigote number into the lesions, were significantly decreased, nanogold solutions were also decreased mortality rate in the mice | [71] |
| Satureja khuzestanica | Essential oil | L. major MRHO/IR/75/ER | Aerial parts | Mice (Balb/c) | 0.01, 0.001, 0.0001% for 7 week | Lesions' size in SKEO treated groups was restrained but not significantly different from the control group the mortality rate in treated groups was clearly less than the control. | [71] |
| A. sativum | Aqueous | L. major | Bulbs | Mice (Balb/c & 57BL/6 and Suri) | Promastigote injection and evaluated For 8 week | The results showed that R10 had good therapeutic efficacy in treatment of lesions in mice (P < 0.05) that this efficacy was significant in sixth, seventh and eighth weeks after the treatment | [72] |
| Echinacea purpurea | Hydro alcoholic | L. major | Aerial parts | Mice (Balb/c) | The mean of lesion size in each group of mice were compared and analyzed. No significant differences in the lesions size were found between the three mice groups. Therefore, E. purpurea extract was not effective against L. major based on the findings of this study. | [74] | |
| Mespilus germanica | Ethanolic | L. major | Leaves | Mice (Balb/c) | 40, 60 and 80% | Extract of M. germanica has the highest effectiveness in concentration of 40%, causing greater reductions in both ulcer diameter and the number of parasites in the lesions compared with other prepared concentrations | [75] |
| A. aucheri Boiss | Methanolic | L. major MRHO/IR/75/ER(IR/75) | NR | Mice (Balb/c) | 0.09, 0.36, 1.44, 6, 28 mg/kg for 30 days | The results indicated that herbal extract was able to affect on lesion size, its performance and to prevent visceralization of the parasite. This is the first report indicating visceralization caused by the cutaneous form of L. major in the Balb/c mice | [76] |
| N. oleander, r. communis, capsicum, almond powder | Unknown | L. major | Stem and seed | Mice (Balb/c) | 1/10,1/100,1/1000,1/10,000 | This skin lesion at the base of the tail of mice under investigation also indicate a significant effect on the composition of the herbal form wound and skin nodule at the base of the tail of mice treated with the control group. | [41] |
| Artemether | Unknown | L. infantum ( MHOM/TN/80/IPI1) | Ointment and injection | Mice (Balb/c) | NR | In vivo experiments indicated that oral artemether treatment of mice, during 3 days and every 6 h (0.625 mg/kg) was more significant than parenteral (0.625 mg/kg IP) treatment | [42] |
| artemether | Unknown | L. major | Ointment and injection | Mice (Balb/c) | NR | Mean diameter of lesion in the infected group treated with ointment of artemether decreased from 1.294 to 0.214 cm mean diameter of lesion in the infected group treated with artemether injection decreased from 0.913 to 0.256 cm | [77] |
| Echium amoenum | Aqueous | L. major (MRHO/75/IR) | Flower | Mice (Balb/c) | 0.1, 0.25, 0,50, 1, 2, 4 and 5 mg/mL | Increased the level of IFN-γ and lowered the parasite burden in the proximal lymph nodes and prevented the necrosis of the footpad as compared with the untreated infected mice. | [78] |
| E. amoenum | Alcoholic | L. major (MRHO/75/IR) | Flower | Mice (Balb/c) | 0.1, 0.25, 0,50, 1, 2, 4 and 5 mg/mL | ||
| Seidlitzia rosmarinus | Hydro alcoholic | L. major (MRHO/75/IR) | leaves | Mice (Balb/c) | 5, 10 and 5 15% | Significant increase in the lesion size of treated mice compared with reference group except for treated group by 15% extract | [79] |
| Peganum harmala | Aqueous | L. major (MRHO/IR/75/ER) | Ground seed | Mice (Balb/c) | NR | In the aqueous extract group only 10% of mice healed. | [80] |
| P. harmala | Ethanolic | L. major (MRHO/IR/75/ER) | Ground seed | Mice (Balb/c) | NR | In the ethanolic extract group only 40% of mice healed. Results showed that ethanol extract of P. harmala had good therapeutic efficacy in treatment of lesions in mice | |
| V. major | Cloroformic | L. major | Leaves and stems | Mice (Balb/c) | NR | Injection was very effective By the prevention of ulcers caused by Leishmania major in Balb/c mice compared to untreated control | [49] |
| S. iastriata | Aqueous | L. major | Aerial parts& root | Mice (Balb/c) | 1, 5, 10,20, 25% for 3 days | Extract concentration (25%) at the concentration at the first day, the extract led to a decrease in the parasite rate to 36 amastigotes. For all of the concentrations (10%, 20%, 25%) we observed completely elimination of amastigotes on the third day. | [44] |
| Hedera helix | Alcoholic | L. major (MHOM/64/IR/ER75) | Leaves | Mice (Balb/c) | 20% and 70% for 30 days. | Study showed that the main lesion size did not decrease significantly, or the small lesions did not completely disappear after treatment by H. helix alcoholic extract. Amastigotes counts (mean ± SD) of the skin lesions decreased in control A and 20% concentration groups, but in negative control and 70% concentration groups the number of parasites did not reduce. | [81] |
| Pistacia khinjuk | Alcoholic | L. tropica (MHOM/IR/2002/Mash2) & L. major (MRHO/IR/75/ER) | Fruits | Mice (Balb/c) | NR | In vivo = after 30 days of treatment, 75 and 87.5% recovery were observed in the infected mice treated with 30% extract and meglumine antimoniate, respectively, while P. khinjuk extract at the concentration of 20% recovered 50% of the infected mice. | [82] |
| C. spinosa | Methanolic | L. major MRHO/IR/75/ER | Root | Mice (Balb/c) | 0.1,0.3,0.5,0.7,0.9 mg/mL for 24, 48 and 72 h | It was determined that 700 μg/ml and 900 g/ml Caparis root extract concentrations were more effective than other concentrations on amastigotes of Leishmania in ulcers. The results were sμggestive that Caparis root extract had significantly similar effect in reduction of ulcer size as compared to Glucantim | [55] |
| Matricaria chamomilla | Hydro alcoholic | L. major | Flower | Mice (Balb/c) | – | The comparison of these three groups revealed that wound healing in group one and group two were 58.3% and 80% respectively, which was significant whereas no healing was seen in the control group | [83] |
| A. absinthium | Methanolic | L. major (MROH/IR/75/IR) | Leaves | Mice (Balb/c) | daily for 30 days repeated 3 times |
A. absinthium extract was statistically significant The lesion size in different groups mice after 30 day = 9.9 ± 2.4 μg/mL |
[58] |
| Vitex agnuscastus | Ethanolic | L. major (MROH/IR/75/IR) | Leaves | Mice (Balb/c) | daily for 30 days repeated 3 times | The lesion size in different groups mice after 30 day = 13.1 ± 2.8 μg/mL | |
| Phytolaca americana | Methanolic | L. major (MROH/IR/75/IR) | Fruits | Mice (Balb/c) | daily for 30 days repeated 3 times | The lesion size in different groups mice after 30 day = 15.3 ± 2.6 μg/mL |
Mixture of Althaea rosa, Althaea officinalis, and Pharmacology, Pathology, and members of the families Leguminosae, Faliaceae, Malvaceae, and Lythraceae.
Table 3.
Results of subgroup meta-analysis for the mean of IC50 separately characteristics.
| Characteristics | n | IC50 | 95% CI |
I-squared | P | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Stem bark | Aerial parts | 44 | 553.10 | 470.09 | 636.11 | 100.00% | P<0.001 |
| Root limb | 8 | 123.43 | 23.18 | 247.07 | 98.7% | ||
| Bulbs | 3 | 22.83 | 4.00 | 41.66 | 100% | ||
| Leaves or twigs | 3 | 422.36 | 393.00 | 451.72 | 99.8% | ||
| Preparation | Ethanol | 9 | 251.33 | 137.64 | 365.03 | 89.32% | P<0.001 |
| Ethyl acetate | 11 | 448.00 | 337.96 | 558.04 | 97.01% | ||
| Dichloromethane | 10 | 531.82 | 457.96 | 605.68 | 100.00% | ||
| Hexane | 11 | 910.92 | 781.92 | 1039.93 | 99.30% | ||
| Methanolic | 3 | 59.75 | 16.12 | 103.38 | 93.28% | ||
| Aqueous | 4 | 23.89 | 5.88 | 41.90 | 89.36% | ||
| Chloroformic | 3 | 132.90 | 12.99 | 252.81 | 100.00% | ||
| Hydroalcoholic | 3 | 510.00 | 490.77 | 529.23 | 100.00% | ||
| Botanic name | Artemisia spp | 49 | 8.0 | 7.4 | 8.6 | 97.1% | P<0.001 |
| Allium spp | 4 | 14.2 | 13.7 | 14.6 | 99.7% | ||
| Alkanna spp | 6 | 9.4 | 8.3 | 10.6 | 98.5 | ||
n: sample size.
Figure 2.
Based on random effect meta-analysis (Q = 1945, df = 61, I-square = 100%, p < 0.001) pooled mean of IC50 was obtained 456.64 (95% CI: 396.15, 517.12). Begg's test showed there is no evidence publishing bias among studies (t = 1.25, p = 0.215).
4. Discussion
Leishmaniasis is an important parasitic disease all around the world. For reducing the resistance in endemic areas, alternative strategies including the use of herbal plants are considered [84], [85]. The present study showed a wide range of plant extracts with antileishmanial properties in vitro and in vivo experiments. Among all medicinal plants, the genus Artemisia (Astraceae) is a large, heterogeneous, and widely dispersed genus all over the world. These species are small shrubs biennial and perennial or annual herbs. The genus Artemisia has 30 species in Iran out of which two are endemic [86]. Artemisia plants contain chemical components such as sesquiterpenes, monoterpenes lactones, flavonoides, coumarins, sterols and polyacetylenes [86]. Artemisis species has cytotoxic and anti-inflammatory activity [86], [87], [88]. The results of a study carried out by Niloofarzadeh et al. (2008), showed that hydroalcoholic extracts of propolis Thymus vulgaris and Achillea millefolium were significantly more effective than systemic glucantime or alcoholic extract for the treatment of dermal leishmaniasis in Balb/c mice. The highest efficacy was observed for propolis, followed by Achillea millefolium and then Thymus vulgaris [65]. The efficacy of ethanol extract of the root leaves and stem of Berberis vulgaris were topically used on experimental dermal lesions of Balb/c mice. The result after two weeks statistically revealed a significant reduction of ulcer size in mice [89]. Doroodgar et al. (2008) reported the effect of various concentrations of Artemisia essence in Balb/c mice. They showed that cutaneous lesions in mice inoculated by L. major were enlarged after the application of higher concentration of the Artemisia essence. As a result, the lesions did not heal, and their size increased. In addition, parasitologic examination also remained positive [67]. The result showed that the size of lesion in mice received 40, 60, and 80% of Rubia. tinctorum extracts revealed no significant difference in comparison with the lesion size in control group [66]. Seidlitzia rosmarinus (S. rosmarinus) has been traditionally used in Mashhad and its suburbs for the treatment of CL. Despite little available data about the possible efficacy of this plant against leishmaniasis, the efficacy of herbal extracts of S. rosmarinus against cutaneous leishmaniasis in Balb/c mice was examined in this study. The natives in Khorasan Province used pure dried leaves' powder of S rosmarinus leaves on their cutaneous lesions. Therefore, alcoholic extract of stem and leaves, which is almost similar to the pure powder, was used in this study. In this study, Eucerine was used as a base for the extracts; however, the results could be different if the researcher used vaseline or lanoline as a base for transdermal delivery of herbal extract [61]. The ulcer size in Balb/c mice received Eucerine alone was significantly increased more than other groups which approved the previous suggestion.
The administration form of a drug is also important. In the present study, the extracts were topically used as an ointment, but the results would be different if the extracts were administered intralesionaly. Recent studies have shown that nanoparticles of anti-leishmanial drugs are highly effective to treat CL. The important advantages of such drugs are low dosage and minimum adverse reactions [90].
In the present systematic review and meta-analysis, the Begg's test showed no publication bias among all studies (t = 1.25, p = 0.215). In addition, subgroup analysis revealed that there was a significant difference in extracting preparation including hexane, dichloromethane, hydroalcoholic and ethyl acetate with higher IC50 values, and aqueous or methanolic with lower IC50 values. (p < 0.001) (Table 3).
However, several studies have demonstrated that the hexane and ether acetate extracts present low or no toxicity to host cells at the effective concentrations [91], [92]. Ribeiro et al. (2014) evaluated anti-leishmania activity of 44 extracts and fractions derived from 16 Brazilian plant species against L. amazonensis. Among them, the most potent extracts were the hexanic extract [92].
In general, the ethanolic extracts were less effective and more toxic than the hexanoic extracts and buthanolic, dichloromethane ethyl acetate and hexanic fractions in the mammalian cells [93]. Thus, the application of the hexanic extract against Leishmania parasites as a potent fraction is recommended in the in vivo experiments.
In the study of Hooshyar et al. (2014), a significant decrease was shown in the main lesion size, or the small lesions were not completely disappeared after treatment by Hedera helix (H.helix) alcoholic extract. Their results disagreed with those of Talari et al. who used, 100 and 50 mg/mL of H. helix extract and observed that all promastigotes of L. major were killed in vitro [94]. This difference of findings may be due to different preparation methods and concentration of the plant extract was used in two studies. Different extract was gathered from eleven Iranian Artemisia species. Their leishmanicidal activities against the growth of L. major showed that ethanol extracts especially those taken from A. ciniformis, A. santolina and A. kulbadica had the strongest effects [86]. In the present study, they demonstrated the inhibitory effect of different extracts from eleven Artemisia species on the growth of L. major promastigotes in vitro. It was previously reported that the aqueous extract and essential oil of A. herbaalba had antileishmanial activity against L. tropica and L. major promastigotes [37]. In addition, the aqueous extract of leaves of A. indica exhibited leishmanicidal activity (IC50 = 430 μg/mL) [95]. Here, some of tested Artemisia spp showed most strong antileishmanial activities. In this study, all tested extracts exhibited antileishmanial activity after incubation, however, ethanol extracts from A. ulbadica and A. ciniformis showed the stronger leishmanicidal activity at value of (IC50 = 25 μg/mL). Growth inhibitory effect of ethanol extract of other plants such as Haplo phylum myrtifolium against L. tropica promastigotes were previously reported (IC50 = 10.9 μg/mL) [96]. Comparing the antileishmanial effect of non-polar extracts revealed that ethyl acetate extract of A. fragrans had less antileishmanial activity against L. major promastigotes. Ethyl acetate extracts of studied Artemisia species (except for A. turanica and A. fragrans) were also more active in comparison with their dichloromethane extract. In vitro antileishmanial activity of ethyl acetate and dichloromethane extracts of Ircinia spinosula (IC50 = 16.09, 47.38 μg/mL) were reported against L. major promastigotes [97]. The lethal dose (LD50) of dichloromethane extract and hexane extract of Calophyllum brasiliense on L. amazonensis promastigotes were 40 mg/mL and 20 mg/mL, respectively [98]. In comparison with other extracts, Artemisia studying species hexane extracts (except for A. fragrans) were less active than L. major. Hexane extracts of A. biennis, A. annua, A. turanica, A. fragrans and A. absinthium were less effective than other species. Other investigators have also reported lower activity of hexane extracts of plants than Leishmania species in comparison with other extracts. For example, ethanol extracts of Arbutus unedo significantly decreased L. tropica promastigotes counts [99].
Leishmanicidal activity of Allium sativum (garlic extract) has been established against infection with L. major, so that it can induce a Th1-type response, stimulate INF-γ and NO production in macrophage and thus prevent the progression of the infection [73], [28]. To improve the therapeutic efficacy and reduce toxicity, above mentioned natural molecules can be applied as either scaffold for producing and exploring new immune drugs or natural immunomodulators in synergy and in combination with existing drugs [100], [101]. Targeting anti-leishmanial drugs to macrophages with drug delivery systems reflects a hopeful strategy overcoming the problems associated with the current treatment protocols.
Another important issue is the safety of natural remedies. Although natural immune therapy in different generations has been tested and approved, it is necessary to prove the overall pharmacological safety of the correction. Chemical agents in Iranian drug market have disadvantages such as high cost and side effects. Considering the effectiveness of these plants would make them as a source of natural and safe agents for the treatment of leishmaniasis. However, anti -leishmanial drugs or natural compounds are safe when their selectivity index is more than 10 [50].
5. Conclusion
In conclusion, the present review showed that a range of plant extracts had effects on promastigote stage of Leishmania and interesting antileishmanial properties exhibited in vitro and in vivo. Therefore, it might be possible to use the extracts instead of chemical drugs. However, almost all of the authors claimed successful results about their investigated plants, but their studies really had limitations which affected with the accuracy of their results. Some of defects included in these studies are described in detail as lacking of randomized double blind clinical trials in all of human based studies. Also some of investigations were performed in vitro and were not performed in vivo [102], [103]. The period of exposure of extracts was not enough in some of the studies [104] and at last in one study, the toxicity level of the plant investigated was very high for testing in volunteer patients [105]. Most of data published were obtained from animal model and were not tested on human [106]. According to all documented data, phytotherapy has provided a large and hopeful vision to new, safe, and effective leishmaniacidal agents. Nevertheless, it needs to generalize all results obtained from in vitro and in vivo studies on the efficacy of plant extracts, metabolites or formulations against different Leishmania species to validate their activities. We concluded that the mechanism of action was enhancing the hosts' cellular immunity.
The present systematic investigation on anti-leishmanial activity of the medicinal plants together with their toxicity, mechanism of action and chemical properties for improvement is the most favorable formulation urgently required to confirm their efficacy in the treatment of leishmaniasis.
As a whole, the present systematic review provide valuable information about the natural products with anti -leishmanial activity which would be very favorable for experimental and clinical trials and herbal combination therapy studies. Consequently, further clinical researches are needed to establish the effective and safe medicinal plants therapy. It is necessary to find their active components, and potential toxic effects would lead to producing the well-tolerated and safe drugs for leishmaniasis.
Ethical approval
This project was funded by Mazandaran University of Medical Sciences (no:2025).
Sources of funding
There has been no financial support for this work that could have influenced its outcome.
Author contribution
Study concept: Mahdi Fakhar.
Data collection: Masoud Soosaraei.
Data interpretation: Masoud Soosaraei, Mahdi Fakhar, Saeed HosseiniTeshnizi, Hajar Ziaei Hezarjaribi.
Writing the paper: Masoud Soosaraei, Mahdi Fakhar.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Guarantor
Mahdi Fakhar.
Research Registration Unique Identifying Number (UIN)
Research registry 2025.
Acknowledgments
The authors wish to acknowledge all researchers that their publications were used in our review. This project was funded by Mazandaran University of Medical Sciences (no:2025).
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ashford R., Desjeux P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 3.Hepburn N.C. Cutaneous leishmaniasis: an overview. J. Postgrad. Med. 2003;49:50. doi: 10.4103/0022-3859.928. [DOI] [PubMed] [Google Scholar]
- 4.Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 5.Camacho M., Phillipson J., Croft S., Solis P., Marshall S., Ghazanfar S. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol. 2003;89:185–191. doi: 10.1016/s0378-8741(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 6.Sen R., Chatterjee M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine. 2011;18:1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Jameel M., Islamuddin M., Ali A., Afrin F., Ali M. Isolation, characterization and antimicrobial evaluation of a novel compound N-octacosan 7 β ol, from Fumaria parviflora Lam. BMC Complement. Altern. Med. 2014;14:1. doi: 10.1186/1472-6882-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfan B., Bahmani M., Hassanzadazar H., Saki K., Rafieian-Kopaei M. Identification of medicinal plants affecting on headaches and migraines in Lorestan Province, West of Iran. Asian Pac. J. Trop. Med. 2014;7:376–379. doi: 10.1016/S1995-7645(14)60261-3. [DOI] [PubMed] [Google Scholar]
- 10.Hadighi R., Mohebali M., Boucher P., Hajjaran H., Khamesipour A., Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izzo A.A., Hoon-Kim S., Radhakrishnan R., Williamson E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016;30(5):691–700. doi: 10.1002/ptr.5591. [DOI] [PubMed] [Google Scholar]
- 12.Asadbeigi M., Mohammadi T., Rafieian-Kopaei M., Saki K., Bahmani M., Delfan M. Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: an ethnobotanical study in the Urmia. Asian Pac. J. Trop. Med. 2014;7:364–368. doi: 10.1016/S1995-7645(14)60259-5. [DOI] [PubMed] [Google Scholar]
- 13.Bahmani M., Saki K., Ezatpour B., Shahsavari S., Eftekhari Z., Jelodari M., Rafieian-Kopaei M., Sepahvand R. Leishmaniosis phytotherapy: review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pac. J. Trop. Biomed. 2015;5(9):695–701. [Google Scholar]
- 14.Karamati S.A., Hassanzadazar H., Bahmani M., Rafieian-Kopaei M. Herbal and chemical drugs effective on malaria. Asian Pac. J. Trop. Dis. 2014;4:599–601. doi: 10.1016/S1995-7645(14)60200-5. [DOI] [PubMed] [Google Scholar]
- 15.Bahmani M., Rafieian-Kopaei M. Medicinal plants and secondary metabolites for leech control. Asian Pac. J. Trop. Dis. 2014;4:315–316. [Google Scholar]
- 16.Amanzadeh Y., Izaddoost M., Soltanpoor A., Mahami M., Taheri M., Kalantari N., Taran M., Sadat Ebrahimi S. Inhibit effect of Allium hirtifolium boiss. (Persian shallot) hydroalcoholic extract on the growth of Leishmania infantum in vitro. J. Med. Plants. 2006;4:48–52. [Google Scholar]
- 17.Sharif M., Ziaei H., Azadbakht M., Daryani A., Ebadattalab A., Rostami M. Effect of methanolic extracts of Artemisia aucheri and Camellia sinensis on Leishmania major (in vitro) Turk. J. Med. Sci. 2006;36(6):365–369. [Google Scholar]
- 18.Shamsedini S.R.A.S., Mirzaei M., Brofei M. Efficacy of Mimosa Tenuiflora extract on growth of Leishmania protozoa in vitro. Iran. J. Dermatol. 2006;9:175–180. [Google Scholar]
- 19.Jaafari M.R., Hooshmand S., Samiei A., Hossainzadeh H. Evaluation of-leishmanicidal effect of Perovskia abrotanoides Karel.root extract by in vitro leishmanicidal assay using promastigotes of Leishmania major. Pharmacol. 2007;1:299–303. [Google Scholar]
- 20.Mirzaie M., Nosratabadi S.J., Derakhshanfar A., Sharif I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Veter. Arh. 2007;77(4):365–375. [Google Scholar]
- 21.Iranshahi M., Arfa P., Ramezani M., Jaafari M.R., Sadeghian H., Bassarello C., Piacente S., Pizza C. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochem. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi R., Ghaffarifar F., Asl A.D. The effect of Alkanna tincturia and Peganum harmala extracts on Leishmania major (MRHO/IR/75/ER) in vitro. Iran. J. Parasitol. 2009;4:40–47. [Google Scholar]
- 23.Barati M., Sharifi I., Sharififar F. Antileishmanial activity of Artemisia aucheri, Ferula asa-foetid and Gossypium hirsutum extracts on Leishmania major promastigotes in vitro. Ann. Mil. Health Sci. Res. 2010;8(3):166–172. [Google Scholar]
- 24.Soudi S., Hashemi S.M., Zavaran Hosseini A., Ghaemi A., Asghari Jafarabadi M. Antileishmanial effect of Echinacea purpurea root extract cultivated in Iran. Iran. J. Pharm. Res. 2010;6(2):147–149. [Google Scholar]
- 25.Maspi N., Ghafarifar F., Bahrami A., Bastaminejad S., Shamsi M. Evaluation of leishmanicidal effect of watery & ethanolic flowers Calendula officinalis extract on promastigotes of leishmania major (MRHO/IR/75/ER) in Vitro. J. Ilam. Univ. Med. Sci. 2010;18:28–33. [Google Scholar]
- 26.Khademvatan S., Saki J., Gharavi M.J., Rahim F. Allium sativum extract induces apoptosis in Leishmania major (MRHO/IR/75/ER) promastigotes. J. Med. Plant Res. 2011;5:3725–3732. [Google Scholar]
- 27.Sadeghi-Nejad B., Saki J., Khademvatan S., Nanaei S. In vitro antileishmanial activity of the medicinal plant Satureja khuzestanica Jamzad. J. Med. Plant Res. 2011;5(24):5912–5915. [Google Scholar]
- 28.Gharavi M., Nobakht M., Khademvatan S., Bandani E., Bakhshayesh M., Roozbehani M. The effect of garlic extract on expression of INFγ and inos genes in macrophages infected with Leishmania major. Iran. J. Parasitol. 2011;6:74. [PMC free article] [PubMed] [Google Scholar]
- 29.Sozangar N., Jeddi F., Reaghi S., Khorrami S., Arzemani K. Abulkhalsa and Yarrow plant effect on Leishmania major in vitro. J. N. Khorasan Univ. Med. Sci. 2012;4:329–333. [Google Scholar]
- 30.Feily A., Saki J., Maraghi S., Moosavi Z., Khademvatan S., Siahpoosh A. In vitro activity of green tea extract against Leishmania major promastigotes. Int. J. Clin. Pharmacol. Ther. 2012;50:233–236. doi: 10.5414/cp201571. [DOI] [PubMed] [Google Scholar]
- 31.Yektaian N., Rafieian M., Khalili-Dehkordi B., Hejazi S.H., Shirani- Bidabadi L., Hosseini S.A. Effect of combination of Achillea millefolium, Artemisia absinthium & Juglans regia leaves extracts on Leishmania major (MRHO/IR/75/ER), in vitro. J. Med. Plants. 2012;11:197–204. [Google Scholar]
- 32.Manjili H.K., Jafari H.R., Ramazani A., Davoudi N. Anti-leishmanial and toxicity activities of some selected Iranian medicinal plants. Parasitol. Res. 2012;111(5):2115–2121. doi: 10.1007/s00436-012-3059-7. [DOI] [PubMed] [Google Scholar]
- 33.Asadi M., Bahrami S., Ansari Samani R., Pakniat N. Effect of hydroalcoholic extracts of Stachys lavandulifolia Vahl and Mespilus germanica leaves on Leishmania major. Bimon. J. Hormozgan Univ. Med. Sci. 2012;15:279–284. [Google Scholar]
- 34.Oskuee R., Jafari M., Farzad S.A., Ramezani M. In vitro Leishmanicidal activity of Calotropis gigantea and its fractions against Leishmania major. J. Med. Plant Res. 2012;6(23):3977–3983. [Google Scholar]
- 35.Heidari F.I., Ghaffarifar F., Dalimi A., Dehkordi N.M., Nikoo S.G. In vitro study of the effect of artimisinin on promastigotes andamastigotes of Leishmania major. Modares J. Med. Sci. Pathobiol. 2012;15:33–43. [Google Scholar]
- 36.Emami S.A., Rabe S.Z.T., Ahi A., Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran. J. basic Med. Sci. 2012;15:807. [PMC free article] [PubMed] [Google Scholar]
- 37.Heydari F.E., Ghaffarifar F., Soflaei S., Dalimi A. Comparison between in vitro effects of aqueous extract of Artemisia seiberi and artemisinin on Leishmania major. Jundishapur J. Nat. Pharm. Prod. 2013;8:70. [PMC free article] [PubMed] [Google Scholar]
- 38.Dalimi A., Arbabi M., Naserifar R. The effect of aqueous extraction of Artemisia sieberi Besser and Scrophularia striata Boiss. on Leishmania major under in vitro conditions. Iran. J. Med. Arom Plant. 2013;29(1):237–246. [Google Scholar]
- 39.Fouladvand M., Barazesh1 A., Tahmasebi R. Evaluation of in vitro antileishmanial activity of curcumin and its derivatives “Gallium curcumin, Indium curcumin and Diacethyle curcumin. Euro Rev. Med. Pharmacol. Sci. 2013;17(24):3306–3308. [PubMed] [Google Scholar]
- 40.Jafari F., Nourian A., Fazaeli A., Yazdinezhad A., Haniloo A. In vitro activity of Alkanna frigida extracts in comparison with glucantime against Leishmania major. Iran. J. Microb. 2013;5(2):177. [PMC free article] [PubMed] [Google Scholar]
- 41.Yakhchali M., Ranjbariki-Jandabeh M. Effects of Nerium oleander leaf, Ricinus communis oil, Capsicum spp. seeds, and almond compound on cutaneous leishmaniasis caused by Leishmania species under laboratory condition and its effect on cutaneous lesion progression in mice. Sci. J. Kurdistan Univ. Med. Sci. 2013;18:13–19. [Google Scholar]
- 42.Dehkordi N.M., Ghaffarifar F., Mohammad Hassan Z., Esavand Heydari F. In vitro and in vivo studies of anti leishmanial effect of artemether on Leishmaniainfantum. Jundishapur J. Microb. 2013;6(5):e6379. [Google Scholar]
- 43.Delavari M., Dalimi A.A., Ghaffarifar F., Sadraei J. Effect of aloe-emodin on growth and induction of apoptosis in Leishmania major promastigotes in vitro. Feyz J. Kashan Univ. Med. Sci. 2013;17:422–428. [Google Scholar]
- 44.Nokhodi F., Bandani E., Kooshki H., Eftekhari M., Mahmoudi R., Mansouri M., Jafari A.A. Medicinal plant Scrophulariastriata evaluation anti-parasitic effects on Leishmania major: in vitro and in vivo study. Biosci. Biotech. Res. Asia. 2014;11(2):627–634. [Google Scholar]
- 45.Pirali K.K., Dehghani S.A., Adel M., Hoseinpour F. The effect of essential oil of nigella sativa and satureia hortensis on promastigot stage of leishmania major. Armaghane-danesh. 2013;18:687–698. [Google Scholar]
- 46.Sadeghi-Nejad B., Saki J., Azish M. Effect of aqueous Allium cepa and Ixora brachiata root extract on Leishmania major promastigotes. Jundishapur J. Nat. Pharm. Prod. 2014;9(2):e15442. doi: 10.17795/jjnpp-15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maleki F., Tabatabaie F., Keighobadi A., Golestani M., Shahmohammad M., Voosoogh M., Noori M., Mokhtarian K. Antileishmanial activity of Hyssopusofficinalis, Tussilagofarfara, Carumcopticum extracts in comparison with glucantime in Iran. J. Med. Plants. 2014;2(5):12–18. [Google Scholar]
- 48.Mahmoudvand H., Sharififar F., Sharifi I., Ezatpour B., Fasihi Harandi M., Makki M.S. In vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, berberine against different Leishmania species. Iran. J. Parasitol. 2014;9:28–36. [PMC free article] [PubMed] [Google Scholar]
- 49.Assmar M., Farahmand M., Aghighi Z., Ghaemi N., Ayatollahi A.M. In vitro and in vivo evaluation of therapeutic effects of Vinca major alkaloids on Leishmania major. J. Sch. Public Health Inst. Public Health Res. 2003;1:1–8. [Google Scholar]
- 50.Mahmoudvand H., Sepahvand P., Jahanbakhsh S., Azadpour M. Evaluation of the antileishmanial and cytotoxic effects of various extracts of garlic (Allium sativum) on Leishmania tropica. J. Parasit. Dis. 2014:1–4. doi: 10.1007/s12639-014-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoudvand H., Ezzatkhah F., Sharififar F., Sharifi I., Dezaki Saedi. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. Korean J. Parasitol. Vol. 2015;53(1):21–27. doi: 10.3347/kjp.2015.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoudvand H., Tavakoli R., Sharififar F., Minaie K., Ezatpour B., Jahanbakhsh S. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm. Biol. 2015;53:1052–1057. doi: 10.3109/13880209.2014.957784. [DOI] [PubMed] [Google Scholar]
- 53.Kheirabadi K.P., Dehkordi S.S., Kheibari P. Effect of Kelussia odoratissima Mozaff essential oil on promastigot form of Leishmania major (in vitro) J. Herb. Med. Pharmacol. 2015;4(1):10–14. [Google Scholar]
- 54.Saki Khademvatan S.H., Pazyar N., Eskandari A., Tamoradi A., Nazari P. In vitro activity of Cordiamyxa Mucilage extract against Leishmania major and L. infantum promastigotes. Jundishapur J. Microbiol. 2015;8(3):e19640. doi: 10.5812/jjm.19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shemshadi B., Ranjbar-Bahadory S., Ahmadi H. Effect of Caparis spinosa root extract on promastigotes and amastigotes of Leishmania major. J. Paramed. Sci. 2015;6(1):18–21. [Google Scholar]
- 56.Ezatpour B., Dezaki E.S., Mahmoudvand H., Azadpour M., Ezzatkhah F. In vitro and in vivo antileishmanial effects of pistacia khinjuk against Leishmania tropica and Leishmania major. Evid. Based Complement. Alter Med. 2015;2:6. doi: 10.1155/2015/149707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nosratabadi S.J., Sharifi I., Sharififar F., Bamorovat M., Daneshvar H., Mirzaie M. In vitro antileishmanial activity of methanolic and aqueous extracts of Eucalyptus camaldulensis against leishmania major. J. Parasit. Dis. 2015;39(1):18–21. doi: 10.1007/s12639-013-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanjani Jafroodi S., Farazmand A., Amin M., Doroodgar A., Shirzadi M., Razavi M. Methanolic Extract’s Activity of Artemisia absinthium, Vitex agnus-castus and Phytolaca americana against Leishmania major; in vitro and in vivo. Int. Arch. Heal Sci. 2015;2:69–74. [Google Scholar]
- 59.Zerehsaz F., Salmanpour R., Handjani F., Ardehali S., Panjehshahin M.R., Tabei S.Z., Tabatabaee H.R. A double-blind randomized clinical trial of a topical herbal extract (Z-HE) vs. systemic meglumine antimoniate for the treatment of cutaneous leishmaniasis in Iran. Int. J. Dermatol. 1999;38:610–612. doi: 10.1046/j.1365-4362.1999.00727.x. [DOI] [PubMed] [Google Scholar]
- 60.Ahmadi-Renani K., Mahmoodzadeh A., Cheraghali A., Esfahani A. Effect of garlic extract on cutaneous leishmaniasis and the role of nitric oxide. IJMS. 2002;27:97–100. [Google Scholar]
- 61.Fata A., Rakhshandeh H., Berenji F., Jalilianfard A. Treatment of cutaneous Leishmaniasis in murine model by alcoholic extract of Berberis vulgaris. Iran. J. Parasitol. 2006;1:39–42. [Google Scholar]
- 62.Babaee Khou L., Mohebali M., Niakan Lahiji M.R., Mehrabi Tavana A. The therapeutic effect of Eucalyptus, Myrtus, Ferula, Aretmisia, Allium and Urtica extracts against cutaneous leishmaniasis caused by Leishmanaia major in small white mice (out-bred) Hakim. 2007;10:21–27. [Google Scholar]
- 63.Jaffary F., Nilforoushzadeh M.A., Moradi S.H., Derakhshan R., Ansari N. The efficacy of topical treatment of concentrated boiled extract and hydroalcoholic extract of Cassia Fistula in comparison to the intralesional injection of meglumine antimoniate in the treatment of acute cutaneous Leishmaniasis. J. Skin. Leishman. 2010;1(1):23–27. [Google Scholar]
- 64.Kazemi E., Talari S., Hooshyar H. The effect of an alcoholic extract of Berberis Vulgaris on Cutaneous leishmaniasis (L. major) in BALB/c mice. J. Sch. Publ. Heal a Inst. Publ. Heal Res. 2007;5:35–42. [Google Scholar]
- 65.Nilforoushzadeh M.A., Shirani-Bidabadi L., Zolfaghari-Baghbaderani A., Saberi S., Siadat A.H., Mahmoudi M. Comparison of Thymus vulgaris (Thyme), Achillea millefolium (Yarrow) and propolis hydroalcoholic extracts versus systemic glucantime in the treatment of cutaneous leishmaniasis in balb/c mice. J. Vector Borne Dis. 2008;45:301–306. [PubMed] [Google Scholar]
- 66.Bafghi A.F., Noorbala M.T., Hejazian S.H. The effect of Rubia tinctorium extract on cutaneous leishmaniasis in BALB/c mice. World J. Zool. 2008;3:25–29. [Google Scholar]
- 67.Doroodgar A., Arbabi M., Razavi M., Mohebali M., Sadr F. Treatment of cutaneous leishmaniasis in murine model by hydro alcoholic essence of Artemisia sieberi. J. Arthropod Dis. 2008;2:42–47. [Google Scholar]
- 68.Shirani-Bidabadi L., Mahmoudi M., Saberi S., Zolfaghari-Baghbaderani A., Nilforoushzadeh M., Abdoli H., Moatar F. The effectiveness of mix extracts of Thyme, Yarrow and Propolis on Cutaneous Leishmaniasis: a comparative study in animal model (Balb/c) Tehran Univ. Med. Sci. 2009;66(11):785–790. [Google Scholar]
- 69.Hejazi S.H., Shirani-Bidabadi L., Zolfaghari-Baghbaderani A., Saberi S., Nilforoushzadeh M.A., Moradi S.H. Comparison effectiveness of extracts of Thyme, yarrow, henna and garlic on cutaneous leishmaniasis caused by L. major in animal model (Balb/c) J. Med. Plants. 2009;8:129–136. [Google Scholar]
- 70.Taran M., Mohebali M., Esmaeli J. In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iran. J. Publ. Health. 2010;39:36. [PMC free article] [PubMed] [Google Scholar]
- 71.Torabi N., Mohebali M., Shahverdi A.R., Rezayat S.M., Edrissian G.H., Esmaeili J., Charehdar S. Nanogold for the treatment of zoonotic cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an animal trial with methanol extract of Eucalyptus camaldulensis. J. Pharm. Sci. 2011:113–116. [Google Scholar]
- 72.Kheirandish F., Delfan B., Farhadi S., Ezatpour B., Khamesipour A., Kazemi B., Ebrahimzade F., Rashidipour M. The effect of Satureja khuzestanica essential oil on the lesions induced by Leishmania major in BALB/c mice. Afr. J. Pharm. Pharmacol. 2011;5:648–653. [Google Scholar]
- 73.Ghaffarifar F., Mahmmoudzadeh Poornaki A., Ghazanfari T., Naseri M., Khmesipoor A., Naeini A., Mojabi F.S. Evaluation treatment of cutaneous Leishmaniasis with Garlic extract and R10 fraction in BALB/c, C57BL/6 and outbred SW mice. Modares J. Med. Sci. Pathobio. 2011;14:25–34. [Google Scholar]
- 74.Sadati M., Sarkari B., Asgari Q., Hatami S., Tavakl E. Effect of Echinacea purpurea on control of leishmania major induced cutaneous leishmaniasis in mice. Armaghan Danesh. 2011;16(1):31–40. [Google Scholar]
- 75.Shariatifar N., Rahimnia R., Jamshidi A., Pirali H.M., Shoeibi S. Effect of ethanolic extract of Mespilus germanica on cutaneous leishmaniasis in balb/c mice. J. Med. Plant. 2011;3(39):76–81. [Google Scholar]
- 76.Rostami M., Nahrevanian H., Farahmand M., Ziaee H., Sharif M., Maghsudloorad F.S. Evaluation of anti-leishmanial efficacy by extract of Artemisia auchery Boiss. on Leishmania major in Balb/c. J. Herb. Drug. 2012;7(2):269–274. [Google Scholar]
- 77.Ebrahimi-Sadr P., Ghaffarifar F., Hassan-Saraf Z.M., Beheshti N. Effect of artemether on the recovery of lesions caused by Leishmania major. KAUMS J. (FEYZ) 2013;16:529–535. [Google Scholar]
- 78.khoshzaban F., Ghaffarifar F., Mahmmoudzadeh Poornaki A., Ghazanfari T., Naseri M., Khmesipoor A., Naeini A., Mojabi F.S. Evaluation treatment of cutaneous Leishmaniasis with Garlic extract and R10 fraction in BALB/c, C57BL/6 and outbred SW mice. Pathobiol. Res. 2011;14(3):25–34. [Google Scholar]
- 79.Ahmadi M., Fata A., Khamesipour A., Rakhshandeh H., Mohammadi A.M., Salehi G., Monavari H. The efficacy of hydro alcoholic extract of Seidlitzia rosmarinus on experimental zoonotic cutaneous leishmaniasis lesions in murine model. Avicenna J. Phytomed. 2014;4:385. [PMC free article] [PubMed] [Google Scholar]
- 80.Khoshzaban F., Ghaffarifar F., Koohsari H.R.J. Peganum harmala aqueous and ethanol extracts effects on Lesions caused by Leishmania major (MRHO/IR/75/ER) in BALB/c mice. Jundishapur J. Microb. 2014;7(7):e10992. doi: 10.5812/jjm.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hooshyar H., Talari S.A., Feyzi F. Therapeutic effect of Hedera helix alcoholic extract against cutaneous Leishmaniasis caused by Leishmania major in Balb/c mice. Jundishapur J. Microb. 2014;7(4) doi: 10.5812/jjm.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ezatpour B., Dezaki E.S., Mahmoudvand H., Azadpour M., Ezzatkhah F. In vitro and in vivo antileishmanial effects of pistacia khinjuk against Leishmania tropica and Leishmania major. Evid. Based Complement. Alter Med. 2015;2:6. doi: 10.1155/2015/149707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dashtpeima A.R., Manzoori L., Arefkhah N., Shahryari S., Mohseni M., Abbasi M. The effect(s) of Matricaria chamomilla on Leishmania major ulcers in Balb/c mice. Armaghane-danesh, Yasuj Univ. Med. Sci. J. (YUMSJ) 2015;20(2):127–137. [Google Scholar]
- 84.Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isnard A., Shio M.T., Olivier M. Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front. celld Inf. Microb. 2012;2:72. doi: 10.3389/fcimb.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emami S.A., Taghizadeh Rabe S.Z., Iranshahi M., Ahi A., Mahmoudi M. Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacol. Immunotoxicol. 2010;32:688–695. doi: 10.3109/08923971003677808. [DOI] [PubMed] [Google Scholar]
- 87.Mahmoudi M., Rabe S.Z.T., Ahi A., Emami S.A. Evaluation of the cytotoxic activity of different Artemisia khorassanica samples on cancer cell lines. Pharmacol. Online. 2009;2:778–786. [Google Scholar]
- 88.Taghizadeh Rabe S.Z., Mahmoudi M., Ahi A., Emami S.A. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm. Biol. 2011;49:962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 89.Fata A.E., Haririzadeh G., Ghahramani S., Tavakoli R. Study the effect of juice, extract and active ingredient of Euphorbia myrsinites on growth of promastigotes in NNN medium. Med. J. Mashhad Univ. Med. Sci. 1993;36:30–36. [Google Scholar]
- 90.Tafaghodi M., Khamesipour A., Jaafari M.R. Immunization against leishmaniasis by PLGA nanospheres loaded with an experimental Autoclaved Leishmania major (ALM) and Quillajasaponins. Trop. Biomed. 2010;27:639–650. [PubMed] [Google Scholar]
- 91.Rodrigues I.A., Azevedo M.M., Chaves F.C., Alviano C.S., Alviano D.S., Vermelho A.B. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. Biomed. Res. Int. 2014:985171. doi: 10.1155/2014/985171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribeiro T.G., Chavez-Fumagalli M.A., Valadares D.G., Franca J.R., Lage P.S., Duarte M.C., Andrade P.H., Martins V.T., Costa L.E., Arruda A.L., Faraco A.A., Coelho E.A., Castilho R.O. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp. Parasitol. 2014;143:60–68. doi: 10.1016/j.exppara.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Oryan A., Alidadi S., Akbari M. Risk factors associated with leishmaniasis. Trop. Med. Surg. 2014;2:e118. [Google Scholar]
- 94.Talari S.A., Dorodgar A., Ouzougi J. In vitro effect of various concentrations of Hedera Helix extract and Glucantime on visceral Leishmaniosis. KAUMS J. 1997;1:61–67. [Google Scholar]
- 95.Ganguly S., Bandyopadhyay S., Bera A., Chatterjee M. Antipromastigote activity of an ethanolic extract of leaves of Artemisia indica. Indian J. Pharmacol. 2006;38:64–65. [Google Scholar]
- 96.Özbel Y., Özbilgin A. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol. 2007;30:439–445. [PubMed] [Google Scholar]
- 97.Kahla-Nakbi AB, Haouas N, El Ouaer A, Guerbej H, Mustapha KB, Babba H. Screening of antileishmanial activity from marine sponge extracts collected off the Tunisian coast. Parasitol. Res. 106: 1281–1286. [DOI] [PubMed]
- 98.Honda P.A., Ferreira I.C.P., Cortez D.A.G., Amado C.A.B., Silveira T.G.V., Brenzan M.A., Lonardoni M.V.C. Efficacy of components from leaves of Calophyllum brasiliense against Leishmania amazonensis. Phytomedicine. 2010;17:333–338. doi: 10.1016/j.phymed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Kivçak B., Mert T., Ertabaklar H., Balcioğlu I.C., Toz S.O. In vitro activity of Arbutus unedo against Leishmania tropica promastigotes. Turk. Parasitol. Derg. 2009;33:114–115. [PubMed] [Google Scholar]
- 100.Jain K., Jain N.K. Novel therapeutic strategies for treatment of visceral leishmaniasis. Drug Discov. Today. 2013;18:1272–1281. doi: 10.1016/j.drudis.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Prajapati V.K., Awasthi K., Gautam S., Yadav T.P., Rai M., Srivastava O.N., Sundar S. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J. Antimicrob. Chemother. 2011;66:874–879. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dutta A., Mandal G., Mandal C., Chatterjee M. In vitro antileishmanial activity of Aloe vera leaf exudate: a potential herbal therapy in leishmaniasis. Glycoconj J. 2007;24:81–86. doi: 10.1007/s10719-006-9014-z. [DOI] [PubMed] [Google Scholar]
- 103.Peraza-Sanchez S.R., Cen-Pacheco F., Noh- Chimal A., May-Pat F., Sima-Polanco P., Dumonteil E., García-Miss M.R., Mut-Martín M. Leishmanicidal evaluation of extracts from native plants of the Yucatan peninsula. Fitoterapia. 2007;78:315–318. doi: 10.1016/j.fitote.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 104.Monzote L., Montalvo A.M., Scull R., Miranda M., Abreu J. Activity, toxicity and analysis of resistance of essential oil from Chenopodium ambrosioides after intraperitoneal, oral and intralesional administration in BALB/c mice infected with Leishmania amazonensis: a preliminary study. Biomed. Pharmacother. 2007;61:148–153. doi: 10.1016/j.biopha.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Cui Z., Guo Z., Miao J., Wang Z., Li Q., Chai X., Li M. The genus Cynomorium in China: an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2013;147:1–15. doi: 10.1016/j.jep.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 106.Ngure P.K., Tonui W.K., Ingonga J., Mutai C., Kigondu E., Ng’ang’a Z. In vitro antileishmanial activity of extracts of Warburgia ugandensis (Canellaceae), a Kenyan medicinal plant. J. Med. Plants Res. 2009;3(2):61–66. [Google Scholar]