Abstract
Objective
To determine risk factors for multi-drug-resistant Acinetobacter baumannii (MDR-AB) nosocomial infections in intensive care units in a tertiary care hospital, Makkah, Saudi Arabia.
Methods
We performed a hospital-based, matched case–control study in patients who were admitted to Al Noor Specialist Hospital between 1 January 2012 and 31 August 2012. The study included cases of A. baumannii nosocomial infection and controls without infection. Controls were matched to cases by age and ward of admission.
Results
The most frequent site of infection was the respiratory tract (77.3%). Susceptibility to antimicrobial MDR-AB was 92.0% for ceftazidime and ciprofloxacin, while it was 83.3% for imipenem, 83.0% for trimethoprim, 79.0% for amikacin, and 72.7% for gentamicin. Multiple logistic regression of risk factors showed that immunosuppression (OR = 2.9; 95% CI 1.5–5.6; p = 0.002), clinical outcome (OR = 0.4; 95% CI 0.3–0.9; p = 0.01), invasive procedures (OR = 7.9; 95% CI 1.8–34.2; p = 0.002), a central venous catheter (OR = 2.9; 95% CI 1.5–5.6; p = 0.000), and an endotracheal tube (OR = 3.4; 95% CI 1.6–7.3; p = 0.001) were associated with MDR-AB.
Conclusions
Acinetobacter nosocomial infections are associated with admission to the ICU (Intensive care unit) and exposure to invasive procedures.
Keywords: Risk factors, nosocomial infection, multi-drug-resistant Acinetobacter baumannii, intensive care unit
Introduction
More than 2 million people, or approximately 5% to 10% of hospitalized patients, are affected by nosocomial infections with an estimated 90,000 deaths every year.1,2 As well as the disease burden regarding significant morbidity and mortality of nosocomial infections, high healthcare costs are incurred in managing nosocomial infections. A study that was performed in Rhode Island Hospital showed that the cost of patients with hospital-acquired infections was more than three times higher than that of those without infectious diseases.3 Nosocomial infections are usually transmitted by poor hygiene practice, followed by the provision of outpatient treatment and invasive medical procedures. Patients’ impaired defence against bacteria (e.g., because of pre-term birth or immunodeficiency) increases the chance of infection.4 Additionally, because medical staff are associated with patients in different units, they may carry and spread pathogens.5
One of the major gram-negative bacteria responsible for nosocomial infections is Acinetobacter. Acinetobacter may cause severe pneumonia and infections of the urinary tract, bloodstream, and other parts of the body. Acinetobacter is primarily found in hospitalized patients with a reduced immune defence who are affected by drug-resistant gram-negative germs. These bacteria can survive on surfaces in the hospital for a long time and attack the body through wounds and invasive devices. According to recent data from the U.S. National Healthcare Safety Network, more than 30% of hospital-acquired infections are due to gram-negative bacteria, and the majority of ventilator-associated pneumonia (47%) and urinary tract infection (45%) cases are associated with these bacteria.6 Acinetobacter baumannii also causes community-acquired infections, albeit at a lower percentage than hospital-acquired infections.
Management of A. baumannii infections may be difficult because of intrinsic resistance to some antibiotics, as well as new mechanisms of resistance throughout treatment.7 These infections form a large proportion of hospital infections. A report from Turkish hospitals showed that 16.6% of nosocomial infections were due to A. baumannii, with the majority (68.9%) of them in ICU (Intensive care unit) patients; more than half (52.5%) of these patients suffered from respiratory tract infections.8 A. baumannii is the most common species of genus,9 which is widely present in the hospital environment. A. baumannii can cause severe or fatal illnesses, especially in critically ill patients with low immune responses,5 and can increase patient mortality along with hospital costs. Therefore, multi-drug resistance is a major public health emergency. Additionally, A. baumannii is a species of pathogenic bacteria and is an aerobic gram-negative bacterium known for resistance to most antibiotics. A. baumannii can survive in dry environments for weeks, which enables transmission through contaminants in hospitals.10 Patients, who are the main reservoirs of A. baumannii, can contaminate attending staff, leading to further cross-transmission11 Therefore, hospitals are thought to be the main source of A. baumannii infections. A study carried out in Spain showed that more than 90% of A. baumannii infections were of nosocomial origin, and only 4% originated from the community.12
While antimicrobial agents are considered as a solution for infectious disease, resistance of microorganisms to various drugs has raised new problems, especially for hospital-acquired infections. There has been an increasing amount of research conducted on factors related to the transmission of such infections. However, multi-drug resistance makes the illnesses more serious because of limited treatment options.
Several studies have identified general characteristics of patients that place them at increased risk for acquisition of multi-drug-resistant outbreak strains.4 However, the diversity of risk factors suggests that separate investigations should be performed in each hospital setting.7 Therefore, associated factors contributing to infection should be assessed to apply basic prevention and control measures.
Methods
Study design and setting
We performed a hospital-based, retrospective case–control study to determine the demographic characteristics and factors that may be associated with mortality and morbidity related to multi-drug-resistant A. baumannii (MDR-AB) nosocomial infection. We included patients in Al Noor Specialist Hospital, Makkah, Kingdom of Saudi Arabia.
The study was conducted at an approximately 500-bed tertiary care hospital providing all major specialties (e.g., adult cardiology, internal medicine, nephrology, urology, neurology, plastic surgery, dental health, emergency medicine, and adult intensive care; 29 beds). Patients requiring paediatrics and maternity care are transferred to the regional maternity and children’s hospital in Makkah. Al-Noor Specialist Hospital is a referral hospital for Hajj and Umrah pilgrims with multiple nationalities.
This hospital deals with a variety of patients with multiple risk factors. Therefore, there are strict infection control measures in hospital in general and intensive care unit to control the spread of infections. Measures are implemented and supervised by the infection control department. Standard precautions are taken (e.g., hand hygiene and use of protective devices) health care professionals to prevent spreading infection.
The study population consisted of all patients aged 18 years and older in the intensive care unit, and surgery, medicine, neurology, and urology wards in this hospital from 1 January 2012 to 31 August 2012.
The cases were patients with one or more clinically positive cultures for MDR-AB, where clinical evidence of infection was present 48 hours after admission.13 Multi-drug resistance was defined as resistance to more than three classes of antibiotics (aminoglycosides, beta-lactams, quinolones, and tetracyclines).13 Therefore, patients who had isolated MDR-AB from their clinical specimen within 48 hours of admission were not included in the case group. Each A. baumannii infection case was matched by age (within 5 years) and ward of admission with two control patients without A. baumannii. Matching of the controls to cases was performed by enrolling the same percentage of patients without isolated A. baumannii in their clinical specimen who were within the same age category and who stayed in the same ward as the cases.
Data collection
Specially designed data collection forms were used to collect demographic and clinical data. Two slightly different forms, specific to the case and control groups, were used. Data on demographic characteristics, host and therapeutic factors, laboratory and bacteriology results, antibiotic therapy, and outcomes were collected using medical and laboratory records. Data on a previous history were collected from previous medical records.
Statistical analysis
Descriptive analysis
In this study, a single variable was analysed at a time. No comparisons were made between variables. For nominal or ordinal (categorical) variables, the frequency and proportion were used, while for continuous or discrete (numerical) variables, the mean and standard deviation are shown. After processing the analysis using SPSS, independent variables are described using tables, pie charts, and bar charts. Distribution of the study sample by demographic and clinical variables was analysed using this method.
Multivariate analysis
Factors with a p value < 0.05 were considered statistically significant and analysed using multivariate regression. A multivariate logistic regression model was created with MDR-AB (and clinical outcomes of these cases) as the dependent variable. All variables with p < 0.05 in univariate analysis were included in the multivariate model as independent variables to identify independent predictors of multi-resistance. A p value was considered to be two-tailed and p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was first approved by the Institutional Review Board of Al-Noor Specialist Hospital, Ministry of Health (approval #: HJ/3065). The study was conducted retrospectively and the patients’ information was retrieved from medical records. Therefore, written consent was not necessary.
Results
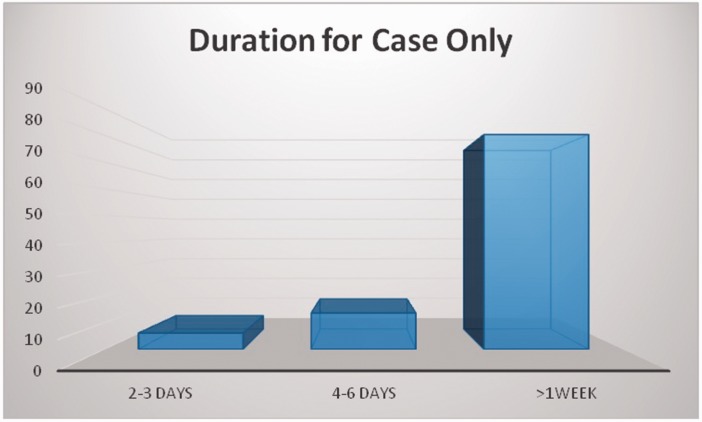
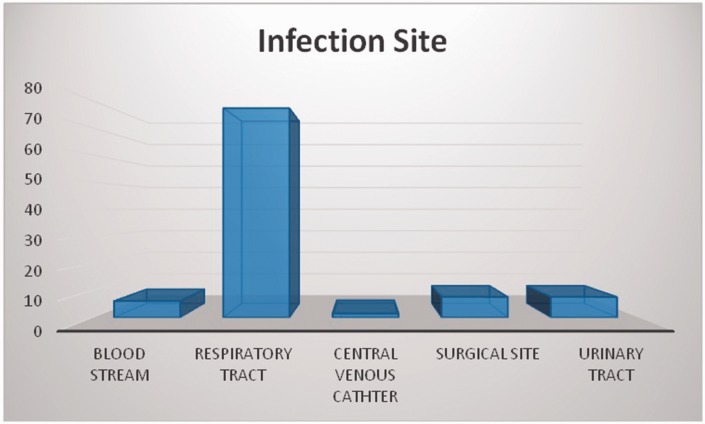
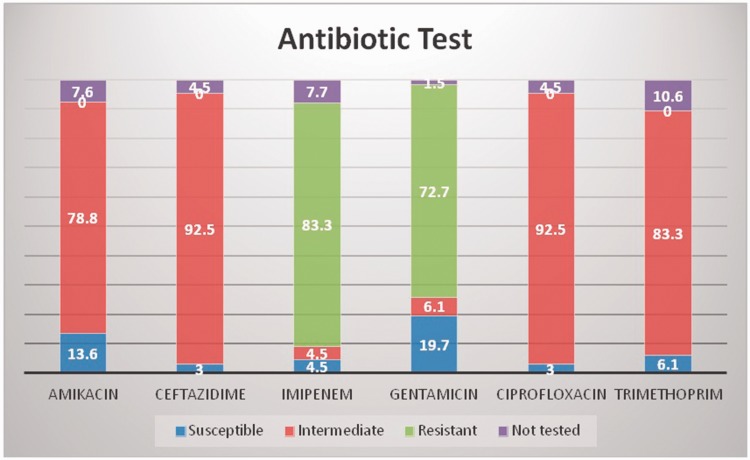
In the study population, 57% were males and 43% were females. The mean age was 53.5 ± 21.9 years for cases and 50.4 ± 22.1 years for controls (Table 1). The majority (80.3%) of patients in the case group remained in hospital for longer than 1 week (Figure 1). The most frequent site of infection was the respiratory tract (77.3%, Figure 2). A total of 92% of A. baumannii cases were susceptible to ceftazidime and ciprofloxacin, 83% to trimethoprim, and 79% to amikacin. The highest resistance (Figure 3) was observed for imipenem (83.3%) and gentamicin (72.7%). All of the isolates were susceptible to colistin and tigecycline.
Table 1.
Demographic variables of the case and control groups.
| Cases, n (%) | Controls, n (%) | |
|---|---|---|
| Age (mean ± SD) | 53.5 (21.9) | 50.4 (22.1) |
| Sex | ||
| Male | 37 (56.1) | 76 (57.6) |
| Female | 29 (43.9) | 56 (42.4) |
| n = 66 | n = 132 | |
Figure 1.
Duration of hospitalization
Figure 2.
Sites of infection
Figure 3.
Susceptibility to antimicrobial drugs
The results of multiple logistic regression of risk factors associated with MDR-AB are shown in Table 2. Immunosuppression was a significant risk factor. Patients with immunosuppression were three times more likely to become infected than those with a good immune status [Odd ratio (OR) = 2.9; 95% Confidence Interval (CI) = 1.5−5.6; p = 0.002]. Furthermore, undergoing invasive procedures had a significant and strong association with A. baumannii infection (OR = 7.9; 95% CI 1.8–34.2; p = 0.002) (Table 2). Patients who had a central venous catheter or endotracheal tube were more susceptible to A. baumannii infection (OR = 2.9; 95% CI 1.5–5.6; p = 0.001 and OR = 3.4; 95% CI 1.6–7.3; p = 0.001, respectively) (Table 3). Other significant factors were a history of hospitalisation, a history of surgery, and infection with bacteraemia (p < 0.01, p < 0.001, and p < 0.01, respectively).
Table 2.
Clinical variables of the case and control groups
| Factors | Cases, n (%) n = 66 | Controls, n (%) n = 132 | P value | Odds ratio |
|---|---|---|---|---|
| Invasive procedure (yes) | 64 (97) | 106 (80) | 0.002 | |
| Arterial catheter | 12 (18) | 21 (16) | 0.4 | 1.8 |
| Central venous catheter | 47 (71) | 60 (46) | 0.001 | 3.0 |
| Abdominal drainage | 2 (3) | 0 (0) | 0.1 | |
| Nasogastric tube | 37 (56) | 79 (60) | 0.3 | 0.9 |
| Urinary catheter | 1.7 | |||
| Peritoneal dialysis | 6 (9) | 8 (6.1) | 0.3 | 1.5 |
| Tracheostomy | 37 (56) | 83 (63) | 0.2 | 0.8 |
| Endotracheal tube | 56 (85) | 82 (61) | 0.001 | 3.4 |
Table 3.
Other contributing factors for A. baumannii infections
| Factors | Cases, n (%) n = 66 | Controls, n (%) n = 132 | P value |
|---|---|---|---|
| History of hospitalization* | 33 (50) | 33 (33) | 0.00 |
| History of surgery* | 7 (11) | 1 (0.8) | 0.00 |
| Other bacteremia* | 22 (33.3) | 1 (0.8) | 0.000 |
| Clinical outcome | |||
| Alive | 21 (32) | 65 (49) | 0.01 |
| Dead | 45 (68) | 67 (51) | |
Yes.
Discussion
This study investigated infections with MDR-AB in a specialist hospital in Makkah. This phenomenon is not specific to this hospital alone because an increased rate of MDR-AB has been reported5,6,9 in health care settings elsewhere. A previous study showed that male sex was among the major predictors for MDR-AB.14 In this study, we found that the majority (80.3%) of patients in the case group stayed in the hospital for longer than 1 week. Another study in Taiwan found that prolonged hospital stay affected disease outcome and increased the mortality rate of patients.10
In the present study, the respiratory tract was the most frequent site of infection. This finding is similar to a previous report.8 The most resistant antibiotics were imipenem and gentamicin. This finding is comparable with previous findings where patients who were treated with imipenem had increased mortality (17.5%) because of underlying illnesses and lowered immunity.15 Our findings are also in agreement with other studies16–18 that reported that A. baumannii was resistant to several antibiotics. In our study, patients with immunosuppression were three times more likely to be infected compared with those without immunosuppression (OR = 2.9; 95% CI 1.5–5.6; p = 0.002). A previous study15 in Riyadh, Saudi Arabia, supports this finding, showing that A. baumannii is more common in patients who have intravascular catheters, have prior antibiotic use, and use ventilator support. Our results are also consistent with findings of study15 that reported the antimicrobial resistance pattern among community-acquired organisms in pilgrims. A. baumannii is highly resistant to carbapenems and quinolones.
Furthermore, in our study, invasive procedures had a significant effect and were strongly associated with A. baumannii infection (Table 2). In particular, patients who had a central venous catheters or endotracheal tube were more susceptible to A. baumannii infection (Table 3). These findings are similar to previous studies19–21 that reported that previous antibiotic treatment, major surgical procedures, burns, immunosuppression and using invasive devices, especially mechanical ventilation, predisposed patients to acquiring A. baumannii infection.
Conclusion
Widespread A. baumannii nosocomial infections are increasing, and the situation is becoming more serious each day, with growing drug resistance. Difficulties in treatment are hard to manage. Some of these infections may be reduced if prevention programmes are timely and effectively applied. Avoidance of unnecessary antimicrobial treatment, invasive devices, and some other procedures, as well as shortening the length of hospital stay by applying effective treatment for underlying illnesses, may make infections easier to control. Furthermore, good sanitation practices and staff hygiene may also contribute to controlling the threat of A. baumannii.
Acknowledgements
The authors wish to thank colleagues from the Department of Infection Prevention and Control Programme, and the staff of the ICU, Al Noor Specialist Hospital Makkah, Kingdom of Saudi Arabia for their support in conduction of this study. We particularly thank Hosham Karar, Lecturer at the College of Public Health and Health Informatics, Umm Al Qura University, Kingdom of Saudi Arabia, for his help and advice in statistical analysis.
Authors’ contributions
MMA designed the study, interpreted the results and drafted the initial manuscript. HSF drafted the manuscript for submission and critically revised it for important content. SSA improved the revised manuscript and made some linguistic revision. AHA performed preliminary statistical analysis, interpreted the results, and drafted the initial manuscript. AH (corresponding author) designed the study tools, performed final statistical analysis of the data for publication, and submitted the final manuscript. TKM collected data and critically revised the manuscript for important content. AM interpreted the results and drafted the initial manuscript. MK critically revised the manuscript for important content. MAH provided constructive advice and guidance in the revised manuscript. All authors read and approved the final manuscript.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Burke JP. Infection control–a problem for patient safety. N Engl J Med 2003; 348: 651–651. [DOI] [PubMed] [Google Scholar]
- 2.Haseeb A, Faidah HS, Bakhsh AR, et al. Antimicrobial resistance among pilgrims: a retrospective study from two hospitals in Makkah, Saudi Arabia. Int J Infect Dis 2016; 47: 92–94. [DOI] [PubMed] [Google Scholar]
- 3.Thompson J, Jefferson J, Mermel LA. Potential economic impact of hospital-acquired infections in uninsured patients: a preliminary investigation. Infection Control Hosp Epidemiol 2008; 29: 764–766. [DOI] [PubMed] [Google Scholar]
- 4.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 2008; 8: 751–762. [DOI] [PubMed] [Google Scholar]
- 5.Lin WR, Lu PL, Siu LK, et al. Rapid control of a hospital-wide outbreak caused by extensively drug-resistant OXA-72-producing Acinetobacter baumannii. Kaohsiung J Med Sci 2011; 27: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al H, Edwards J, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National healthcare safety network at the centers for disease control and prevention, 2006-2007. Infect Control Hosp Epidemiol 2008; 29: 996–1011. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Kopterides P. Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 2006; 64: 7–15. [DOI] [PubMed] [Google Scholar]
- 8.Dizbay M, Tunccan OG, Sezer BE, et al. Nosocomial imipenem-resistant Acinetobacter baumannii infections: epidemiology and risk factors. Scand J Infect Dis 2010; 42: 741–746. [DOI] [PubMed] [Google Scholar]
- 9.El Shafie S, Alishaq M, Garcia ML. Investigation of an outbreak of multidrug-resistant Acinetobacter baumannii in trauma intensive care unit. J Hosp Infect 2004; 56: 101–105. [DOI] [PubMed] [Google Scholar]
- 10.Jang TN, Lee SH, Huang CH, et al. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case–control study. J Hosp Infect 2009; 73: 143–150. [DOI] [PubMed] [Google Scholar]
- 11.Towner K. Acinetobacter: an old friend, but a new enemy. J Hosp Infect 2009; 73: 355–363. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Baño J, Cisneros JM, Fernández-Cuenca F, et al. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 2004; 25: 819–824. [DOI] [PubMed] [Google Scholar]
- 13.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol 2006; 55: 1619–1629. [DOI] [PubMed] [Google Scholar]
- 14.Abbo A. Multidrug-Resistant Acinetobacter baumannii-Volume 11, Number 1—January 2005-Emerging Infectious Disease journal-CDC 2005. [DOI] [PMC free article] [PubMed]
- 15.Babay HA, Kambal AM, Al-Anazy AR, et al. Acinetobacter blood stream infection in a teaching hospital–Riyadh, Saudi Arabia. Kuwait Med J 2003; 35: 196–201. [Google Scholar]
- 16.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21: 538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navon-Venezia S, Leavitt A, Carmeli Y. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother 2007; 59: 772–774. [DOI] [PubMed] [Google Scholar]
- 18.Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother 2007; 59: 128–131. [DOI] [PubMed] [Google Scholar]
- 19.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42: 692–699. [DOI] [PubMed] [Google Scholar]
- 20.Coelho J, Woodford N, Turton J, et al. Multiresistant acinetobacter in the UK: how big a threat? J Hosp Infect 2004; 58: 167–169. [DOI] [PubMed] [Google Scholar]
- 21.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 2007; 65: 204–211. [DOI] [PubMed] [Google Scholar]