Abstract
Background
Dexmedetomidine (DEX), an α2-adrenergic receptor agonist, produces ideal sedation and early postoperative recovery for premedication in paediatric surgery, reducing preoperative anxiety and facilitating smooth induction of anaesthesia. We performed a meta-analysis to compare the effects of DEX and midazolam (MDZ) in paediatric anaesthesia with sevoflurane.
Methods
PubMed, Ovid, Web of Science, and Public Health Management Corporation were searched through December 2016 for randomized controlled trials (RCTs) that compared DEX and MDZ in children undergoing sevoflurane anaesthesia. The risk ratio (RR) with 95% incidence interval (95%CI) was used for dichotomous variables.
Results
Twelve RCTs involving 422 patients in the DEX group and 448 patients in the MDZ group were included. Patients in the DEX group had a significantly lower incidence of unsatisfactory sedation (RR [95%CI] = 0.71 [0.57–0.89]), unsatisfactory parental separation (RR [95%CI] = 0.56 [0.35–0.87]), and rescue analgesia (RR [95%CI] = 0.52 [0.35–0.77]) than patients in the MDZ group. However, both groups had a similar incidence of unsatisfactory mask acceptance, emergence agitation, and postoperative nausea and vomiting.
Conclusion
Compared with MDZ, DEX is beneficial in paediatric anaesthesia with sevoflurane because of its lower incidence of unsatisfactory sedation, parental separation, and rescue analgesia.
Keywords: Dexmedetomidine, midazolam, paediatric anaesthesia, sevoflurane, meta-analysis
Introduction
Paediatric anaesthesia may be accompanied by significant anxiety, uncooperative behaviour, distress, fear, and physical resistance during preoperative preparation, times of parental separation, and invasive diagnostic procedures.1 Thus, paediatric anaesthesia is always considered a principal challenge for anaesthetists. Sevoflurane is characterized by more rapid onset and offset than other inhaled anaesthetics because of its low solubility in blood, relatively low airway irritation, and stable hemodynamic effects, all of which are ideal for induction and maintenance of anaesthesia in children.2,3 However, inhaled sevoflurane in paediatric anaesthesia tends to lead to a high occurrence of emergence agitation (EA) or delirium accompanied by restlessness, crying and moaning, agitation or thrashing, and incoherence.4
Because of the particularity and arduous work of paediatric anaesthesia, it is necessary to administer premedication using drugs such as dexmedetomidine (DEX) or midazolam (MDZ) for sedation, analgesia, relief of the stress response, and elimination of nervousness, anxiety, and fear. DEX, a highly selective α2-receptor agonist with a ratio of affinity between α2 and α1 receptors 7.36 times higher that of clonidine,5 is the most prevalent premedication used in paediatric anaesthesia because of its sedative, analgesic, amnesic, anxiolytic, and sympatholytic properties without respiratory depression.6 Various studies have demonstrated that DEX more effectively decreases anxiety and sedation, reduces EA, and provides postoperative analgesia than does MDZ in children;7–10 however, the definitive effects of DEX versus MDZ in paediatric anaesthesia with sevoflurane remain unclear.
Although some randomized controlled trials (RCTs) have compared the efficacy of DEX versus MDZ in paediatric anaesthesia with sevoflurane, the sample size in all of these trials was too small to provide a definite conclusion. Moreover, some of their results were inconsistent. Therefore, the present meta-analysis was performed to confirm their conclusions using a large sample size.
Methods
Search strategy and process
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 All relevant references that compared the effect of DEX versus MDZ in paediatric anaesthesia with sevoflurane were identified. PubMed, Ovid, Web of Science, and Public Health Management Corporation (updated December 2016) were systematically searched for all articles that may be included. The following search terms were used to identify comparative studies: “dexmedetomidine” or “DEX” or “α2 receptor agonist,” and “midazolam” or “MD.” The primary references were filtered to include only RCTs or clinical trials involving humans without publication year restrictions but with the published language limited to English. Relevant references were manually searched to identify additional studies.
Selection and quality assessment of included studies
Citations selected from the initial search were subsequently screened for eligibility using the follow criteria: (1) all subjects were children undergoing sevoflurane anaesthesia, (2) details of the comparison between the DEX and MDZ groups were included, and (3) the study was designed as an RCT. Conference abstracts and other forms of summary publications were also excluded. In the case of multiple studies apparently based on the same population, we included only the study with the largest number of participants. The methodological quality of the included RCTs was assessed according to the tool established by the Cochrane Collaboration. This tool was used to examine the following items: (1) description of random sequence generation, (2) allocation concealment, (3) blinding of outcome assessment and participants, (4) incomplete outcome data, and (5) selective reporting. A judgment of unclear, low, or high risk of material bias was executed for each item.9
Data extraction and sorting
The eligibility of the included trials was independently assessed by two co-authors (X.-X.W. and Y.-Y.L.). The titles and abstracts of the studies were screened independently by these two authors. Full texts were examined for any trial that appeared qualified. Disagreements and contradictions were resolved by discussion with a third author (J.-F.F.) to attain a consensus. For each study, the following data were collected and sorted: first author; publication year; patient age; American Society of Anesthesiologists physical status; type of surgery; dose, route, and timing of DEX or MDZ administration; incidence of unsatisfactory sedation, parental separation, and mask acceptance; and incidence of postoperative complications involving EA, rescue analgesia, and postoperative nausea and vomiting (PONV).
Outcome measures
The primary outcomes in this meta-analysis were the incidences of unsatisfactory sedation, unsatisfactory parental separation, and unsatisfactory mask acceptance. The secondary outcomes were the incidences of postoperative complications involving EA, rescue analgesia, and PONV.
Statistical analysis
The statistical analysis of all included RCTs was performed using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark). Pooled estimates of risk ratio (RRs) with corresponding 95% confidence intervals (95%CIs) were calculated for dichotomous data using the Mantel–Haenszel method. The I-square (I2) test was conducted to estimate heterogeneity; if heterogeneity was present at I2 > 50%, a random-effects model was selected; otherwise, a fixed-effects model was used according to the Cochrane Review guidelines. Potential publication bias was assessed using a funnel plot. Point estimates of RR were considered statistically significant when P < 0.05.
Results
Literature search
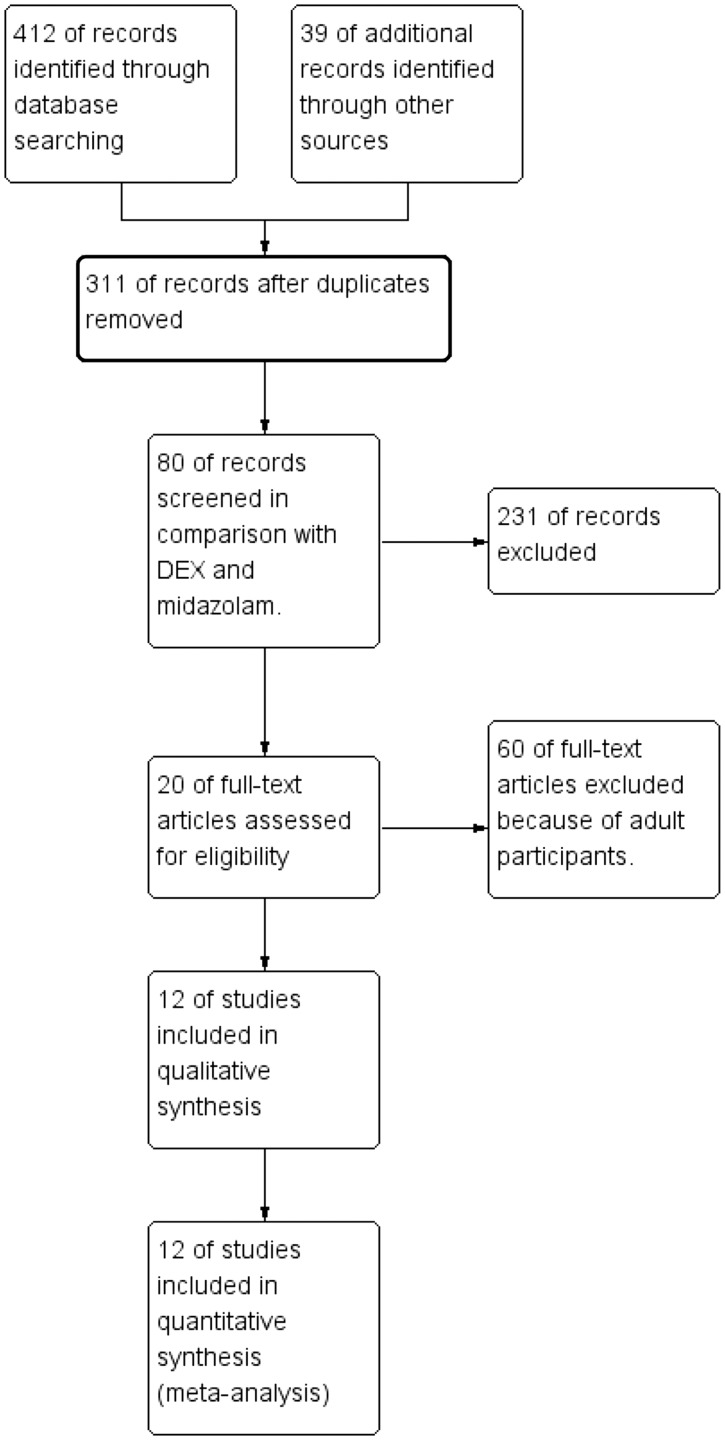
In total, 451 references were identified. Among them, 140 were duplicates. After excluding 231 irrelevant studies, 60 studies involving adults, and 8 RCTs without sevoflurane anaesthesia, the remaining 12 RCTs were included (Figure 1).1,4,13–22 These 12 RCTs included 454 patients in the DEX group and 480 patients in the MDZ group. Clinical heterogeneity was mostly derived from the type of surgery and the dose, route, and timing of drug administration (Table 1). Two routes of DEX administration were used: intranasal in five trials1,13,16,17,19 and oral in seven trials.4,14,15,18,20–22 DEX was administered at different doses: ≤2 µg/kg in nine trials,1,13,15–17,19–22 2.5 µg/kg in one trial,4 and 4 µg/kg in two studies.14,18 The route and dose of MDZ administration also varied among the RCTs (Table 1). Yuen et al.22 compared 0.5- and 1.0 -µg/kg doses of DEX with MDZ.
Figure 1.
Flow chart of study selection.
Table 1.
General characteristics of included studies.
| Author | Age (y) | ASA | Type of surgery | DEX dose | MDZ dose | Route/timing of DEX | Route/timing of MDZ | Sedation/anxiety scores |
|---|---|---|---|---|---|---|---|---|
| Akin, 2012 | 2–9 | I | ADT | 1.0 µg/kg | 0.2 mg/kg | Intranasal 45–60 min | Intranasal 45–60 min | Modified observer’s assessment |
| Arora, 2014 | 1–4 | I–II | Urogenital surgery | 4.0 µg/kg | 0.5 mg/kg | Oral 60 min | Oral 30 min | 4-point scale |
| Faritus, 2015 | 2–12 | I–II | On-pump heart surgery | 2.0 µg/kg | 0.5 mg/kg | Oral 45 min | Oral 45 min | Ramsay |
| Ghali, 2011 | 4–12 | I–II | ADT | 1.0 µg/kg | 0.5 mg/kg | Intranasal 60 min | Oral 30 min | MOAA/S mYPAS |
| Hosokaw, 2010 | 1/12–12 | I–II | Cardiac surgery | 0.6 µg/kg per h | 0.5 mg/kg | Intranasal 30 min | Intranasal 30 min | Ramsay |
| Mountain, 2011 | 1–6 | I–II | Dental surgery | 4.0 µg/kg | 0.5 mg/kg | Oral 45 min | Oral 45 min | 4-point scale |
| Ozcengiz, 2011 | 3–9 | I–II | Oesophageal dilatation | 2.5 µg/kg | 0.5 mg/kg | Oral 45 min | Oral 45 min | Emergence agitation |
| Pant, 2014 | 1–12 | I–II | Inguinal hernia repair, orchidopexy, circumcision | 1.5 µg/kg | 0.25 mg/kg | Intranasal >45 min | Intranasal >20 min | MOAA/S |
| Savla, 2013 | 1–6 | I–II | Short elective surgery | 2.0 µg/kg | 0.5 mg/kg | Oral 30 min | Intranasal 30 min | Ramsay |
| Schmidt, 2007 | 7–12 | I-II | Ambulatory surgery | 1.0 µg/kg | 0.5 mg/kg | Oral 45 min | Oral 30 min | STAIC STAI |
| Sheta, 2013 | 3–6 | I–II | Dental surgery | 1.0 µg/kg | 0.5 mg/kg | Intranasal 45–60 min | Intranasal 45–60 min | 4-point scale |
| Yuen, 2008 | 2–12 | I–II | Minor surgery | 0.5 or 1.0 µg/kg | 0.5 mg/kg | Oral 30 min | Oral 30 min | MOAA/S |
ASA, American Society of Anesthesiologists physical status; DEX, dexmedetomidine; MDZ, midazolam; ADT, adenotonsillectomy; MOAA/S, modified from the observer assessment of alertness and sedation scale; mYPAS, modified Yale preoperative anxiety scale; STAIC, State-Trait Anxiety Inventory for Children; STAI, State-Trait Anxiety Inventory for Adults.
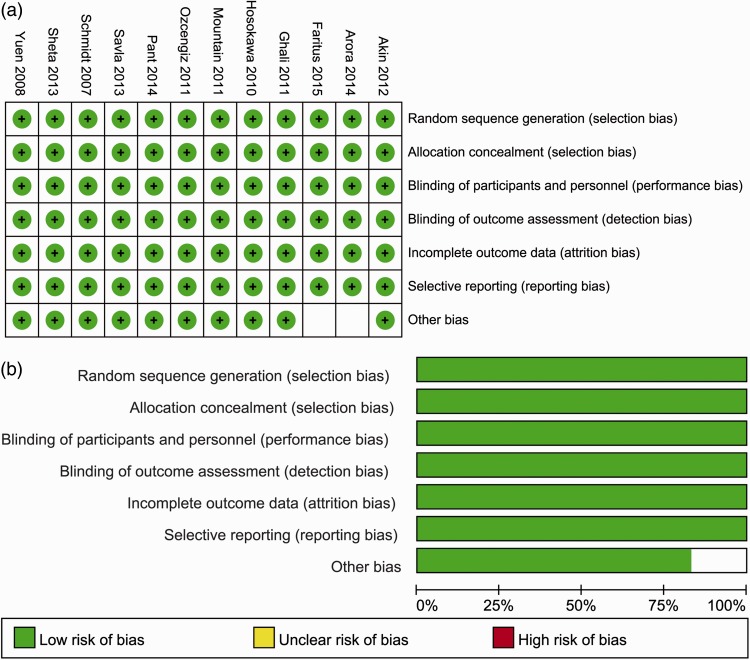
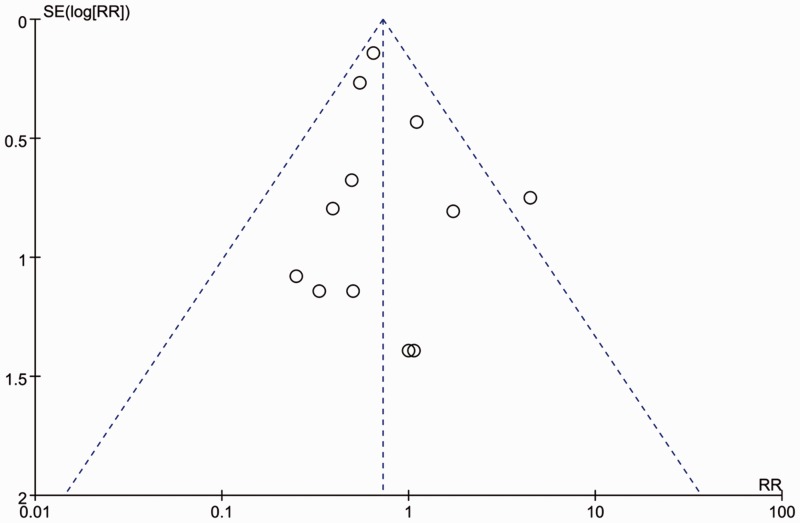
As shown in Figure 2, a risk of bias remained in some studies. A funnel plot was employed to evaluate publication bias with respect to unsatisfactory sedation. Only one RCT showed evident publication bias based on the visual distribution of the funnel plot (Figure 3).13
Figure 2.
Quality assessment of included randomized controlled trials. (a) Risk-of-bias summary: review authors’ judgments regarding each risk-of-bias item for each included study. (b) Risk-of-bias graph: review authors’ judgments regarding each risk-of-bias item presented as percentages across all included studies.
Figure 3.
Funnel plot for incidence of unsatisfactory sedation to assess publication bias. RR: risk ratio.
Quantitative data analysis
Primary outcomes
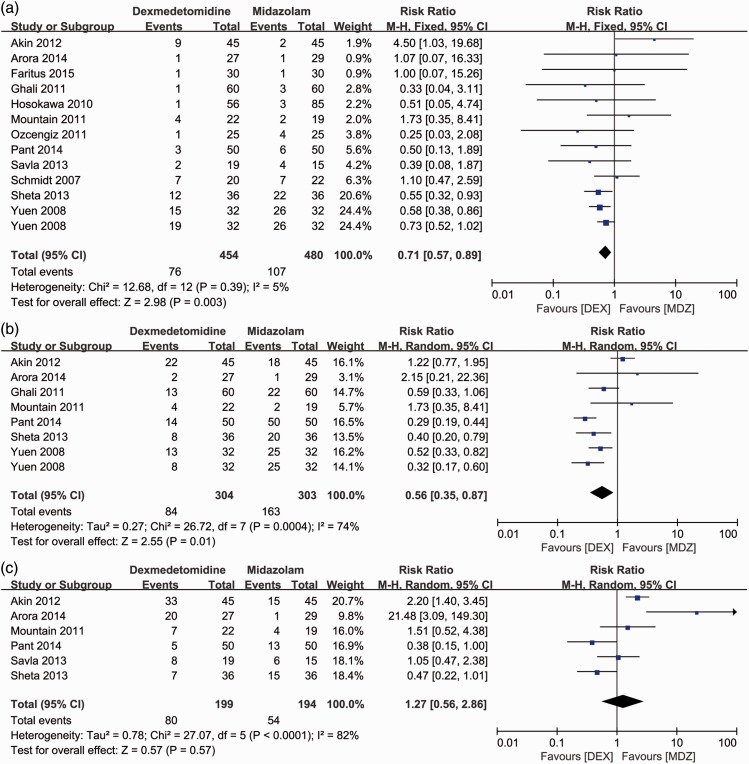
Unsatisfactory sedation was reported in all included studies.1,4,13–22 Low heterogeneity was present between the studies (I2 = 5%). The incidence of unsatisfactory sedation in the DEX group was significantly lower than that in the MDZ group (RR [95%CI] = 0.71[0.57–0.89], P = 0.003) (Figure 4(a)). Similarly, the incidence of unsatisfactory parental separation1,13,14,16,18,19,22 was significantly lower in the DEX than MDZ group (RR [95%CI] = 0.56 [0.35–0.87], P = 0.01) with high heterogeneity (I2 = 74%, P < 0.001) (Figure 4(b)). However, the patients in the two groups had a similar incidence of unsatisfactory mask acceptance (Figure 4(c)).1,13,14,18–20
Figure 4.
Forest plot for primary outcomes: incidence of unsatisfactory (a) sedation, (b) parental separation, and (c) mask acceptance during induction between the dexmedetomidine and midazolam groups. M-H: Mantel–Haenszel method; 95%CI: 95% confidence interval.
Secondary outcomes
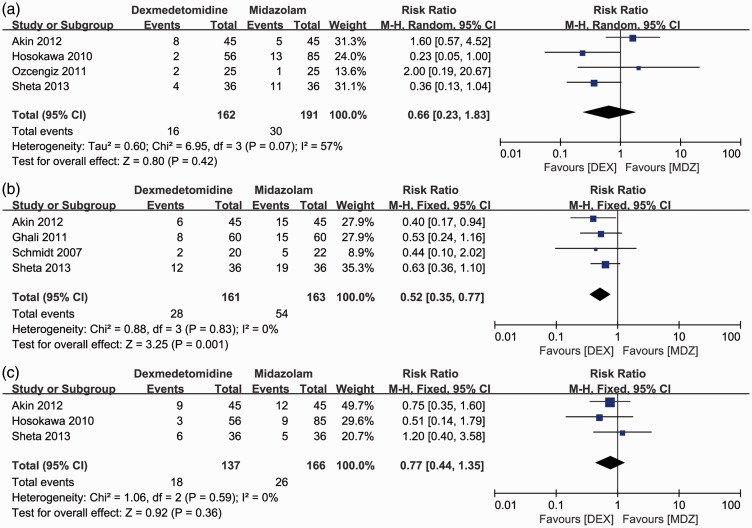
Four RCTs1,4,13,17 reported the incidence of sevoflurane-related EA by comparison of the DEX and MDZ groups. Surprisingly, the incidence of postoperative EA was similar between the DEX and MDZ groups (Figure 5(a)).The incidence of rescue analgesia was significantly lower in the DEX than MDZ group (RR [95%CI] = 0.52 [0.35–0.77]; P = 0.001) (Figure 5(b)). However, there was no significant difference in the prevalence of PONV1,13,17 between the two groups (Figure 5(c)).
Figure 5.
Forest plot for secondary outcomes: comparison of postoperative complications including (a) emergence agitation, (b) rescue analgesia, and (c) postoperative nausea and vomiting between the dexmedetomidine and midazolam groups. M-H: Mantel–Haenszel method; 95%CI: 95% confidence interval; IV: inverse variance.
Discussion
This meta-analysis included 12 RCTs that compared the pharmacological effect of DEX versus MDZ in children undergoing anaesthesia with sevoflurane. The results suggest that DEX is associated with a significantly lower incidence of unsatisfactory sedation, parental separation, and rescue analgesia than is MDZ.
Various drugs have been used for premedication to eliminate the adverse events associated with sevoflurane-inhaled anaesthesia in children. Oral MDZ was historically used as a common preanaesthetic medication because it effectively reduced anxiety and allowed for uneventful parental separation in children undergoing induction of anaesthesia without impacting the recovery time.23,24 Oral MDZ has since been substituted with other drugs, such as the α2-agonists clonidine and DEX, for premedication in children.
DEX, a highly selective α2-agonist with sedative and analgesic functions, is an effective adjuvant medication that can be given before anaesthetic induction in children without inducing respiratory or hemodynamic effects. Children with DEX-induced sedation are characterized as cooperative and semi-arousable, in contrast to the indistinct consciousness induced by MDZ or propofol via the γ-aminobutyric acid receptor. DEX was recently recommended as an anxiolytic and sedative medication for use in the intensive care unit and during procedural sedation. Like MDZ, DEX may be associated with satisfactory parental separation because of ideal sedation. One study showed that children who received DEX before induction had a lower incidence of unsatisfactory sedation and parental separation, which is consistent with our results.8
Sevoflurane, an inhaled anaesthetic with a fragrant and fruity smell, creates fewer airway stimuli and has a prompt onset and offset without hemodynamic effects. Thus, it is often considered to be the preferred inhaled anaesthetic for procedures that range from outpatient procedures to elective surgery. However, increasing evidence has shown that sevoflurane for anaesthesia in children is associated with a high incidence of EA.4,25 Singh et al.26 compared the incidence of EA among isoflurane, desflurane, and sevoflurane in children and found that sevoflurane was associated with a higher incidence of EA. DEX was recently associated with a lower incidence of postoperative EA than placebo in children undergoing general anaesthesia, especially with sevoflurane.12,27–30 However, the present findings do not prove that DEX effectively prevents the occurrence of postoperative sevoflurane-associated EA.
Compared with other premedications, DEX has potential analgesic properties and is opioid-sparing. The analgesia may be concentrated on the alleviation of inflammatory and oxidation reactions,31 activation of central α2-adrenergic receptors in the locus coeruleus,32 sedation, and prevention of EA. Consistent with other studies,9,33,34 our results also prove that DEX favours reduction in the need for rescue analgesia during anaesthesia.
Moreover, DEX is associated with a shorter stay in the post-anaesthesia care unit following sevoflurane anaesthesia in paediatric patients.35 Some trials have shown that premedication with DEX in children clearly reduces the incidence of EA, improves sedation and parental separation, and shortens the stay in the post-anaesthesia care unit compared with other sedatives such as midazolam or propofol.9,35–37 Our results are in accordance with their findings.
Compared with previous meta-analyses, our study has some different findings.8,38,39 This is the first study to analyse the most recent RCTs of only children (<12 years of age) undergoing general anaesthesia with sevoflurane. We comprehensively compared DEX and MDZ with respect to the quality of recovery, including the incidence of unsatisfactory sedation, parental separation, and mask acceptance as well as the incidence of postoperative complications such as EA, rescue analgesia, and PONV.
Our findings should be interpreted with caution because of the limitations in this meta-analysis. Although 12 RCTs were included, the sample size of the included studies was small. Moreover, fewer than 12 studies were included in some outcome analyses. Therefore, more RCTs with larger sample sizes are needed to confirm our findings. Second, the patients underwent different types of surgery and received different doses of DEX or MDZ, which may also limit the reliability of our findings. However, a subgroup analysis based on different types of surgery or different doses of drugs was unable to be performed because of the small sample size and lack of original data.
In conclusion, premedication with DEX significantly promotes the recovery quality during sevoflurane-inhaled anaesthesia in paediatric patients, reducing unsatisfactory sedation and parental separation and the need for rescue analgesia compared with MDZ.
Acknowledgements
The authors thank Dr. R. Jing for the excellent technical assistance and discussion of this work.
Authors’ contributions
Dr. J.-F. Feng designed the study; Drs. X.-X. Wang, Y.-Y. Lu, and D.-G. Pang checked the data and statistical analysis; Drs. W. Peng and J.-L. Mo wrote the manuscript; and Dr. J.-F. Feng reviewed the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Guangxi Natural Science Foundation of China [2014GXNSFAA 118181].
References
- 1.Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth 2014; 24: 181–189. [DOI] [PubMed] [Google Scholar]
- 2.Lerman J, Davis PJ, Welborn LG, et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery. A comparison with halothane. Anesthesiology 1996; 84: 1332–1340. [DOI] [PubMed] [Google Scholar]
- 3.Baum VC, Yemen TA, Baum LD. Immediate 8% sevoflurane induction in children: a comparison with incremental sevoflurane and incremental halothane. Anesth Analg 1997; 85: 313–316. [DOI] [PubMed] [Google Scholar]
- 4.Ozcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth 2011; 25: 184–188. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee A, Das A, Basunia SR, et al. Emergence agitation prevention in paediatric ambulatory surgery: A comparison between intranasal Dexmedetomidine and Clonidine. J Res Pharm Pract 2015; 4: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch E, Tobias JD. Hemodynamic and respiratory changes following dexmedetomidine administration during general anesthesia: sevoflurane vs desflurane. Paediatri Anaesth 2007; 17: 438–444. [DOI] [PubMed] [Google Scholar]
- 7.Bong CL, Yeo AS, Fabila T, et al. A pilot study of dexmedetomidine sedation and caudal anesthesia for inguinal hernia repair in infants. Paediatr Anaesth 2016; 26: 621–627. [DOI] [PubMed] [Google Scholar]
- 8.Pasin L, Febres D, Testa V, et al. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth 2015; 25: 468–476. [DOI] [PubMed] [Google Scholar]
- 9.Tong Y, Ren H, Ding X, et al. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Paediatr Anaesth 2014; 24: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Kang DL, Na HY, et al. Consequence of dexmedetomidine on emergence delirium following sevoflurane anesthesia in children with cerebral palsy. Int J Clin Exp Med 2015; 8: 16238–16244. [PMC free article] [PubMed] [Google Scholar]
- 11.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647–g7647. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Lu Y, Huang Y, et al. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Paediatr Anaesth 2014; 24: 863–874. [DOI] [PubMed] [Google Scholar]
- 13.Akin A, Bayram A, Esmaoglu A, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth 2012; 22: 871–876. [DOI] [PubMed] [Google Scholar]
- 14.Arora S, Saini K, Bhardwaj N. A comparative evaluation of midazolam, clonidine and dexmedetomidine as oral premedicants in children: A double blind randomized clinical trial. APIC 2014; 18(4): 355–360. [Google Scholar]
- 15.Faritus SZ, Khazaee-Koohpar M, Ziyaeifard M, et al. Oral dexmedetomidine versus midazolam as anesthetic premedication in children undergoing congenital heart surgery. Anesth Pain Med 2015; 5(3): 2015–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghali AM, Mahfouz AK, Al-Bahrani M. Preanesthetic medication in children: A comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J Anaesth 2011; 5: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosokawa K, Shime N, Kato Y, et al. Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med 2010; 11: 39–43. [DOI] [PubMed] [Google Scholar]
- 18.Mountain BW, Smithson L, Cramolini M, et al. Dexmedetomidine as a Pediatric Anesthetic Premedication to Reduce Anxiety and to Deter Emergence Delirium. AANA J 2011; 79: 219–224. [PubMed] [Google Scholar]
- 19.Pant D, Sethi N, Sood J. Comparison of sublingual midazolam and dexmedetomidine for premedication in children. Minerva Anestesiol 2014; 80: 167–175. [PubMed] [Google Scholar]
- 20.Savla JR, Ghai B, Bansal D, et al. Effect of intranasal dexmedetomidine or oral midazolam premedication on sevoflurane EC50 for successful laryngeal mask airway placement in children: a randomized, double-blind, placebo-controlled trial. Paediatr Anaesth 2014; 24: 433–439. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt AP, Valinetti EA, Bandeira D, et al. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Pediatr Anesth 2007; 17: 667–674. [DOI] [PubMed] [Google Scholar]
- 22.Yuen VM, Hui TW, Irwin MG, et al. A Comparison of Intranasal Dexmedetomidine and Oral Midazolam for Premedication in Pediatric Anesthesia: A Double-Blinded Randomized Controlled Trial. Anesth Analg 2008; 106: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 23.Cox RG, Nemish U, Ewen A, et al. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anaesth 2006; 53: 1213–1219. [DOI] [PubMed] [Google Scholar]
- 24.Kapur A, Jain K, Goyal A, et al. Oral Midazolam Sedation for uncooperative children in outpatient paedodontics: time for reappraisal. SAAD Dig 2016; 32: 14–16. [PubMed] [Google Scholar]
- 25.Wei J, Wu T, Yang Q, et al. Nitrates for stable angina: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol 2011; 146: 4–12. [DOI] [PubMed] [Google Scholar]
- 26.Singh R, Kharbanda M, Sood N, et al. Comparative evaluation of incidence of emergence agitation and post-operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anaesthesia. Indian J Anaesth 2012; 56: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni J, Wei J, Yao Y, et al. Effect of dexmedetomidine on preventing postoperative agitation in children: a meta-analysis. PloS One 2015; 10: e0128450–e0128450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand 2014; 58: 642–650. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Hu J, Liu X, et al. Effects of intravenous dexmedetomidine on emergence agitation in children under sevoflurane anesthesia: a meta-analysis of randomized controlled trials. PloS One 2014; 9: e99718–e99718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M, Wang H, Zhu A, et al. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PloS One 2015; 10: e0123728–e0123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Yang Y, Yu C, et al. Dexmedetomidine analgesia effects in patients undergoing dental implant surgery and its impact on postoperative inflammatory and oxidative stress. Oxid Med Cell Longev 2015; 2015: 186736–186736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whittington RA, Virag L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg 2006; 102: 448–455. [DOI] [PubMed] [Google Scholar]
- 33.Oza VP, Parmar V, Badheka J, et al. Comparative study of postoperative analgesic effect of intraperitoneal instillation of dexmedetomidine with bupivacaine and bupivacaine alone after laparoscopic surgery. J Minim Access Surg 2016; 12: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma A, Kumar NJ, Azharuddin M, et al. Evaluation of low-dose dexmedetomidine and neostigmine with bupivacaine for postoperative analgesia in orthopedic surgeries: a prospective randomized double-blind study. J Anaesthesiol Clin Pharmacol 2016; 32: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasin L, Greco T, Feltracco P, et al. Dexmedetomidine as a sedative agent in critically ill patients: a meta-analysis of randomized controlled trials. PloS One 2013; 8: e82913–e82913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: A comparison of dexmedetomidine and propofol. Saudi J Anaesth 2013; 7: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadi SM, Saleh AJ, Tang YZ, et al. The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int J Pediatr Otorhinolaryngol 2015; 79: 671–676. [DOI] [PubMed] [Google Scholar]
- 38.Peng K, Wu SR, Ji FH, et al. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics (Sao Paulo) 2014; 69: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Lu Y, Huang Y, et al. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Pediatr Anesth 2014; 24: 863-–874. [DOI] [PubMed] [Google Scholar]