Abstract
Objective
This study aimed to investigate the risk factors and clinical value of lymph node metastasis (LNM) and missed central lymph node metastasis (CLNM) using preoperative ultrasound (US) in patients with papillary thyroid microcarcinoma (PTMC).
Methods
This retrospective study included 521 patients who underwent thyroidectomy for confirmed PTMC based on a final histological examination between January 2014 and June 2015. Based on the presence of LNM, 521 cases were divided into two groups: metastasis (218) and non-metastasis (303). Univariate and multivariate logistic regression analyses were used to analyse the US and clinical characteristics of the primary tumour.
Results
We defined LNM based on the tumour diameter with an optimal critical value of 0.55 cm using ROC analysis with a sensitivity of 65.6% and specificity of 59.6%. We defined US-missed CLNM based on the optimal critical value of 0.65 cm using diagnostic ROC analysis with a sensitivity of 66.0% and specificity of 73.0%. The odds ratios of significant factors with LNM by US were 10.3 (95% confidence interval [95% CI], 6.2–17.0), 5.3 (95% CI, 3.3–8.7), 2.7 (95% CI, 1.1–6.5), 4.3 (95% CI, 1.7–10.5), 2.5 (95% CI, 1.5–4.1), and 2.7 (95% CI, 1.7–4.4) for extrathyroidal invasion, blood flow, multifocality, tumour diameter greater than 0.55 cm, male sex, and age younger than 47 years, respectively.
Conclusions
US characteristics, such as extrathyroidal invasion, blood flow, tumour diameter, sex, and age, may improve the efficacy of predicting LNM and facilitating diagnosis of PTMC. Furthermore, tumour invasion to the extracapsular thyroid and a diameter greater than 0.65 cm indicate CLNM.
Keywords: Papillary thyroid microcarcinoma, lymph node metastasis, central lymph node metastasis, ultrasound
Abbreviations
LNM Lymph node metastasis CLNM Central lymph node metastasis
PTMC Papillary thyroid microcarcinoma
PTC Papillary thyroid carcinoma
ROC Receiver-operating characteristic
FNAC Fine-needle aspiration cytology
LND Lymph node dissection CCND Central neck lymph node dissection
Introduction
The increased detection of thyroid nodules of papillary thyroid carcinoma (PTC) in recent years has been attributed to the widespread use of high-resolution thyroid ultrasound (US) and fine-needle aspiration cytology (FNAC) under US guidance. PTC is the most common histological type of differentiated thyroid cancer, and is characterized by early spread to regional lymph nodes and extracapsular invasion. However, PTC has a highly favourable prognosis, with a low incidence of distant metastasis and mortality.1,2 Papillary thyroid carcinomas smaller than 1 cm at the greatest dimension are classified as papillary thyroid microcarcinoma (PTMC) according to the World Health Organization classification system for thyroid tumours.3 The role of surgery for PTC is controversial.4,5 The revised American Thyroid Association (ATA) guidelines recommend that central neck lymph node dissection (CCND) may be considered in patients with high-risk thyroid cancer.6 However, there is no evidence that prophylactic CCND improves loco-regional control or disease-specific survival, particularly in PTMC. Therefore, the efficacy of prophylactic CCND in patients with PTMC is controversial. High-resolution US shows lymph node metastasis (LNM) and the findings affect the extent of surgery. The accuracy of preoperative US in early diagnosis of LNM is often limited, despite the high positive detection rate.7
Although, the detection of PTC has increased in the past few years, the incidence of distant metastasis and mortality has not increased. PTMC without cervical metastasis has a reported risk of mortality of < 1%, loco-regional recurrence rate of 2% to 6%, and distant recurrence rate of 1% to 2%.8 Some researchers question the value of early detection and treatment of PTMC. The 2015 ATA guidelines8 no longer recommend a biopsy for thyroid nodules less than 1 cm, even if US results are suspicious, without high-risk features, such as cervical adenopathy or extrathyroidal invasion. Therefore, prediction of LNM using preoperative US is of great clinical significance, especially for CLNM. If there are any suspicious characteristics of LNM, FNAC and further surgery should be performed. Our retrospective study aimed to investigate the US and clinical characteristics of PTMC that was confirmed by pathological diagnosis on frozen sections. Our findings could provide more guidance and help in management by improving prediction of LNM.
Materials and methods
Patients
This study was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University. All of the patients signed informed consent. Between January 2014 and June 2015, a total of 956 patients were pathologically diagnosed with thyroid cancer based on frozen sections at the Affiliated Hospital of Qingdao University, including 521 patients with PTMC. A retrospective review of US features was also conducted.
Instruments
US was performed with the GE LOGIQ E8, Siemens 2000, equipped with a commercially available 6 to 15-MHz linear array transducer. Board-certified radiologists with more than 5 years of experience in thyroid imaging performed US twice (including preliminary and preoperative locations). All of the patients underwent surgery after a diagnosis of papillary carcinoma based on fine-needle aspiration.
US and clinical characteristics
Various US prognostic factors, such as tumour size, shape, composition, blood flow, calcification, extrathyroidal extension, multifocality, and coexistence of chronic lymphocytic thyroiditis or nodular goiter were observed and recorded. After PTMC was confirmed surgically, the largest nodule was analysed. The internal echo of composition of the tumour was divided into solid, cystic, and solid mixed. Microcalcification was defined as coexistence of microcalcification or thick microcalcification. Absence of calcification was ascertained by nodules without calcification and only coarse calcification. An irregular shape was defined as burrs and a lobulated aspect ratio greater than 1. Extracapsular invasion was defined by tumour contact greater than 25% with the adjacent capsule.9
Using ROC curves, we identified the best point with high sensitivity and a low false-negative rate (1-specificity).
Surgery and pathology
In our hospital, the extent of thyroid surgery is determined according to the guidelines issued by the ATA.7 Total and near-total thyroidectomy with bilateral CCND was performed in patients with multiple, bilateral tumours, extrathyroidal invasion, LNM upon preoperative evaluation or intraoperative findings, first-degree familial history of thyroid cancer, or a history of head or neck radiotherapy. Patients with a single intrathyroidal lesion (≤ 1 cm), without evidence of LNM by US, without a personal history of radiation therapy to the head and neck, and without a family history of thyroid cancer, were treated with hemithyroidectomy combined with prophylactic ipsilateral CCND. The present study included 283 (54.3%) patients undergoing total thyroidectomy with bilateral CCND and 227 (43.6%) treated with hemithyroidectomy with ipsilateral CCND. Before surgery, 21 patients were diagnosed with preoperative US for suspected metastatic lymph nodes in the lateral compartment, which was proven to be negative in four patients.
Statistical analysis
The predicted tumour size in LNM and US-missed CLNM of PTMC was measured using receiver-operating characteristic (ROC) curves. Statistical analysis was performed with SPSS (version 17.0). Univariate analysis with the χ2 test and Fisher’s exact test was used to analyse the statistical correlation between risk factors and LNM, and missed CLNM of PTMC. Multivariate logistic regression analysis was performed to identify the multivariate correlates of US factors with LNM and missed CLNM of PTMC. Results are shown as odds ratios (ORs) with 95% confidence intervals (CIs). P < 0.05 was considered statistically significant.
Results
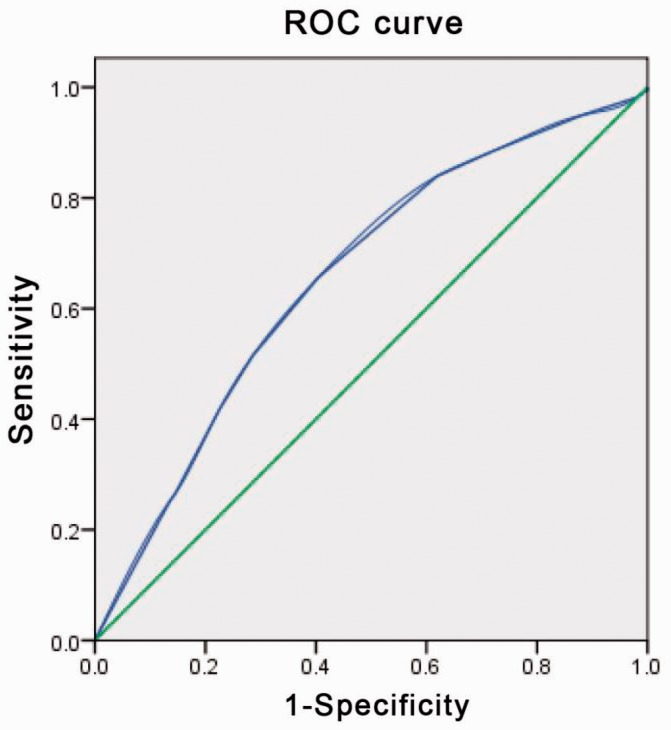
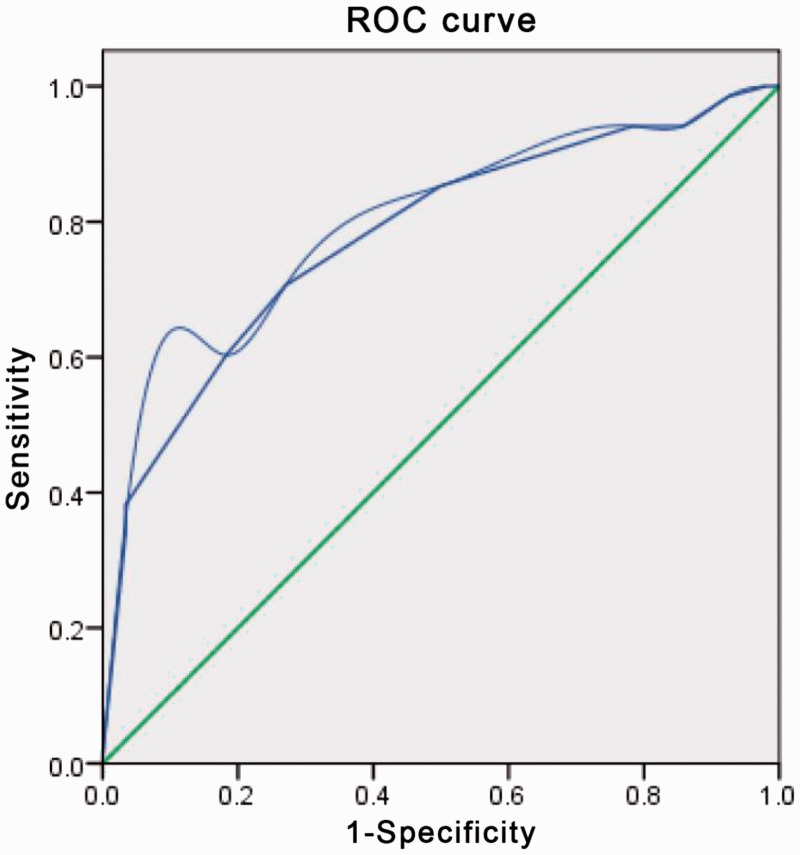
LNM was present in 218 (41.8%) patients, including 149 (68.3 %) who had LNM missed on US and 69 (31.7%) (of whom 21 had lateral LNM) who had LNM detected. The median age was 42 years. We defined LNM by the optimal critical value of the tumour diameter (0.55 cm) using ROC analysis, with a sensitivity of 65.6% and a specificity of 59.6% (Figure 1). We defined ultrasound-missed CLNM by the optimal critical value of the tumour diameter (0.65 cm) using ROC analysis, with a sensitivity of 66.0% and a specificity of 73.0% (Figure 2).
Figure 1.
Receiver-operating characteristics (ROC) curve analysis for lymph node metastasis (LNM)
An index point ≥ 2 was found to be the optimal point to distinguish between PTMC with and without LNM. The sensitivity and specificity were 65.6% and 59.6%, respectively, with an area under the ROC curve of 0.655
Figure 2.
Receiver-operating characteristics (ROC) curve analysis for cervical lymph node metastasis (CLNM) missed by US
An index point ≥ 2 was found to be the optimal point to distinguish between PTMC with and without CLNM. The sensitivity and specificity were 66.0% and 73.0%, respectively, with an area under the ROC curve of 0.770.
Univariate analysis and multivariate logistic regression analysis of PTMC with LNM
Univariate analysis showed that patients with PTMC and LNM were significantly associated with tumour characteristics of microcalcification, extrathyroidal invasion, an irregular shape, blood flow, coexistence of nodular goiter, multifocality, and a diameter greater than 0.55 cm, male sex, and age younger than 47 years (Table 1).
Table 1.
Univariate and multivariate analyses of LNM and ultrasonographic and clinical characteristics of patients with PTMC.
| LNM |
Univariate analysis |
Multivariate logistic regression analysis |
95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound and clinical characteristics | Points | Total (n = 521) | (+) | (−) | χ2 | P value | P value | Odds ratio | Lower | Upper |
| Primary tumour microcalcification | ||||||||||
| Present | 1 | 443 | 152 | 291 | 68.97 | 0.000 | 0.122 | 1.4 | 0.9 | 2.4 |
| Absent | 0 | 78 | 66 | 12 | ||||||
| Extrathyroidal Invasion | ||||||||||
| Present | 1 | 387 | 124 | 263 | 59.34 | 0.000 | 0.000 | 10.3 | 6.2 | 17.0 |
| Absent | 0 | 134 | 94 | 40 | ||||||
| Defined | ||||||||||
| Well-defined | 1 | 19 | 8 | 11 | 5.59 × 10−4 | 1 | ||||
| Ill-defined | 0 | 502 | 210 | 292 | ||||||
| Shape | ||||||||||
| Irregular | 1 | 447 | 206 | 241 | 23.28 | 0.000 | 0.139 | 0.6 | 0.3 | 1.2 |
| Regular | 0 | 74 | 12 | 62 | ||||||
| Blood flow | ||||||||||
| Absent | 1 | 212 | 111 | 101 | 16.24 | 0.000 | 0.000 | 5.3 | 3.3 | 8.7 |
| Present | 0 | 309 | 107 | 202 | ||||||
| Coexisting lymphocytic thyroiditis | ||||||||||
| Present | 1 | 67 | 24 | 43 | 1.15 | 0.750 | ||||
| Absent | 0 | 454 | 194 | 260 | ||||||
| Coexisting nodular goiter | ||||||||||
| Present | 1 | 264 | 93 | 171 | 9.62 | 0.000 | 0.978 | 1.0 | 0.6 | 1.6 |
| Absent | 0 | 257 | 125 | 132 | ||||||
| Composition | ||||||||||
| Cystic and solid mixed | 1 | 4 | 1 | 3 | 9.0 × 10−4 | 1 | ||||
| Solid | 0 | 517 | 217 | 300 | ||||||
| Multifocality | ||||||||||
| Present | 1 | 46 | 28 | 18 | 7.5 | 0.006 | 0.019 | 2.8 | 1.1 | 6.5 |
| Absent | 0 | 475 | 190 | 285 | ||||||
| Age (y) | ||||||||||
| <47 | 1 | 218 | 60 | 158 | 31.59 | 0.000 | 0.000 | 0.4 | 0.2 | 0.6 |
| >47 | 0 | 303 | 158 | 145 | ||||||
| Sex | ||||||||||
| Female | 1 | 381 | 134 | 247 | 25.9 | 0.000 | 0.003 | 0.5 | 0.3 | 0.8 |
| Male | 0 | 140 | 84 | 56 | ||||||
| Tumour size | ||||||||||
| >0.55 | 1 | 422 | 131 | 145 | 16.94 | 0.000 | 0.002 | 4.3 | 1.7 | 10.5 |
| <0.55 | 0 | 89 | 87 | 158 | ||||||
Significant results that were obtained in univariate analysis were subjected to multivariate logistic regression analysis. Tumour characteristics of extrathyroidal invasion, blood flow, multifocality, and a diameter greater than 0.55 cm, male sex, and age younger than 47 years were predictive factors for LNM in multivariate analysis. The odds ratios of these significant factors with LNM by US were 10.3 (95% CI, 6.2–17.0), 5.3 (95% CI, 3.3–8.7), 2.7 (95% CI, 1.1–6.5), 4.3 (95% CI, 1.7–10.5), 2.5 (95% CI, 1.5–4.1), and 2.7 (95% CI, 1.7–4.4), respectively (Table 1).
Univariate analysis and multivariate logistic regression analysis of CLNM missed by US
In univariate analysis, unifocal lesions without calcification or extrathyroidal invasion measuring less than 0.65 cm in diameter were significantly independently correlated with CLNM missed by US (Table 2).
Table 2.
Univariate and multivariate analyses of missed CLNM by US and US and clinical characteristics of patients with PTMC.
| Central LNM |
Univariate analysis |
Multivariate logistic regression analysis |
95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound and clinical characteristics | Points | Total (n = 197) | (+) | (−) | χ2 | P value | P value | Odds ratio | Lower | Upper |
| Primary tumour microcalcification | ||||||||||
| Present | 1 | 132 | 41 | 91 | 9.7 | 0.000 | 0.163 | 2.0 | 0.8 | 5.4 |
| Absent | 0 | 65 | 7 | 58 | ||||||
| Extrathyroidal invasion | ||||||||||
| Present | 1 | 53 | 27 | 26 | 27.7 | 0.000 | 0.000 | 9.6 | 4.3 | 21.6 |
| Absent | 0 | 144 | 21 | 123 | ||||||
| Defined | ||||||||||
| Well-defined | 1 | 8 | 2 | 6 | 1.8 × 10−3 | 1 | ||||
| Ill-defined | 0 | 189 | 46 | 143 | ||||||
| Shape | ||||||||||
| Irregular | 1 | 162 | 39 | 123 | 0.04 | 1 | ||||
| Regular | 0 | 35 | 9 | 26 | ||||||
| Blood flow | ||||||||||
| Absent | 1 | 116 | 24 | 92 | 2.07 | 1 | ||||
| Present | 0 | 81 | 24 | 57 | ||||||
| Coexisting lymphocytic thyroiditis | ||||||||||
| Present | 1 | 25 | 5 | 20 | 0.29 | 1 | ||||
| Absent | 0 | 172 | 43 | 129 | ||||||
| Coexisting nodular goiter | ||||||||||
| Present | 1 | 88 | 23 | 65 | 2.62 | 1 | ||||
| Absent | 0 | 109 | 25 | 84 | ||||||
| Composition | ||||||||||
| Cystic and solid mixed | 1 | 1 | 1 | 0 | 3.1 | 1 | ||||
| Solid | 0 | 196 | 47 | 149 | ||||||
| Multifocality | ||||||||||
| Present | 1 | 24 | 4 | 20 | 5.3 | 0.00 | 0.167 | 0.4 | 0.1 | 1.5 |
| Absent | 0 | 173 | 44 | 129 | ||||||
| Age (y) | ||||||||||
| <47 | 1 | 80 | 17 | 63 | 1.83 | 1 | ||||
| >47 | 0 | 117 | 31 | 86 | ||||||
| Sex | ||||||||||
| Female | 1 | 75 | 17 | 58 | 0.71 | 1 | ||||
| Male | 0 | 122 | 31 | 91 | ||||||
| Tumour size | ||||||||||
| >0.55 | 1 | 164 | 32 | 41 | 7.21 | 0.005 | 0.041 | 5.0 | 1.1 | 23.7 |
| <0.55 | 0 | 33 | 16 | 108 | ||||||
Significant results that were obtained in univariate analysis were subjected to multivariate logistic regression analysis. Tumours without extracapsular invasion and measuring less than 0.65 cm in diameter were independent predictive factors for missed CLNM by US in multivariate analysis. A tumour diameter less than 0.65 cm was a critical factor (Table 2).
Discussion
PTMC is defined by the World Health Organization as a tumour measuring 1.0 cm or less in diameter.4 Although PTMC is generally associated with an excellent prognosis, LNM, extrathyroidal invasion, and distant metastasis are common in patients with PTMC compared with those with PTM. Despite the small size and accidental discovery, the incidence of LNM in patients with PTMC ranges from 4% to 65%, with a loco-regional recurrence rate of up to 20%.10–12 Pathological studies have shown similar biological behaviour in terms of BRAFV600E, frequency of mutation, invasiveness, patients’ age, and sex.13 However, excessive surgical therapy may increase the risks and complications postoperatively. Therefore, preoperative US is invaluable for predicting LNM in patients with PTMC, to serve as a reference and guideline for surgery.
Several factors affect the risk of LNM in patients with PTCM. In the present study, the incidence of LNM increased to 42% (218/521), which is consistent with previous studies.14,15 LNM was significantly associated with independent factors, such as age younger than 47 years, male sex, and the tumour characteristics of extrathyroidal invasion, blood flow, presence of microcalcification, and multifocality. Zhang et al.14 showed that male sex, age less than 45 years, tumour size larger than 0.6 cm, extracapsular invasion, and multifocality were associated with LNM, similar to our study. Lee et al.15 reported that LNM mainly occurred in patients with a primary tumour diameter exceeding 7 mm. Kim et al.16 showed that a threshold of 5 mm for the tumour diameter was significant. We also investigated the tumour diameter by ROC curve analysis, with an optimal cutoff of 0.55 cm, and showed a sensitivity and specificity of 65.6% and 59.6%, respectively. Univariate and multivariate logistic regression analyses showed that a primary tumour size greater than 0.55 cm on US was associated with CLNM (OR 4.3, 95% CI 1.7–10.5). The risk of LNM is known to increase with tumour size. In our study, univariate and multivariate logistic regression analyses showed that extrathyroidal extension was an independent factor for LNM. This finding may be attributed to easy dissemination of cancer cells to other sites by the destroyed capsule. LNM was found in 77% of patients with extrathyroidal extension in our study, consistent with previous studies.17 Therefore, we focussed on PTMC, which destroyed the thyroid capsule. In the present study, multifocality (P = 0.027, OR = 2.1) was an independent predictor of LNM, as shown by other studies.14,21 The left and right lobes of the thyroid are connected by the lymphatic duct,18 facilitating metastasis via lymphatic spread. Tumour blood vessels are also an independent factor for LNM. Blood flow may feed growth and spread of cancer cells. The present study showed significant associations between male sex (P = 0.003< 0.05, OR = 0.5) and age younger than 47 years (P = 0.000, OR = 0.4) and LNM, which is consistent with previous reports.19,20
Some studies have reported that ill-defined tumours and microcalcification are associated with LNM in patients with PTMC.8 However, a few studies reported that tumour composition, microcalcification, definition, and shape were not associated with LNM.21 In the current study, these factors were analysed. Our findings suggested that although microcalcification, ill-defined tumours, and coexisting nodular goiter were risk factors in univariate analyses, they were not significant when adjusted for other factors in multivariate analysis. A previous report showed that coexisting lymphocytic thyroiditis in patients with PTMC was correlated with LNM.22,23 However, So et al.19 reported that 24.9% of 551 patients with PTMC had lymphocytic thyroiditis and there was no association between lymphocytic thyroiditis and LNM. Kim et al.22 showed that lymphocytic thyroiditis did not affect the frequency of LNM in all PTMC cases. The present study showed that the frequency of LNM was 35% (24/69 cases) in the lymphocytic thyroiditis group, which was lower compared with the control group. However, underlying lymphocytic thyroiditis was not significantly associated with CLNM.
The widespread use of US and US-FNAB has resulted in an increased number of preoperative diagnoses of PTMC without palpable thyroid nodes. Recently, studies have reported a good prognosis of PTMC. However, the rate of LNM has increased from 41% to 64%.24 Although preoperative US is widely used, pathologically confirmed lymph nodes are not discovered before surgery. Kim et al.21 showed that the sensitivity and specificity of CLNM were 27.3%–55% and 69%–90%, and those of lateral LNM (LLNM) were 65%–90.3% and 82%–94.8%, respectively. The sensitivity and specificity of CLNM were 24.4% and 97.1%, and those of LLNM were 100.0% and 95.1%, respectively, in our study. The reduced sensitivity was largely due to failure to detect positive nodes in the central neck. Because of the high incidence and low detection of CLNM, experts have proposed routine prophylactic dissection of cervical lymph nodes. Other experts oppose intervention because of the higher risks of surgery and postoperative complications of CLNM, especially in patients with PTMC without any features that are diagnostic of LNM either on a physical examination or preoperative US.25 Our study investigated the preoperative predictive factors of central LNM that was missed by US in patients with PTMC, using clinicopathological and US features. This facilitated management of patients with PTMC. In our study, 149 of 218 patients with PTMC and LNM manifested central LNM, and 69 were detected, including 21 cases of LLNM. We analysed the clinicopathological and US features of patients who were missed. Larger tumour size (0.65 cm) and extrathyroidal invasion showed significant associations with central LNM, which is consistent with previous studies.25,26 Tumours with a diameter less than 0.65 were similar to those reported by Kim et al.21 Therefore, suspicion of PTMC with a larger tumour size (0.65 cm) or extracapsular invasion warrants further scrutiny. Early detection of these features is essential. Selective CCND may be helpful in patients who are diagnosed with PTMC.
There are several limitations in this study. First, our study only included patients with thyroid cancer from a similar environment. Second, although we reported the predictive factors of US images and discussed the clinical features of CLNM in patients with PTMC, we did not investigate the factors associated with long-term survival rates and loco-regional recurrence by continuous follow-up. Additional studies are required to address these issues.
In conclusion, our study shows that thyroid nodules with extrathyroidal invasion, the presence of microcalcification and multifocality, male sex, and age less than 47 years are predictors of LNM. Primary tumours without extrathyroidal invasion and measuring less than 0.65 cm are risk factors for CLNM. Therefore, prediction of CLNM based on clinical and US factors is essential for appropriate surgical decisions and interventions.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Witt RL. What is the best treatment of incidental papillary thyroid microcarcinoma? Laryngoscope 2016; 126: 2203–2204. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26. [DOI] [PubMed] [Google Scholar]
- 3.Zuo H, Tang W, Yasuoka H, et al. A review of 227 cases of small papillary thyroid carcinoma. Eur J Surg Oncol 2007; 33: 370–375. [DOI] [PubMed] [Google Scholar]
- 4.Lang BH, Ng SH, Lau LL, et al. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid 2013; 23: 1087–1098. [DOI] [PubMed] [Google Scholar]
- 5.Sosa JA. Is routine prophylactic central neck dissection indicated for low-risk papillary thyroid cancer: can we determine cost-effectiveness if we are unsure about its effectiveness and safety? Surgery 2013; 154: 1146–1147. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19: 1167–1214. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Yun JS, Kook SH, et al. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg 2010; 34: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 8.Haugan BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak JY, Kim EK, Youk JH, et al. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid 2008; 18: 609–614. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Lee E, Nam SJ, et al. Ultrasound texture analysis: Association with lymph node metastasis of papillary thyroid microcarcinoma. PloS one 2017; 12: e0176103. [DOI] [PMC free article] [PubMed]
- 11.Yu X, Song X, Sun W, et al. Independent risk factors predicting central lymph node metastasis in papillary thyroid microcarcinoma. Hormone & Metabolic Research 2017; 49: 201–207. [DOI] [PubMed]
- 12.Pelizzo MR, Boschin IM, Toniato A, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun 2004; 25: 547–552. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Chen C, Chen Z, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol (Oxf) 2014; 81: 282–288. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012; 97: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 15.Lee KJ, Cho YJ, Kim SJ, et al. Analysis of the clinicopathologic features of papillary thyroid microcarcinoma based on 7-mm tumor size. World J Surg 2011; 35: 318–323. [DOI] [PubMed] [Google Scholar]
- 16.Kim BY, Jung CH, Kim JW, et al. Impact of clinicopathologic factors on subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Yonsei Med J 2012; 53: 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varshney R, Pakdaman MN, Sands N, et al. Lymph node metastasis in thyroid papillary microcarcinoma: a study of 170 patients. J Laryngol Otol 2014; 128: 922–925. [DOI] [PubMed] [Google Scholar]
- 18.Salter KD, Andersen PE, Cohen JI, et al. Central nodal metastases in papillary thyroid carcinoma based on tumor histologic type and focality. Arch Otolaryngol Head Neck Surg 2010; 136: 692–696. [DOI] [PubMed] [Google Scholar]
- 19.So YK, Son YI, Hong SD, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery 2010; 148: 526–531. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012; 97: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 21.Kim KE, Kim EK, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg 2013; 37: 385–391. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Choi YJ, Yun JS. Features of papillary thyroid microcarcinoma in the presence and absence of lymphocytic thyroiditis. Endocr Pathol 2010; 21: 149–153. [DOI] [PubMed] [Google Scholar]
- 23.Lin KL, Wang OC, Zhang XH, et al. The BRAF, mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol 2010; 17: 3294–3300. [DOI] [PubMed] [Google Scholar]
- 24.Xiang Y, Lin K, Dong S, et al. Prediction of central lymph node metastasis in 392 patients with cervical lymph node-negative papillary thyroid carcinoma in Eastern China. Oncol Lett 2015; 10: 2559–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab 2009; 94: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi CP, Bellantone R, De Crea C, et al. Papillary thyroid microcarcinoma: extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter area. World J Surg 2010; 34: 1214–1221. [DOI] [PubMed] [Google Scholar]