Abstract
Purpose
The aim of this study was to compare the postoperative clinical and radiological data of patients with vestibular schwannomas who were initially managed by near total resection (NTR) or subtotal resection (STR). The Ki-67 analysis results were compared with tumor regrowth to determine the presence of a correlation between this proliferative index and postoperative tumor regrowth.
Study Design
Seventeen adult patients (7 male, 10 female) were retrospectively reviewed. Nine (52.9%) and eight (47.1%) patients underwent NTR and STR, respectively. Postoperative clinical and radiological data associated with vestibular schwannoma growth were compared with the Ki-67 immunohistochemical analysis results.
Results
Evidence of clinically significant regrowth was observed in four (23.5%) patients. Patients who underwent NTR had a lower rate/incidence of tumor regrowth than did patients who underwent STR. Patients with a higher Ki-67 index had the highest tumor regrowth rates.
Conclusions
Our study indicates that assessment of the Ki-67 index may be useful for determining the probability of regrowth of vestibular schwannomas when only partial removal is accomplished.
Keywords: Vestibular schwannoma, subtotal resection, near total resection, facial paralysis, Ki-67 index
Introduction
Vestibular schwannoma (VS) is the most common tumor of the cerebellopontine angle, and microsurgical removal remains central to its management.1,2 The goals of surgical removal are complete tumor eradication with preservation of the facial nerve and thus cochlear function.1,2 However, achieving complete tumor eradication without compromising the function of important structures such as the brain stem, vessels, and other nerves in the cerebellopontine angle is impossible in some patients because of the tumor’s intimate relationship with these structures.1–6
Increasing attention is being given to the risk of postoperative facial palsy because of the high importance of aesthetics to many patients. This means that even minimal facial paralysis may be poorly tolerated by some patients.2,4–6
The surgeon faces the dilemma of whether to preserve the nerve at the cost of leaving a small remnant of tumor or to achieve total eradication at the cost of increasing the risk of facial injury.1,2,7 Historically, complete tumor resection was often prioritized at the expense of normal facial nerve function. At present, however, the decision regarding whether to perform total surgical removal may be a source of debate for patients during the consultation phase.
Reviews of the literature have revealed that in the event of partial removal, the residual VS in patients who had undergone near total resection (NTR) versus subtotal resection (STR) showed an incidence of regrowth ranging from 0.0% to 3.5% versus 18.4% to 73.9%, respectively.1,8–15 The ability to monitor the residual tumor with magnetic resonance imaging (MRI), advances in revision microsurgery, and the development of stereotactic radiosurgery allow for adequate management when a VS regrows after partial resection.1,10,15
The molecular mechanisms associated with the formation of a VS secondary to impaired growth regulation remain unclear.16,17 Identification of these mechanisms could be of great clinical value for planning the postoperative management of patients who have undergone incomplete tumor resection.18
Although numerous immunohistochemical tests are able to provide information that can be used to estimate tumor growth rates and patterns, the Ki-67 index is still one of the most widely used markers of cell proliferation.17–20 Some researchers have analyzed the Ki-67 proliferative index of VS.16–24 Charabi et al.,21,22 who were the first to describe the growth rate of VSs as expressed by Ki-67 in relation to symptom duration, reported that tumors with a high proliferative status showed a short preoperative symptom duration, while tumors with a low proliferative status had a long symptom duration. Subsequently, other authors assumed that tumor growth was positively associated with a higher proliferative status as expressed by Ki-67.16–18,23,24 However, no studies have been performed to analyze the correlation between partial VS resection and the Ki-67 index.
We herein report the results obtained from a review of patients with a sporadic unilateral VS who were initially managed by NTR or STR and subsequent periodic follow-up with MRI. The aim of this paper is to report the results of partial VS resection and compare the postoperative clinical and radiological data with the immunohistochemical analysis of Ki-67 to determine the presence of a correlation between this proliferative index and postoperative tumor regrowth.
Materials and methods
This retrospective study included 17 consecutive adult patients (7 male, 10 female) surgically treated for a VS in the Otorhinolaryngology Division of Sapienza University of Rome from 2002 to 2012. The patients selected for this study underwent partial resection of the tumor by a translabyrinthine approach or retrosigmoid approach because of a high risk of intraoperative and postoperative complications. Either NTR or STR was performed.
Ninety-eight patients who underwent total VS resection (100% tumor clearance according to the surgeon’s subjective observation and the 6-month postoperative MRI findings) in our institute during the same period of time were excluded from the study.
In the present study, NTR was defined as >95% resection with an intracanalicular tumor remnant or adherence of the tumor to the facial nerve or brain stem. STR was defined as <95% tumor resection. This classification was adopted in accordance with a recent study by El-Kashlan et al.15
The initial tumor size was defined as the tumor diameter on preoperative gadolinium-enhanced MRI.
The preoperative symptom duration, postoperative complications, any subsequent treatment, and the degree of postoperative facial paralysis were assessed. All patients underwent postoperative MRI at 6 months and annually throughout the observation period to evaluate any possible growth of the residual tumor.
Long-term VS regrowth was assessed in each patient by MRI performed 6 months postoperatively and annually thereafter until before the date of the study. The duration of follow-up ranged from 2 to 12 years (median, 6.7 years). No patients underwent postoperative radiosurgery.
Immunohistochemical staining for the nuclear proliferation-associated antigen Ki-67 was performed using the mouse monoclonal antibody MIB1. The Ki-67 index was estimated as the percentage of stained cell nuclei (marked antigen Ki-67) among all nuclei visible in the field. Ki-67 analysis was performed by the same researcher (C.D.G.) for minimum variability and later confirmed by a second scientist (R.C.) in a blinded fashion. The specimens of patients who underwent operations at the second hospital were evaluated by the same pathologists. We considered a Ki-67 index of >2.5% to indicate high cell proliferation as reported in similar studies.18–24
Outcomes were calculated starting from the date of surgery. Growth of the residual tumor was determined by an increase in its maximum diameter on follow-up imaging studies. Only tumor growth of >0.5 cm of the estimated postoperative size was considered to indicate clinical tumor recurrence.
Postoperative clinical and radiological data associated with VS growth were compared with the Ki-67 immunohistochemical analysis results, and the efficacy of this proliferative tumor index in identifying tumor growth in cases of partial resection was estimated.
The statistical analysis was performed using the chi-square test and regression analysis.
The study was performed in accordance with the Declaration of Helsinki and received prior approval from our institutional ethics committee.
Results
Preoperative, intraoperative, and postoperative clinical data
The mean age of the patients at surgery was 58.1 years (range, 48–74 years). Seven tumors were located on the right side and 10 on the left side. The most common symptom at the time of presentation was progressive hearing loss, which was reported in all patients of the study (100%). Other symptoms included tinnitus (58.8%), vertigo (52.9%), and aural fullness (17.6%). Preoperative facial paralysis (grade II) was reported in two patients (11.7%) (Table 1). Five (29.4%) patients underwent hearing preservation surgery.
Table 1.
Preoperative, intraoperative, and postoperative clinical data.
| Preoperative symptoms | Patients | Reason for intraoperative subtotal resection | Patients | Postoperative facial palsy (grade) | Patients |
|---|---|---|---|---|---|
| Facial palsy | 2 (11.7) | Tumor adherent to facial nerve | 14 (82.3) | I | 13 (76.4) |
| Hearing loss | 17 (100.0) | Tumor adherent to brain stem | 6 (35.3) | II | 3 (17.6) |
| Tinnitus | 10 (58.8) | Tumor adherent to brain stem and facial nerve | 3 (17.6) | III | 1 (5.8) |
| Vertigo | 9 (52.9) | IV–VI | – | ||
| Aural fullness | 3 (17.6) | ||||
| Hydrocephalus | 1 (5.8) |
Data are presented as n (%).
Nine (52.9%) and eight (47.1%) patients underwent NTR and STR, respectively.
The preoperative tumor size ranged from 1.5 to 4.0 cm with an estimated mean of 2.3 cm.
Intraoperatively, tumor adherence to the facial nerve was present in 14 (82.3%) patients, to the brain stem in 6 (35.3%), and to both of these nervous structures in 3 (17.6%). Complete resection of the tumor without complications such as facial dissection or serious life-threatening risks was considered impossible in all patients (Table 1).
Three (17.6%) patients developed postoperative grade II facial palsy according to the House–Brackmann (HB) classification, while only one (5.8%) patient developed grade III palsy. The remaining 13 (76.4%) patients developed grade I paralysis (Table 1). No patients received postoperative radiation therapy or other surgical treatments.
Ki-67 index evaluation
The Ki-67 labeling index in our study varied from 0.5% to 4.3% with a mean of 1.8%. In five patients, the Ki-67 proliferation index was >2.5% (Figure 1).
Figure 1.
Percentage of Ki-67 in the study group.
Ki-67 index and preoperative tumor size
No correlation was found between the Ki-67 index and preoperative tumor size in our group of patients. Only one patient with a VS size of 3 cm showed a relatively high Ki-67 index of 2.8%, whereas another patient with a low Ki-67 index of 0.8% had a VS of 4 cm. No significant correlation was found between the Ki-67 index and patient age or sex.
Postoperative regrowth
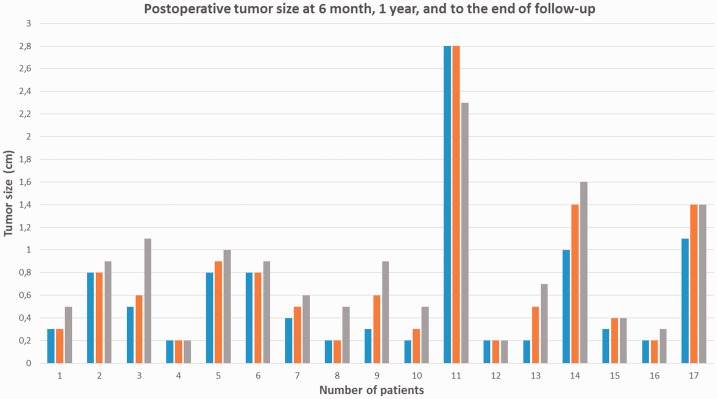
The postoperative tumor size was evaluated at 6 months, 1 year, and shortly before the date of the study (Figure 2). The median long-term follow-up duration was 6.7 years (range, 2–12 years).
Figure 2.
Postoperative tumor size at 6 months, 1 year, and end of follow-up.
Evidence of regrowth was observed in four (23.5%) patients; the tumor size increased by 0.6 cm in three patients and by 0.5 cm in one patient. In 12 (70.5%) patients, no evidence of regrowth was evident (Table 2). A reduction of 0.5 cm in the tumor size in was observed in one patient (Patient 11).
Table 2.
Tumor regrowth after NTR and STR.
| Patients | No regrowth | Regrowth of ≥0.5 cm | |
|---|---|---|---|
| NTR | 9 (52.9) | 8 (47.1) | 1 (5.8) |
| STR | 8 (47.1) | 5 (29.4) | 3 (17.6) |
| Total | 17 | 12 (70.5) | 4 (23.5) |
Data are presented as n (%).
Patients who underwent NTR had a lower tumor regrowth rate than did patients who underwent STR (5.8% vs. 17.6%, respectively) (Table 2). However, the chi-square test revealed no significant difference between the two groups.
Ki-67 index and postoperative tumor regrowth
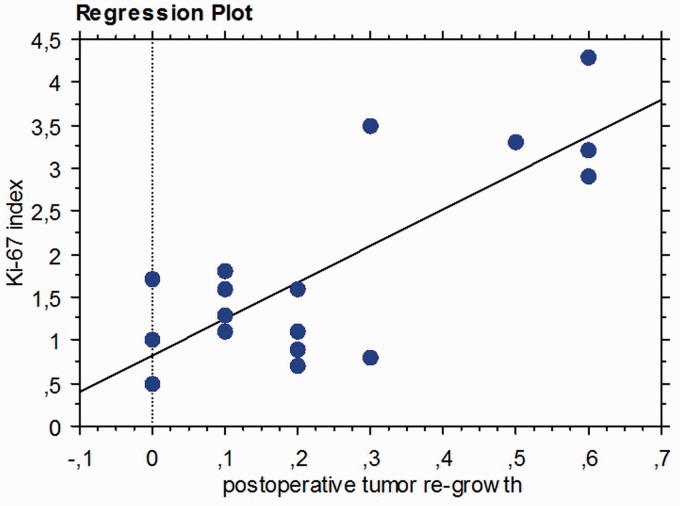
Finally, we evaluated the correlation between the Ki-67 proliferation index with the percentage of tumor regrowth observed at the last follow-up. The regression analysis results showed that patients with a higher Ki-67 index had the highest tumor regrowth rates (p = 0.0002) (Figure 3). The five patients with tumor regrowth of >0.5 cm showed an average Ki-67 index of 3.2%.
Figure 3.
Comparison between Ki-67 proliferation index and postoperative tumor regrowth; regression plot.
Discussion
The goal of microsurgical VS removal is complete tumor eradication with preservation of the facial nerve function and eventual hearing preservation.1,2 Total resection of VSs, especially larger tumors, increases the risk of facial nerve injury with a negative impact on patients’ quality of life.1–7 Falcioni et al.3 analyzed a series of 1052 patients with anatomically preserved facial nerves and total tumor removal. The authors reported a postoperative HB grade of I or II in 684 (65%) patients, a grade III FP in 309 (29.4%) patients, and unsatisfactory results (HB grade IV–VI) in the remaining 59 (5.6%) patients.
In certain circumstances, if complete surgical eradication of a VS is impossible, NTR or STR is indicated.1,2,10–12 Most authors suggest that the rationale for complete removal may evolve toward NTR or STR when the risk of facial nerve paralysis becomes unacceptably high. If regrowth is demonstrated, the patients should be monitored by MRI for residual tumor growth postoperatively and eventually treated with revision microsurgery or radiation therapy if regrowth is demonstrated.1,10–14,25,26
In the present study, NTR and STR were defined in accordance with a recent study by El-Kashlan et al.15 Because NTR was defined as >95% resection, even a very small tumor residue or a thin tumor capsule (<0.2 cm) remaining on the facial nerve or brain stem was considered as intraoperative NTR.
Increasing attention is being given to aesthetics in the present surgical era, and postoperative facial paralysis seriously compromises patients’ quality of life. Thus, during the preoperative consultation, increasing numbers of patients are becoming seriously concerned about the possibility that this complication may occur.4–6 Lee et al.27 noted that the social impact of facial nerve disability in patients is not correlated with the degree of facial nerve damage and that this varies from one individual to another. The authors showed how even minimal facial paralysis may be poorly tolerated by some patients.
Unfortunately, the available data regarding tumor regrowth, facial palsy, and the need for subsequent re-intervention after incomplete VS resection is limited because of the small number of published studies of these topics.1,8–15 Chen et al.1 analyzed facial nerve outcomes after incomplete excision of VSs and reported a significant benefit of this type of surgery in terms of postoperative facial nerve function. Among 105 patients with normal preoperative facial nerve function, the postoperative facial nerve function was HB grade I and II in 51 patients (48.57%), HB grade III in 34 patients (32.38%), and HB grade IV to VI in 20 patients (19.05%). Intraoperative adherence between the VS and the facial nerve was found in 82.3% of the patients in the present study, and partial resection of the tumor was performed in all of these patients to preserve the nerve. Three patients developed postoperative grade II facial paralysis, and only one developed postoperative grade III facial paralysis. The remaining 13 patients developed postoperative grade I facial paralysis. No differences in facial nerve outcomes were observed between patients who underwent NTR and those who underwent STR.
The possibility of worsening facial paralysis after adjuvant radiotherapy should be considered. Virk et al.14 performed a series of 16 STRs and demonstrated that two patients with an initial HB grade of I/II facial paralysis developed grade V/VI palsy following adjuvant radiation treatment.
Despite the satisfactory outcomes in terms of postoperative complications and minimal facial paralysis obtained after partial VS removal, the possibility of tumor regrowth should be considered and evaluated.1,2,11,28–31
Several studies including only patients who underwent either STR or NTR have indicated that most incomplete VS resections are not associated with a significant increase in the recurrence rate.1,8–15 Chen et al.1 reported that the incidence of VS regrowth in their NTR subset ranged from 0.0% to 3.5%, while that in their STR ranged from 18.4% to 73.9%. Bloch et al.8 classified 52 patients who had undergone incomplete VS resection into either NTR or STR. Recurrence was observed in 1 (3%) of the 33 patients who underwent NTR versus 6 (32%) of the 19 patients who underwent STR.
Evidence of clinically significant regrowth was observed in 23.5% of the patients in the present study. In accordance with published results, patients who underwent NTR had a lower tumor regrowth rate (5.8%) than did patients who underwent STR (17.6%).1,8–15
In contrast to previous studies,1,8–10,13 despite an evident increase in tumor regrowth in patients who underwent STR, no significant differences were found between the two groups. This finding was probably influenced by the limited sample of patients in our study.
The current management strategy after NTR or STR includes monitoring of the residual tumor with MRI and, when VS regrowth occurs, performing microsurgical revision or stereotactic radiosurgery.1,2,10–12,32–34
In published studies involving only partial VS removal (analyzed in Table 3), VS regrowth occurred in 189 (10.04%) cases. Among these patients, 58 (30.6%) underwent revision microsurgery, 111 (58.7%) underwent stereotactic radiosurgery, and 20 (10.5%) were only observed.
Table 3.
Incomplete resection of vestibular schwannoma: literature review according to near total resection (NTR) and subtotal resection (STR) classification.
| Authors | Total patients | Type of resection/ patients | Mean preoperative tumor size (cm) | Postoperative facial paralysis (House–Brackmann grade) | Tumor regrowth | Median time of recurrence | Recurrence management |
||
|---|---|---|---|---|---|---|---|---|---|
| Revision microsurgery | Radiation therapy | Observation | |||||||
| Vakilian et al. 2012 | 40 | NTR 10 (25.0%) STR 30 (75.0%) | 2.92 | Not analyzed | NTR 0 (0.0%) STR 12 (40.0%) | NTR 6.3 y STR 6.8 y | – | – | – |
| 5 | 4 | 3 | |||||||
| Chen et al. 2014 | 111 | NTR 73 (65.8%) STR 38 (34.2%) | NTR 2.9 STR 3.2 | 51 pz (48.6%) I–II 34 pz (32.4%) III 20 pz (19.0%) IV–VI | NTR 0 (0.0%) STR 7 (18.4%) | 140 months | – | – | – |
| 3 | 3 | 1 | |||||||
| Seol et al. 2006 | 116 | GTR 26 (22.0%) NTR 32 (28.0%) STR 58 (50.0%) | GTR 3.7 NTR 4.1 STR 3.9 | 44 pz (37.9%) I–II 63 pz (54.3%) III 9 pz (7.8%) IV–VI | GTR 1 (3.8%) NTR 3 (9.4%) STR 16 (27.6%) | 22 months | 10 | 16 | – |
| Schwartz et al. 2013 | 400 | GTR 325 (81.3%) NTR 44 (11.0%) STR 31 (7.7%) | GTR 3.2 NTR 3.2 STR 3.4 | I 45.9% II 11.3% III 4.0% V 10.3% VI 20.3% | GTR 3 (2.8%) NTR 5 (20.8%) STR 6 (22.2%) | GTR 6.5 y NTR 4.0 y STR 4.3 y | – | – | – |
| – | 1 | 4 | |||||||
| 1 | 2 | – | |||||||
| Fukuda et al. 2011 | 74 | GTR 41 (55.0%) STR 25 (34.0%) PR 8 (11.0%) | GTR 2.3 STR 3.2 PR 4.1 | Grade I–II GTR 35 (85.4%) STR 15 (60.0%) PR 7 (87.5%) | GTR 1 (2.4%) STR 13 (52.0%) PR 5 (62.0%) | GTR 76 months STR 34.2 months PR 17.2 months | – | – | 1 |
| 4 | 9 | – | |||||||
| 1 | 5 | – | |||||||
| Bloch et al. 2004 | 79 | NTR 50 (63.0%) STR 29 (37.0%) | NTR 2.4 STR 3.1 | Grade I–II NTR 37 (80.0%) STR 20 (83.0%) Grade III–IV NTR 8 (17.0%) STR 3 (12.0%) Grade V–VI NTR 1 (2.0%) STR 1 (4.0%) | NTR 1 (3%) of 33 patients STR 6 (32%) of 19 patients | NTR 3 years STR 3.1 years | 1 | – | – |
| 1 | 5 | – | |||||||
| Sughrue et al. 2011 | 772 | GTR 571 (74.0%) NTR 89 (11.5%) STR 112 (14.5%) | GTR 1.9 NTR 2.7 STR 3.1 | Not analyzed | Total regrowth 58 (7.5%) | 5 years | 21 | 40 | 7 |
| Virk et al. 2014 | 16 | STR 16 | 14.7 cm3 | 11 pz (68.7%) I–II 0 (0.0%) III–IV 5 (31.3%) V–VI | 7 (43.7%) | 20.2 months | 1 | 6 | – |
| El-Kashlan et al. 2000 | 39 | STR 23 (59.0%) NTR 16 (41.0%) | 2.6 | I 23 (59.0%) II 5 (12.8%) III 5 (12.8%) IV 1 (2.6%) V 0 (0.0%) VI 5 (12.8%) | NTR 2 STR 15 | 4.6 years | 8 | 2 | 7 |
| Jacob et al. 2016 | 103 | NTR 50 (48.5) STR 53 (51.5) | NTR 2.7 STR 2.9 | I 64 (62.1%) II 20 (19.4%) III 13 (12.6%) IV 5 (4.8%) V 0 (0.0%) VI 1 (0.9%) | 14 (13.5%) | – | 1 | 11 | 2 |
| Monfared et al. 2016 | 132 | GTR 12 (16.0%) NTR 24 (33.0%) STR 30 (41.0%) Not recorded 7 (10%) | 3.33 ± 0.70 | – | GTR 1 NTR 2 STR 11 | 35 months | 1 | 11 | 2 |
In the management of large VS, the initial combination of subtotal resection and Gamma Knife® surgery should also be considered. This strategy might be reasonable for decreasing the risk of both nerve damage and growth of the residual VS. However, only a few authors have evaluated the results of this treatment combination.35,36
Assessment of the cellular growth rate may help to determine an adequate strategy for managing patients undergoing partial removal of VS. Several authors have observed a relationship between tumor growth and a higher proliferative status expressed through Ki-67.16–18,21,22,24 Ki-67 is a proliferative marker that may be expressed in numerous neoplastic conditions, and antibodies to the Ki-67 protein have been increasingly used as diagnostic tools in different types of neoplasms.
Some researchers have analyzed the proliferative Ki-67 index of VS.16–24 Bedavanija et al.16 showed that large VSs exhibit enhanced proliferative activity and higher growth rates than do smaller tumors and defined a tumor size of 18 mm as highly significant. Charabi et al.21,22 found a significant inverse correlation between the duration of symptoms and proliferation index in two series of 21 and 124 VSs but no correlation between tumor size and proliferation.
Yokoyama et al.23 evaluated the correlation between various clinical parameters and the Ki-67 staining index in 58 cases of VS. The index ranged from 0.37% to 6.61% (mean, 1.70%) and was not correlated with age, sex, or initial tumor volume. Niemczyk et al.24 subsequently used immunohistochemical tests to demonstrate significant differences in the Ki-67 index between radiologically stable neuromas and evolving tumors.
Our study confirmed the absence of a correlation between the Ki-67 index and preoperative tumor size. Moreover, no significant correlation was found between the Ki-67 index and the age or sex of the patients. This finding does not mean that the Ki-67 index is not a good marker of cell proliferation in patients with VS; rather, it confirms the unpredictable growth of neuromas, which may sometimes evolve very slowly to a large size in the absence of clinical symptoms that allow for establishment of a diagnosis.
However, evaluating the proliferation activity of VS could have practical significance when there are doubts about the effectiveness of partial removal of a VS.24 Understanding the pattern of residual tumor regrowth would allow the surgeon to more easily make decisions regarding the best type of postoperative management.
In an attempt to evaluate the effectiveness of partial resection in terms of a lower risk of facial paralysis, the postoperative radiological data regarding tumor regrowth in our study were compared with the results of immunohistochemical analysis of Ki-67. The aim of this comparison was to correlate the tumor index of proliferative activity with the incidence/rate of postoperative tumor regrowth following STR after an average long-term follow-up observation period of 6.7 years. The results of the regression analysis showed that patients with a higher Ki-67 index had the highest rates of tumor regrowth.
In our opinion, Ki-67 should be used as a marker of cell proliferation of VSs, and when this index is high after partial resection, follow-up must be constant and protracted.
Unfortunately, the disadvantage of immunohistochemical tests is that they can only be conducted after surgery. The ability to evaluate the Ki-67 index intraoperatively could provide further useful information to orient the surgeon toward partial resection when the risk of postsurgical facial paralysis is high. Further studies are underway to evaluate this possibility. Obviously, a significant correlation between the intraoperative Ki-67 index and tumor regrowth would have an impact on the management of VS.
Postoperative Ki-67 evaluation is routinely performed in our clinical practice for patients undergoing partial VS resection because it is a low-cost immunohistochemical analysis that provides useful information regarding regrowth. Based on our findings, if NTR or STR is performed and the Ki-67 index is >2.5%, we recommend careful radiological follow-up because of the greater statistical probability that these patients have tumor regrowth. If regrowth occurs, we believe that a new surgical treatment or radiation therapy should be discussed between the patient and surgeon because of the increased risk of facial nerve injury.
Conclusion
During the consultation phase, another surgical option should be offered to the patient as an alternative to total tumor removal to reduce the risk of minor and major postoperative complications.
Our study has shown that assessment of the cellular growth rate using the Ki-67 index may help to determine the probability of VS regrowth when only partial removal is accomplished. Larger series would provide definitive conclusions on this issue.
Authorship
Giannicola Iannella: Design of the study, analysis and interpretation of the data, drafting of the article, approval of the version to be published.
Marco de Vincentiis: Design of the study, analysis and interpretation of the data, drafting of the article, approval of the version to be published.
Cira Di Gioia: Conception of the study, analysis and interpretation of the data, drafting of the article, approval of the version to be published.
Raffaella Carletti: Conception of the study, analysis and interpretation of the data, revision of the article, approval of the version to be published.
Benedetta Pasquariello: Acquisition and analysis of the data, revision of the article, approval of the version to be published.
Alessandra Manno: Acquisition and analysis of the data, revision of the article, approval of the version to be published.
Diletta Angeletti: Acquisition and analysis of the data, revision of the article, approval of the version to be published.
Ersilia Savastano: Acquisition and analysis of the data, revision of the article, approval of the version to be published.
Giuseppe Magliulo: Conception and design of the study, analysis and interpretation of the data, approval of the version to be published.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Chen Z, Prasad SC, Di Lella F, et al. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg 2014; 120: 1278–1287. [DOI] [PubMed] [Google Scholar]
- 2.Spielmann PM, Sillars H. Assessing the threshold for vestibular schwannoma resection and the behavior of residual tumor. Otol Neurotol 2013; 34: 935–938. [DOI] [PubMed] [Google Scholar]
- 3.Falcioni M, Fois P, Taibah A, et al. Facial nerve function after vestibular schwannoma surgery. J Neurosurg 2011; 115: 820–826. [DOI] [PubMed] [Google Scholar]
- 4.Leong SC, Lesser TH. A national survey of facial paralysis on the quality of life of patients with acoustic neuroma. Otol Neurotol 2015; 36: 503–509. [DOI] [PubMed] [Google Scholar]
- 5.Magliulo G, Zardo F, Damico R, et al. Acoustic neuroma: postoperative quality of life. J Otolaryngol 2000; 29: 344–347. [PubMed] [Google Scholar]
- 6.Tufarelli D, Meli A, Alesii A, et al. Quality of life after acoustic neuroma surgery. Otol Neurotol 2006; 27: 403–409. [DOI] [PubMed] [Google Scholar]
- 7.Magliulo G, Zardo F. Facial nerve function after cerebellopontine angle surgery and prognostic value of intraoperative facial nerve monitoring: a critical evaluation. Am J Otolaryngol 1998; 19: 102–106. [DOI] [PubMed] [Google Scholar]
- 8.Bloch DC, Oghalai JS, Jackler RK, et al. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg 2004; 130: 104–112. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Oishi M, Hiraishi T, et al. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg 2011; 114: 1224–1231. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MS, Kari E, Strickland BM, et al. Evaluation of the increased use of partial resection of large vestibular schwanommas: facial nerve outcomes and recurrence/regrowth rates. Otol Neurotol 2013; 34: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 11.Seol HJ, Kim CH, Park CK, et al. Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo) 2006; 46: 176–180. [DOI] [PubMed] [Google Scholar]
- 12.Vakilian S, Souhami L, Melançon D, et al. Volumetric measurement of vestibular schwannoma tumour growth following partial resection: predictors for recurrence. J Neurol Surg B Skull Base 2012; 73: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sughrue ME, Kaur R, Rutkowski MJ, et al. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg 2011; 114: 1218–1223. [DOI] [PubMed] [Google Scholar]
- 14.Virk JS, Tripathi S, Randhawa PS, et al. Tumour resection volumes and facial nerve outcomes for vestibular schwannomas. Indian J Otolaryngol Head Neck Surg 2014; 66: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Kashlan HK, Zeitoun H, Arts HA, et al. Recurrence of acoustic neuroma after incomplete resection. Am J Otol 2000; 21: 389–392. [DOI] [PubMed] [Google Scholar]
- 16.Bedavanija A, Brieger J, Lehr HA, et al. Association of proliferative activity and size in acoustic neuroma: implications for timing of surgery. J Neurosurg 2003; 98: 807–811. [DOI] [PubMed] [Google Scholar]
- 17.Light JP, Roland JT, Jr, Fishman A, et al. Atypical and low-grade malignant vestibular schwannomas: clinical implications of proliferative activity. Otol Neurotol 2001; 22: 922–927. [DOI] [PubMed] [Google Scholar]
- 18.Lesser TH, Janzer RC, Kleihues P, et al. Clinical growth rate of acoustic schwannomas: correlation with the growth fraction as defined by the monoclonal antibody ki-67. Skull Base Surg 1991; 1: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cafer S, Bayramoglu I, Uzum N, et al. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol 2008; 122: 125–127. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly BF, Kishore A, Crowther JA, et al. Correlation of growth factor receptor expression with clinical growth in vestibular schwannomas. Otol Neurotol 2004; 25: 791–796. [DOI] [PubMed] [Google Scholar]
- 21.Charabi S, Engel P, Charabi B, et al. Growth of vestibular schwannomas: in situ model employing the monoclonal antibody Ki-67 and DNA flow cytometry. Am J Otol 1996; 17: 301–306. [PubMed] [Google Scholar]
- 22.Charabi S, Engel P, Jacobsen GK, et al. Growth rate of acoustic neuroma expressed by Ki-67 nuclear antigen versus symptom duration. Ann Otol Rhinol Laryngol 1993; 102: 805–809. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama M, Matsuda M, Nakasu S, et al. Clinical significance of Ki-67 staining index in acoustic neurinoma. Neurol Med Chir (Tokyo) 1996; 36: 698–702. [DOI] [PubMed] [Google Scholar]
- 24.Niemczyk K, Vaneecloo FM, Lecomte MH, et al. Correlation between Ki-67 index and some clinical aspects of acoustic neuromas (vestibular schwannomas). Otolaryngol Head Neck Surg 2000; 123: 779–783. [DOI] [PubMed] [Google Scholar]
- 25.Sanna M, Taibah A, Russo A, et al. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol 2004; 25: 379–386. [DOI] [PubMed] [Google Scholar]
- 26.Heman-Ackah SE, Golfinos JG, Roland JT., Jr Management of surgical complications and failures in acoustic neuroma surgery. Otolaryngol Clin North Am 2012; 45: 455–470. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Fung K, Lownie SP, et al. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg 2007; 133: 56–60. [DOI] [PubMed] [Google Scholar]
- 28.Martin TP, Fox H, Ho EC, et al. Facial nerve outcomes in functional vestibular schwannoma surgery: less than total tumour excision significantly improves results. J Laryngol Otol 2012; 126: 120–124. [DOI] [PubMed] [Google Scholar]
- 29.Kameyama S, Tanaka R, Kawaguchi T, et al. Long-term follow-up of the residual intracanalicular tumours after subtotal removal of acoustic neurinomas. Acta Neurochir (Wien) 1996; 138: 206–209. [DOI] [PubMed] [Google Scholar]
- 30.Kemink JL, Langman AW, Niparko JK, et al. Operative management of acoustic neuromas: the priority of neurologic function over complete resection. Otolaryngol Head Neck Surg 1991; 104: 96–99. [DOI] [PubMed] [Google Scholar]
- 31.Magliulo G, Gagliardi M, Ciniglio Appiani G, et al. Preservation of the saccular nerve and of the vestibular evoked myogenic potential during vestibular schwannoma surgery. Otol Neurotol 2003; 24: 308–311. [DOI] [PubMed] [Google Scholar]
- 32.Monfared A, Corrales CE, Theodosopoulos PV, et al. Facial nerve outcome and tumor control rate as a function of degree of resection in treatment of large acoustic neuromas: preliminary report of the acoustic neuroma subtotal resection study (ANSRS). Neurosurgery 2016; 79: 194–203. [DOI] [PubMed] [Google Scholar]
- 33.Carlson ML, Van Abel KM, Driscoll CL, et al. Magnetic resonance imaging surveillance following vestibular schwannoma resection. Laryngoscope 2012; 122: 378–388. [DOI] [PubMed] [Google Scholar]
- 34.Jacob JT, Carlson ML, Driscoll CL, et al. Volumetric analysis of tumor control following subtotal and near-total resection of vestibular schwannoma. Laryngoscope 2016; 126: 1877–1882. [DOI] [PubMed] [Google Scholar]
- 35.Brokinkel B, Sauerland C, Holling M, et al. Gamma knife radiosurgery following subtotal resection of vestibular schwannoma. J Clin Neurosci 2014; 21: 2077–2082. [DOI] [PubMed] [Google Scholar]
- 36.van de Langenberg R, Hanssens PE, van Overbeeke JJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 2011; 115: 875–884. [DOI] [PubMed] [Google Scholar]