Abstract
Objective
To describe the characteristics of the perforator vessel in the peroneal artery of the lower leg and to explore the use of perforator pedicle propeller flaps to repair soft tissue defects in the lower leg, heel and foot.
Methods
This retrospective study enrolled patients with soft tissue defects of the distal lower leg, heel and foot who underwent surgery using peroneal perforator-based propeller flaps. The peroneal artery perforators were identified preoperatively by colour duplex Doppler ultrasound. The flap was designed based on the preoperatively-identified perforator location, with the posterior border of the fibula employed as an axis, and the perforator vessel as the pivot point of rotation. Patients were followed-up to determine the outcomes.
Results
The study analysed 36 patients (mean age, 39.7 years). The majority of the soft tissue defects were on the heel (20; 55.6%). The donor-site of the flap was closed in 11 patients by direct suturing and skin grafting was undertaken in 25 patients. Postoperative complications included venous congestion (nine patients), which was managed with delayed wound coverage and bleeding therapy. All wounds were eventually cured and the flaps were cosmetically acceptable.
Conclusions
The peroneal perforator pedicle propeller flap is an appropriate choice to repair soft tissue defects of the distal limbs.
Keywords: Perforator-based propeller flaps, soft tissue reconstruction, peroneal artery, congestion
Introduction
Soft tissue reconstruction still presents challenges to modern day medicine.1–5 The limitation often arises from the availability of overlying skin at the lower leg after trauma, thus a free flap is often recommended to repair the wound, but this is extremely time consuming and requires years of training and experience. Nevertheless, extraction and reconstruction based on perforator flaps has provided the next step in reconstructive surgery with improved understanding of the vascular anatomy and cutaneous circulation.6–9
According to the Gent consensus,10 perforator flaps comprise of skin and subcutaneous fat that are provided with nutrition by perforators rising from deep vascular arteries embedded in the muscle and intramuscular septa. Because of the anatomical characteristics of ‘skin and bone’ in the lower leg, soft tissue defects are manifested due to trauma and surgery, and the resulting deep tendon and bone exposure all need flap repair. Muscle tissues in the upper leg are very abundant, more supple, and not easily damaged. Therefore, the application of perforator flaps or neurocutaneous vascular flap transposition can enable repair of a soft tissue defect wound in one-third of the lower leg, foot and ankle. With further studies, some researchers proposed the concept of propeller flaps.11–13 Because the texture of these flaps is similar to the recipient site, in addition to their good appearance and their ability to partially or completely repair the donor-site, their application has become widely adopted.4,11,14–18 However, as with all pedicle flaps, venous congestion is still the major postoperative complication and the primary cause of flap necrosis.19,20 Ever since the propeller flap concept was proposed in the 1990s for skin tissue reconstruction of the body,21 it has gained popularity in the reconstruction of lower extremities due to its high flexibility and versatility. The propeller flap pertaining to the peroneal artery enables an initial incision from either the medial or lateral side, and a reliable perforator can be designed just by visual inspection.22–24 In addition, modification of the flap can be easily facilitated based on a perforator being chosen (one perforator is sufficient as it is able to provide an effective supply of blood to the entire flap). On the contrary, more perforators hinder rotation and may even result in the kinking of blood vessels.
Various issues have been raised on the use of perforator pedicle propeller flaps for soft tissue reconstruction, such as the challenge faced by soft tissue reconstruction, the advantages of this technique, anatomical and physiological details of perforator-based propeller flaps, as well as venous congestion of the flap.4,11,21 This current report aims to illustrate some of the surgical success that has been achieved at our institution based on the understanding of these concepts. Perforator pedicle propeller flaps can be surgically implemented for various medical conditions that urgently require skin tissue reconstruction.12–15 Using observations in clinical practice, this current report describes the characteristics of the perforator vessel in the peroneal artery of the lower leg and explores the methods used to reduce the postoperative complication of inverse venous flow.
Patients and methods
Study population
This retrospective observational study enrolled consecutive patients with soft tissue defects of the distal lower leg, heel and foot who underwent surgery using peroneal perforator-based propeller flaps in the Department of Orthopaedic Surgery, Zhejiang Province Tongde Hospital, Hangzhou, Zhejiang Province, China between June of 2011 to June of 2013. Patients with vascular disease were excluded from this study.
This study did not require ethical approval as it was retrospective. Each patient provided written informed consent prior to surgery.
Surgical procedures for peroneal artery perforator-based propeller flaps
Anatomical definitions
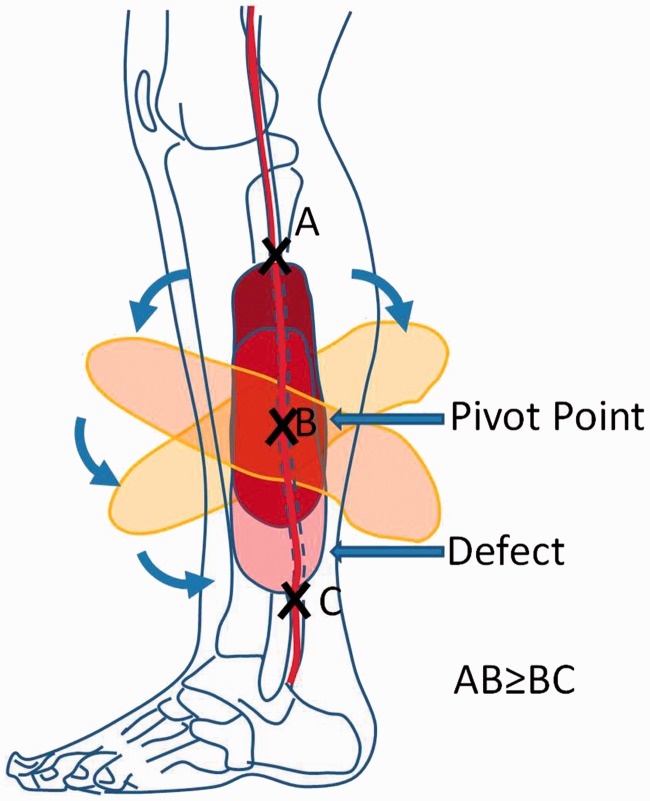
Dissection causes the peroneal artery perforator-based flap to be easily raised, so that it can be used as a propeller flap.16 Such a flap is a local island fasciocutaneous flap arising from a dissected perforator. Here, the retrieved skin of the proximal leg may be used for surgical reconstruction at the regions of the distal lower extremity, based on a 180° perforator-based propeller flap for a reliable coverage of the soft tissue defect. This surgical process is illustrated in a Figure 1.
Figure 1.
Schematic diagram illustrating the surgical procedure for peroneal artery perforator-based propeller flap isolation. Dissection of the perforator enables rotation and allows sufficient mobility so that a fasciocutaneous flap can be isolated and is then rotated 180° like a ‘propeller’. The opened section caused by the rotated flap being moved away from its point of origin is then closed with direct suturing or skin grafting. (A) The proximal point of the propeller flap; (B) the pivot point of the flap (the position of the peroneal perforator); and (C) the distal point of the wound. The arrows indicate the clockwise or anticlockwise rotation of the flap. The colour version of this figure is available at: http://imr.sagepub.com.
The surgical procedure
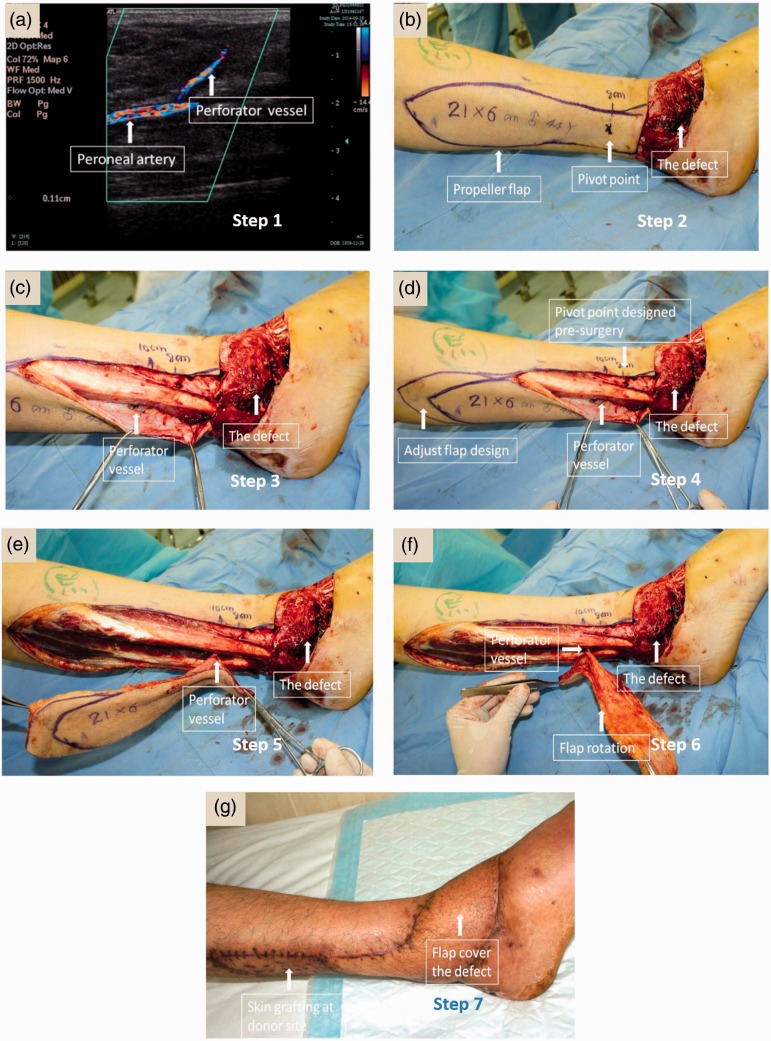
Typically, the peroneal artery perforator-based propeller flaps can be identified preoperatively by colour duplex Doppler ultrasound scanning and are located along the lateral aspect of the lower leg from the lateral malleolus (Figure 2a, Figure 3 – step 1). The next step is to plan the shape and size of the propeller flap near regions of the major perforator proximal to the defect. The longitudinal length of the flap is the distance between the perforator nearest to the defect region added to the longitudinal length of the defect (Figure 2b, Figure 3 – step 2). Based on the defect location, an option of either an anterior or posterior incision is made. The surgical incision can be executed from one side to ensure that the major perforator is well nourished (Figure 2c, Figure 3 – step 3). If the position of the real perforator is different from what was identified by the preoperative Doppler ultrasound scan, the size of the flap is adjusted appropriately according to the real position of the perforator vessel (Figure 2d, Figure 3 – step 4).
Figure 2.
Surgical procedure for peroneal artery perforator-based propeller flap isolation. Steps 1 to 7 illustrate the procedure of harvesting a peroneal perforator-based propeller flap by an experienced surgeon. Representative images were taken during surgery on the same patient. The colour version of this figure is available at: http://imr.sagepub.com.
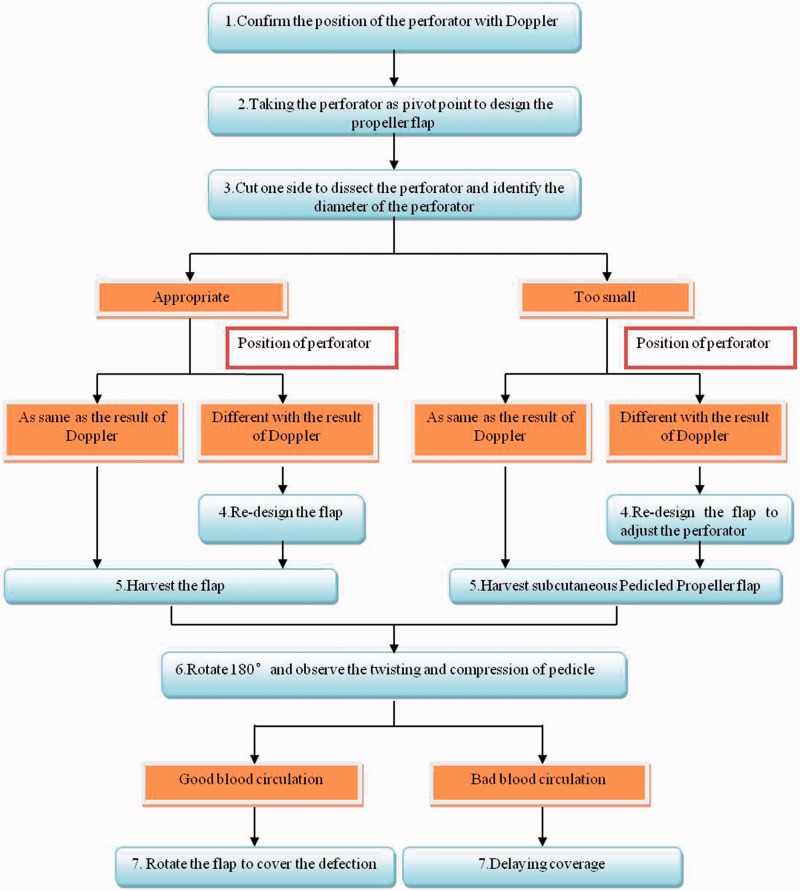
Figure 3.
Operation flowchart showing the surgical processes undertaken and the surgical decisions that need to be made during the procedure. An operation flowchart is important in depicting the process and decisions made during surgery and may serve as a reference for surgeons. This illustrates the chain of decision making by an experienced surgeon during the entire course of the surgery and has been used as a standard operating procedure in our hospital.
The perforator-based propeller flap varies in shape and size, depending on the limiting spatial constraint of the perforator and on the defect region. As demonstrated in Figure 2e and Figure 3 – step 5, the skin is surgically extracted from the proximal section of the lateral leg by having the major perforator as the pivot. The perforator is dissected to facilitate rotation and mobility, so that an isolated and mobilized fasciocutaneous flap can be formed and then rotated 180° (Figure 2f, Figure 3 – step 6); this is what makes it to a ‘propeller’, and hence it turns to fit the defect (Figure 3 – step 7). The opened donor-site area due to the rotation of the flap can then be skin grafted to close it, as shown by the lighter patch of skin (Figure 2g), or it can be sutured directly.
In summary, the details of the surgical procedures are described as follows: (i) step 1: the colour duplex Doppler ultrasound was used to examine and mark the position of the peroneal perforator, in order to design the flap before surgery; (ii) step 2: the flap was designed based on the perforator place examined prior to surgery, with the posterior border of the fibula employed as an axis, and the perforator vessel adopted as an axis point of rotation. The big propeller is proximal to the axis point. The length of the big propeller is 0.5–1 cm longer than the distance from the pivot point to the distal point of the wound. The small propeller is the area from axis point to the defect. The width of flap is 1–2 cm larger than the defect, and the thickness of the subcutaneous fat determines this size difference; (iii) step 3: the anterior edge of the flap was dissected according to the preoperative design. Then, the dissection was performed at a site further from the lower part of the deep fascia to the intermuscular septum in the rear of the peroneus longus and brevis; thereby, the peroneal perforator was dissected; (iv) step 4: the size of flap was appropriately adjusted according to the real position of the perforator vessel; (v) step 5: after fulfilling the flap harvesting, the perforator vessel was dissected to the main part of the peroneal artery, so as to increase the length of the vascular pedicle. The fascia tissues of the intermuscular septum around the vascular pedicle were also carefully removed to decrease the venous drainage obstacle caused by the compression of the vascular pedicle after rotation; (vi) step 6: the conditions of the vascular pedicle were observed after its twisting and compression due to the 180° rotation; (vii) step 7: the flap was sutured to the wound after the rotation if the blood circulation was good, and the donor-site of the flap was sutured directly. Free-skin-grafting could be carried out to repair the donor-site in the case of the failure of direct suturing. Two weeks after the operation, the flaps had completely survived and the wound was healed. If the venous return was bad after the rotation, which indicates that the capillary hyperaemia reaction was quicker than normal, then the wound coverage was delayed. Delayed coverage meant that the flap was rotated and covered the wound, but the wound was not sutured around the pedicle of the flap; and 3–5 days later, when the flap swelling was reduced, delayed suturing was undertaken around the pedicle of the flap.
If the flap had venous congestion when the patient came back from the ward, then ‘bleeding therapy’ was used to manage it. This involved cutting small incisions (approximately 5 mm in size) at the peripheral area of the flap and bleeding from these incisions was maintained using heparin saline (25 U/ml). Careful monitoring was undertaken to prevent excessive blood loss.
During follow-up, the flap and donor-sites were checked by the physicians and the patients were asked their views on the appearance of both sites.
Statistical analyses
Limited descriptive statistical analyses were performed using the SPSS® statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows® and data are presented as mean ± SD.
Results
From June of 2011 to June of 2013, 36 patients with soft tissue defects of the distal lower leg, heel and foot were successfully treated using peroneal perforator-based propeller flaps. None of the patients had diabetes mellitus or vascular disease. The demographic and clinical data for each patient are presented in Table 1. There were 29 men (80.6%) and seven women (19.4%), with a mean ± SD age of 39.7 ± 20.3 years (range, 6–83 years). The majority of the soft tissue defects were on the heel (20 of 36, 55.6%); with seven on the ankle (19.4%), five on the distal lower leg (13.9%), and four on the dorsal foot (11.1%). The size of the soft tissue defects ranged from 4 × 2 cm to 20 × 6 cm. The reason for the soft tissue defect was trauma in all patients. The sizes of the flaps ranged from 10 × 5 cm to 34 × 18 cm.
Table 1.
Demographic and clinical data for 36 patients with soft tissue defects of the distal lower leg, heel and foot who were successfully treated using peroneal perforator-based propeller flaps.
| Patient | Sex | Age | Position | Soft tissue defect size*, cm2 | Flap size*, cm2 | Distance from the perforator to the lateral malleolus, cm | Postoperative complication | Additional processing | Donor-site closure method |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 29 | Heel | 16 × 5 | 34 × 18 | 12 | Skin grafting | ||
| 2 | M | 37 | Heel | 4 × 2.5 | 18 × 3 | 7 | Direct suturing | ||
| 3 | M | 30 | Heel | 7 × 3 | 12 × 5 | 10 | Skin grafting | ||
| 4 | M | 49 | Foot | 6 × 4 | 24 × 4 | 8 | Venous congestion | Delayed wound coverage | Direct suturing |
| 5 | F | 68 | Heel | 6 × 4 | 20 × 5 | 10 | Direct suturing | ||
| 6 | M | 41 | Heel | 5 × 3 | 26 × 5 | 9 | Direct suturing | ||
| 7 | M | 60 | Ankle | 6 × 5 | 30 × 8 | 10 | Skin grafting | ||
| 8 | M | 41 | Lower leg | 9 × 7 | 14 × 9 | 12 | Infection | Skin grafting | Skin grafting |
| 9 | M | 7 | Lower leg | 10 × 6 | 18 × 6 | 11 | Venous congestion | Delayed wound coverage | Skin grafting |
| 10 | M | 49 | Heel | 12 × 6 | 24 × 13 | 10 | Venous congestion | Without further processing | Skin grafting |
| 11 | M | 53 | Lower leg | 7 × 4 | 14 × 5 | 16 | Skin grafting | ||
| 12 | M | 17 | Lower leg | 10 × 3 | 15 × 4 | 15 | Skin grafting | ||
| 13 | M | 60 | Heel | 7 × 4 | 16 × 6 | 11 | Skin grafting | ||
| 14 | M | 62 | Ankle | 7 × 5 | 14 × 7 | 7 | Venous congestion | Bleeding therapy | Skin grafting |
| 15 | M | 83 | Heel | 7 × 4 | 23 × 7 | 8 | Skin grafting | ||
| 16 | M | 64 | Ankle | 12 × 7 | 27 × 13 | 8 | Skin grafting necrosis at the donor-site | Debridement and repeated skin grafting | Skin grafting |
| 17 | M | 48 | Heel | 12 × 9 | 29 × 13 | 11.5 | Skin grafting | ||
| 18 | M | 51 | Heel | 6 × 5 | 32 × 5 | 10 | Direct suturing | ||
| 19 | M | 54 | Ankle | 6 × 6 | 25 × 7 | 13 | Haematocele | Dressing change | Direct suturing |
| 20 | M | 17 | Heel | 10 × 7 | 20 × 7 | 10 | Venous congestion | Bleeding therapy, debridement, skin grafting | Skin grafting |
| 21 | M | 43 | Heel | 4 × 4 | 10 × 5 | 8 | Direct suturing | ||
| 22 | M | 49 | Heel | 8 × 7 | 17 × 8 | 11 | Skin grafting | ||
| 23 | M | 7 | Ankle | 9 × 7 | 17 × 8 | 10 | Skin grafting | ||
| 24 | F | 51 | Heel | 8 × 3 | 20 × 4 | 6 | Direct suturing | ||
| 25 | F | 8 | Heel | 6 × 5 | 17 × 8 | 8 | Skin grafting | ||
| 26 | M | 17 | Foot | 12 × 7 | 26 × 8 | 9 | Venous congestion | Bleeding therapy, debridement, skin grafting | Skin grafting |
| 27 | M | 37 | Heel | 4 × 2 | 16 × 3 | 6 | Venous congestion | Bleeding therapy | Direct suturing |
| 28 | M | 49 | Ankle | 12 × 6 | 25 × 8 | 13 | Skin grafting | ||
| 29 | M | 57 | Lower leg | 8 × 3 | 20 × 5 | 18 | Skin grafting | ||
| 30 | M | 26 | Heel | 7 × 3 | 22 × 8 | 10 | Venous congestion | Delayed wound coverage | Skin grafting |
| 31 | M | 8 | Heel | 8 × 7 | 15 × 8 | 8 | Venous congestion | Bleeding therapy | Skin grafting |
| 32 | F | 45 | Foot | 20 × 6 | 28 × 7 | 14 | Direct suturing | ||
| 33 | F | 53 | Foot | 5 × 3 | 23 × 5 | 8 | Skin grafting | ||
| 34 | F | 8 | Heel | 6 × 4 | 15 × 7 | 8 | Skin grafting | ||
| 35 | M | 6 | Heel | 5 × 5 | 15 × 5 | 7 | Skin grafting | ||
| 36 | M | 44 | Ankle | 13 × 5 | 25 × 6 | 10 | Direct suturing |
Size shown as length × width.
The donor-site of the flap was closed by direct suturing in 11 of 36 patients (30.6%) and skin grafted in 25 of 36 patients (69.4%) (Table 1). All of the wounds were eventually cured. As the appearance of these flaps was similar to the tissues around the wounds and there were no ‘dog ears’ at the pedicles of the flaps, the flaps were considered to be cosmetically acceptable by both the patients and doctors. The mean ± SD distance between the perforator vessels of the flaps and the lateral malleolus was 10.1 ± 2.8 cm (range, 6–18 cm), and 24 patients had perforators that were 8.0 ± 2.0 cm from the lateral malleolus. Complications included venous congestion (nine patients), haematocele (one patient), infection (one patient), and skin grafting necrosis at the donor-site (one patient). The flaps in seven of the nine patients with venous congestion survived, but two patients had necrosis at the distal site requiring debridement and skin grafting. The methods used to treat the venous congestion included delaying the wound coverage in the three patients in whom the flap survived and bleeding therapy in five patients, of which two patients developed necrosis. One of the nine patients had slight venous congestion and the flap survived without any further processing.
Discussion
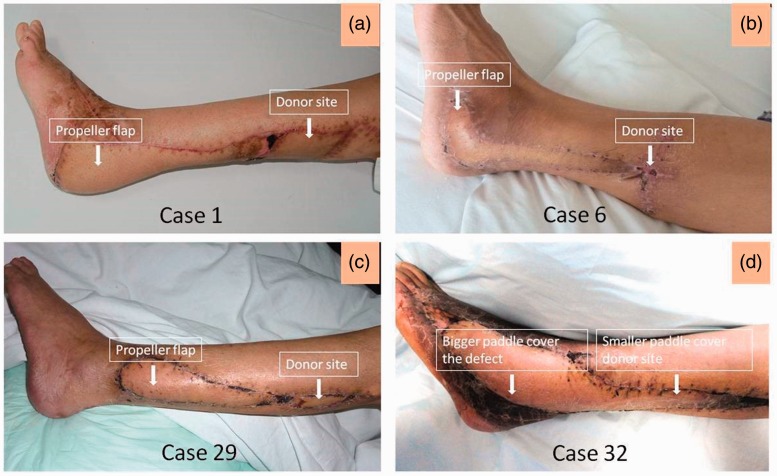
There are a number of important issues to consider when implementing perforator-based propeller flaps for soft tissue defect repair of the lower limbs. When the soft tissue defect in the leg is so severe that it has exposed the internal tendons or bone structures, the situation is complicated and requires more surgical planning than with the repair of less severe soft tissue defects. As a result of the limited local cutaneous and muscle flaps available for grafting around the ankle, the preferred option is to have tissue transfer from the middle and upper leg area. The perforator-based propeller flap is comparable to a local flap in terms of the amount and firmness of the subcutaneous tissue, skin texture, and the possibility of reconstructing ‘like with like’. Similar to a local perforator flap, it is characterized by freedom of choice of skin island shape and dimension, and safety of perfusion. Using a free flap might be a more common procedure, but it is well known that the free flap procedure needs a higher technical level of surgical expertise and more complex surgery.4,18,25 Compared with a free flap, using a perforator-based propeller flap requires a simpler operation and shorter operating times, and can be performed without the staff expertise and complex logistical setup.4 The use of a fibula osteoseptocutaneous flap provides good evidence of how peroneal artery perforator-based propeller flaps are a better option for skin harvesting of a larger area.23,24 Furthermore, perforator-based propeller flaps offer versatility in terms of providing different shapes and sizes, and they can be easily retrieved from other parts of the body for covering small defects in the lower leg.21,24 Most importantly, this procedure ensures blood supply by avoiding the major blood vessels. In particular, its greater rotation versatility allows an easier distal lower leg soft tissue reconstruction. Detailed examination of the perforator-based propeller flap technique has shown it to be a highly reliable and the preferred surgical option for treating soft tissue defects of the lower leg.12–14 It also provides a good cosmetic postoperative appearance as shown in Figure 4.
Figure 4.
Examples of the aesthetics of the postoperative local flap reconstruction in four patients with soft tissue defects of the distal lower leg, heel and foot who were successfully treated using peroneal perforator-based propeller flaps. These images demonstrate successful recovery and the good cosmetic appearance of the soft tissue reconstruction in each of these patients. Each of the patients had no issue with wearing socks or shoes after the operation due to the nicely fitted contours of the flap over the defect. The colour version of this figure is available at: http://imr.sagepub.com.
The traditional fasciocutaneous flap always has a skin bridge at the pedicle. Keeping the skin bridge at the base of a peninsular flap may in fact kink the pedicle, which might compromise the vascular supply of the flap. This makes flap rotation difficult and calls for the need to harvest a bigger flap.26–31 The propeller flap always needs a skeleton of pedicle vessels, so that it can rotate freely from 0° to 180°. Flap rotation will work successfully as long as the pedicle does not become tight after the flap rotation.
Alternative local flaps are also used, such as the distally-based sural flap or the distally-based peroneus brevis flap.32–34 Wounds that can to be covered by a peroneal perforator-based pedicle propeller flap can also be covered by both the distally-based sural flap and the distally-based peroneus brevis flap; and these two flaps are easier to undertake surgically than the propeller flap at the pedicles of the flaps.32–34 However, these two types of flaps produce ‘dog ears’ at the pedicle after flap rotation. It is evident that the bulky ‘dog ears’ of the traditional fasciocutaneous flap is visually unappealing and if the aesthetics of the leg are poor, then the operation would need to be performed again, especially if the ankle is distorted and wearing footwear is not possible. In this case, cosmetic surgery may also be necessary. Furthermore, application of the distally-based sural flap would damage the nerve, which then leads to sensation dysfunction.27,28 In contrast, the peroneal perforator-based pedicle propeller flap does not damage the nerve.16 In terms of postoperative complications, such as venous congestion, haematocele, infection, and skin grafting necrosis at the donor-site, the rates of these are similar for the distally-based sural flap, the distally-based peroneus brevis flap and the peroneal perforator-based pedicle propeller flap.16,17,19,20,33,34
There are a number of disadvantages associated with using perforator-based propeller flaps. Unfortunately, venous congestion cannot be totally avoided during the propeller flap operation because the walls of the perforator vessels are more delicate compared with the perforator arterial wall and it is difficult to control venous wall damage after the 180° rotation of the pedicle.35 Venous congestion can give rise to necrosis at the distal flap (Figure 5). After rotation of the flap, if the pedicle is pulled tightly it will lead to venous congestion. If the pedicle is not bared, the deep fascia around the pedicle will compress the vessels in the pedicle when the flap is rotated.36 Venous congestion is one of the most difficult problems to deal with and constitutes one of the main reasons for flap necrosis.19,20 A precise preoperative plan can reduce the rate of venous congestion.37,38 In this present series of patients, colour duplex Doppler ultrasound was used preoperatively to: (i) determine the position, diameter and length of the peroneal artery perforator; and (ii) determine which perforator would be suitable for the flap. In the current group of 36 patients, the mean ± SD distance between the perforator and lateral malleolus was 10.1 ± 2.8 cm (range, 6–18 cm), and 24 patients had perforators that were 8.0 ± 2.0 cm from the lateral malleolus. The perforator always had ascending and descending branches, so that it can make the flap longer.
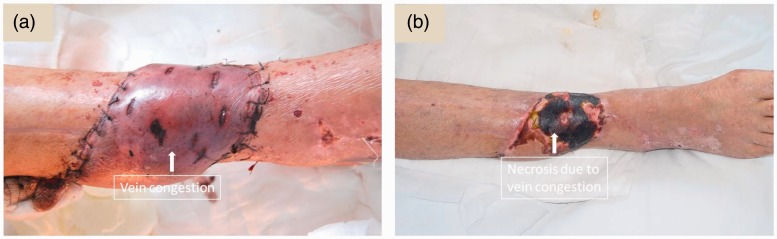
Figure 5.
Representative images showing venous congestion and tissue necrosis. The colour of the skin patch turns purple due to occlusion of the vein, which is known as venous congestion (a). On some occasions, it may lead to necrosis of the distal part of the flap (b). The colour version of this figure is available at: http://imr.sagepub.com.
The typical length of the flap may range from approximately 5 to 25 cm and have an approximate width from 3 to 8 cm. The perforator flap extraction is mostly determined by the nature of the soft tissue defect and is usually around 3 to 25 cm above the lateral malleolus. However, to repair a soft tissue defect around the ankle, it is not practical to use a propeller flap if the pedicle is far from the wound. The septocutaneous vessels from a peroneal artery can supply a skin island of up to a length and width of 22–25 cm and 10–14 cm, respectively.30,39 In the current group of 36 patients, the length of the flap ranged from 10 to 34 cm. The pivot point of the peroneal artery perforator-based flap is preferably based on the distal perforators, as it can facilitate a larger section of the proximal healthy skin to be positioned over the soft tissue defect.38 The performance of the flap relies on: (i) the flap length; (ii) the pedicle size; and (iii) the rotation angle.21
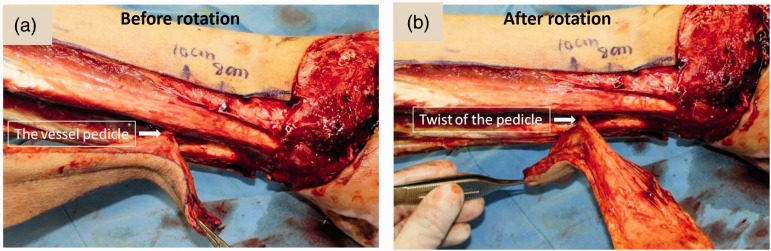
In terms of the method used for pedicle rotation, the perforator artery is examined before and after rotation during surgery as shown in Figure 6. The strain on the blood vessel is carefully reduced. During the operation, the surgeon determines the diameter and number of concomitant veins of the perforator artery. Usually, there are two concomitant veins, but sometimes only a single vein is available. Based on our experience, if the concomitant vein is single or if its diameter is narrow, it is better that a superficial vein of the flap should be microsurgically anastomosed to a recipient vein in order to increase the venous drainage from the flap. The pedicle should be slack when flap rotation is performed. It is better to dissect the pedicle as much as possible, especially when the rotation angle is more than 120°. A skeleton pedicle may be necessary. It has been demonstrated that the risk of vessel buckling is decreased when the perforator is of 1-mm diameter and the vessel length is more than 3 cm.35 The direction of rotation is also very important and it is crucial to try different directions of rotation during the operation (i.e. clockwise or anticlockwise rotation) in order to increase the slackness of the pedicle.
Figure 6.
Exposure of the perforator artery during perforator-based propeller flap isolation. The strain placed on the blood vessels following the surgical procedure was examined before rotation (a) and after rotation (b) of the flap. The colour version of this figure is available at: http://imr.sagepub.com.
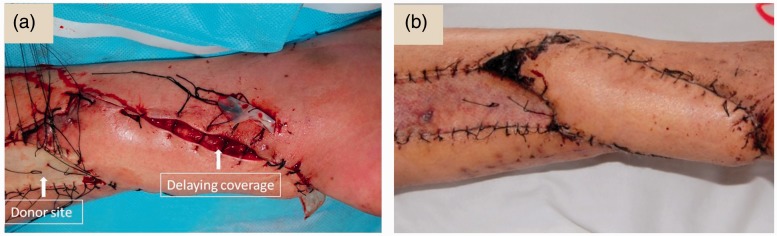
In this current series of patients, the flap was sutured in situ, then the tourniquet was relaxed. The blood circulation of the flap was then closely observed for 3–5 minutes, carefully checking for the colour and capillary hyperaemia reaction. If the blood circulation was good, the flap was rotated to cover the defect. If the blood circulation was insufficient, a delayed coverage method was employed, which means that the flap was rotated to cover the wound, but the wound was not sutured around the pedicle of the flap. When the swelling of the flap was reduced 3–5 days later, suturing was then performed (Figure 7).
Figure 7.
Delaying coverage and suturing of the flap due to venous congestion. (a) If the surgeon finds that the flap is too tight to suture during the operation, then the flap may not be completely sutured. (b) After 3–5 days, when the swelling of the flap has reduced, the surgeon can complete the suturing and wound coverage. This helps to avoid the skin tearing. The colour version of this figure is available at: http://imr.sagepub.com.
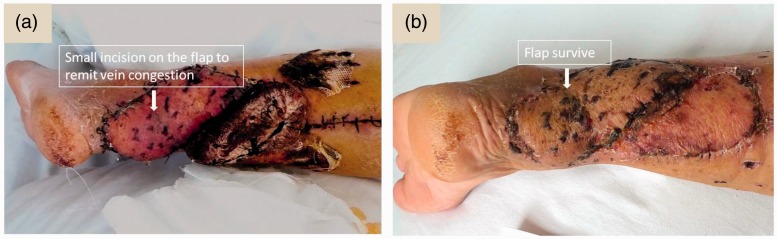
Another way to rectify the problem of venous congestion is to massage the flap from the peripheral margins towards the centre. If the venous congestion persists, a few sutures can be loosened at the flap edge and adequate massaging can be performed to relieve the venous congestion.24 If the venous congestion can’t be released by massaging, then bleeding therapy can be used as follows. Some small incisions (approximately 5 mm in size) can be made around the flap to allow continuous bleeding of the flap. If this method is employed, then physicians must monitor blood pressure and heart rate, because in the case of excessive bleeding, blood transfusion will need to be given immediately (Figure 8).
Figure 8.
Analysis of the flap after the use of bleeding therapy to control venous congestion. (a) A surgical expert made some small incisions at the peripheral area of the flap when postoperative venous congestion occurred in order to release the blood. (b) The flap can be saved as a result of this procedure. The colour version of this figure is available at: http://imr.sagepub.com.
In conclusion, the perforator-based propeller flap technique provides a similar texture of skin as that of the recipient area, a large range of possible rotation, an easy operation and a small wound. In addition, the donor-site of the flap can be closed directly without influencing the appearance. Due to the aforementioned advantages associated with using a peroneal perforator-based propeller flap, it has been widely used in clinical practice to repair soft tissue defects of the lower part of the leg, foot and ankle. A precondition of using this type of flap is the ability to preoperatively locate an appropriate perforator using colour duplex Doppler ultrasound. If one cannot be located, then it is more appropriate to use a distally-based sural flap or a free flap technique. Locating the precise position of the perforator preoperatively is one of the most important issues when planning to use this type of flap technique and in our opinion, colour Doppler is the best way to achieve this. Alternative methods, such as computed tomography angiogaphy37,40 and magnetic resonance angiography,41 have also been suggested to identify the precise position of the perforator. Venous congestion remains the main complication postoperatively and proper pedicle processing and flap design can improve venous congestion. Based on our experience, when venous congestion occurs after surgery, delaying the wound coverage and bleeding therapy are useful methods for controlling it.
Acknowledgements
The authors express their appreciation for the clinical support from and valuable discussions with surgeons at the Southern Medical University, Guangzhou, Guangdong Province, China.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Science and Technology Plan Projects in Zhejiang Province (grant no. 2015C33195) and the Nature Science Foundation of China (grant no. 81271993).
References
- 1.Chang SM, Hou CL, Xu DC. An overview of skin flap surgery in the mainland china: 20 years’ achievements (1981 to 2000). J Reconstr Microsurg 2009; 25: 361–367. [DOI] [PubMed] [Google Scholar]
- 2.Chen YL, Zheng BG, Zhu JM, et al. Microsurgical anatomy of the lateral skin flap of the leg. Ann Plast Surg 1985; 15: 313–318. [DOI] [PubMed] [Google Scholar]
- 3.Chesnier I, Bali D, Casanova D, et al. Flaps in lower limb reconstruction: a 10-year retrospective review of 157 pedicled flaps. Ann Chir Plast Esthet 2012; 57: 328–335. [in French, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 4.Jakubietz RG, Jakubietz DF, Gruenert JG, et al. Reconstruction of soft tissue defects of the Achilles tendon with rotation flaps, pedicled propeller flaps and free perforator flaps. Microsurgery 2010; 30: 608–613. [DOI] [PubMed] [Google Scholar]
- 5.Lo CH, Leung M, Baillieu C, et al. Trauma centre experience: flap reconstruction of traumatic lower limb injuries. ANZ J Surg 2007; 77: 690–694. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Matsumura H, Miyaki T, et al. An anatomic study of the intermuscular septum of the lower leg; branches from the posterior tibial artery and potential for reconstruction of the lower leg and the heel. J Plast Reconstr Aesthet Surg 2006; 59: 835–838. [DOI] [PubMed] [Google Scholar]
- 7.Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 2003; 30: 331–342. [DOI] [PubMed] [Google Scholar]
- 8.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987; 40: 113–141. [DOI] [PubMed] [Google Scholar]
- 9.Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg 1998; 102: 599–616. [PubMed] [Google Scholar]
- 10.Blondeel PN, Van Landuyt KH, Monstrey SJ, et al. The “Gent” consensus on perforator flap terminology: preliminary definitions. Plast Reconstr Surg 2003; 112: 1378–1382. [DOI] [PubMed] [Google Scholar]
- 11.Jakubietz RG, Jakubietz MG, Gruenert JG, et al. The 180-degree perforator-based propeller flap for soft tissue coverage of the distal, lower extremity: a new method to achieve reliable coverage of the distal lower extremity with a local, fasciocutaneous perforator flap. Ann Plast Surg 2007; 59: 667–671. [DOI] [PubMed] [Google Scholar]
- 12.Pignatti M, D'Arpa S, Cubison TCS. Novel fasciocutaneous flaps for the reconstruction of complicated lower extremity wounds. Techniques in Orthopedics 2009; 24: 88–95. [Google Scholar]
- 13.Pignatti M, Pasqualini M, Governa M, et al. Propeller flaps for leg reconstruction. J Plast Reconstr Aesthet Surg 2008; 61: 777–783. [DOI] [PubMed] [Google Scholar]
- 14.Chang SM, Tao YL, Zhang YQ. The distally perforator-pedicled propeller flap. Plast Reconstr Surg 2011; 128: 575e–577e. [DOI] [PubMed] [Google Scholar]
- 15.Jiga LP, Barac S, Taranu G, et al. The versatility of propeller flaps for lower limb reconstruction in patients with peripheral arterial obstructive disease: initial experience. Ann Plast Surg 2010; 64: 193–197. [DOI] [PubMed] [Google Scholar]
- 16.Lu TC, Lin CH, Lin YT, et al. The peroneal artery perforator-based propeller flap for distal lower limb reconstruction. JTSPS 2011; 20: 196–202. [Google Scholar]
- 17.Ono S, Sebastin SJ, Yazaki N, et al. Clinical applications of perforator-based propeller flaps in upper limb soft tissue reconstruction. J Hand Surg Am 2011; 36: 853–863. [DOI] [PubMed] [Google Scholar]
- 18.Pinsolle V, Reau AF, Pelissier P, et al. Soft-tissue reconstruction of the distal lower leg and foot: are free flaps the only choice? Review of 215 cases. J Plast Reconstr Aesthet Surg 2006; 59: 912–917. [DOI] [PubMed] [Google Scholar]
- 19.Innocenti M, Menichini G, Baldrighi C, et al. Are there risk factors for complications of perforator-based propeller flaps for lower-extremity reconstruction? Clin Orthop Relat Res 2014; 472: 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Blacam C, Colakoglu S, Ogunleye AA, et al. Risk factors associated with complications in lower-extremity reconstruction with the distally based sural flap: a systematic review and pooled analysis. J Plast Reconstr Aesthet Surg 2014; 67: 607–616. [DOI] [PubMed] [Google Scholar]
- 21.Pignattii M, Ogawa R, Hallock GG, et al. The “Tokyo” consensus on propeller flaps. Plast Reconstr Surg 2011; 127: 716–722. [DOI] [PubMed] [Google Scholar]
- 22.Akita S, Mitsukawa N, Rikihisa N, et al. Descending branch of the perforating branch of the peroneal artery perforator-based island flap for reconstruction of the lateral malleolus with minimal invasion. Plast Reconstr Surg 2013; 132: 461–469. [DOI] [PubMed] [Google Scholar]
- 23.John JR, Tripathy S, Sharma RK, et al. Peroneal artery perforator-based flaps for reconstruction of middle and lower third post-traumatic defects of the leg. ANZ J Surg 2015; 85: 869–872. [DOI] [PubMed] [Google Scholar]
- 24.Lu TC, Lin CH, Lin CH, et al. Versatility of the pedicled peroneal artery perforator flaps for soft-tissue coverage of the lower leg and foot defects. J Plast Reconstr Aesthet Surg 2011; 64: 386–393. [DOI] [PubMed] [Google Scholar]
- 25.Basheer MH, Wilson SM, Lewis H, et al. Microvascular free tissue transfer in reconstruction of the lower limb. J Plast Reconstr Aesthet Surg 2008; 61: 525–528. [DOI] [PubMed] [Google Scholar]
- 26.Chai Y, Zeng B, Zhang F, et al. Experience with the distally based sural neurofasciocutaneous flap supplied by the terminal perforator of peroneal vessels for ankle and foot reconstruction. Ann Plast Surg 2007; 59: 526–531. [DOI] [PubMed] [Google Scholar]
- 27.Chang SM, Zhang K, Li HF, et al. Distally based sural fasciomyocutaneous flap: anatomic study and modified technique for complicated wounds of the lower third leg and weight bearing heel. Microsurgery 2009; 29: 205–213. [DOI] [PubMed] [Google Scholar]
- 28.Fraccalvieri M, Bogetti P, Verna G, et al. Distally based fasciocutaneous sural flap for foot reconstruction: a retrospective review of 10 years experience. Foot Ankle Int 2008; 29: 191–198. [DOI] [PubMed] [Google Scholar]
- 29.Parodi P, De Biasio F, Rampino Cordaro E, et al. Distally-based superficial sural flap: advantages of the adipofascial over the fasciocutaneous flap. Scand J Plast Reconstr Surg Hand Surg 2010; 44: 37–43. [DOI] [PubMed] [Google Scholar]
- 30.Wei FC, Seah CS, Tsai YC, et al. Fibula osteoseptocutaneous flap for reconstruction of composite mandibular defects. Plast Reconstr Surg 1994; 93: 294–304. [PubMed] [Google Scholar]
- 31.Zhang FH, Chang SM, Lin SQ, et al. Modified distally based sural neuro-veno-fasciocutaneous flap: anatomical study and clinical applications. Microsurgery 2005; 25: 543–550. [DOI] [PubMed] [Google Scholar]
- 32.Al-Qattan MM. A modified technique for harvesting the reverse sural artery flap from the upper part of the leg: inclusion of a gastrocnemius muscle “cuff” around the sural pedicle. Ann Plast Surg 2001; 47: 269–278. [DOI] [PubMed] [Google Scholar]
- 33.Ajmal S, Khan MA, Khan RA, et al. Distally based sural fasciocutaneous flap for soft tissue reconstruction of the distal leg, ankle and foot defects. J Ayub Med Coll Abbottabad 2009; 21: 19–23. [PubMed] [Google Scholar]
- 34.Le Fourn B, Caye N, Pannier M. Distally based sural fasciomuscular flap: anatomic study and application for filling leg or foot defects. Plast Reconstr Surg 2001; 107: 67–72. [DOI] [PubMed] [Google Scholar]
- 35.Wong CH, Cui F, Tan BK, et al. Nonlinear finite element simulations to elucidate the determinants of perforator patency in propeller flaps. Ann Plast Surg 2007; 59: 672–678. [DOI] [PubMed] [Google Scholar]
- 36.Selvaggi G, Anicic S, Formaggia L. Mathematical explanation of the buckling of the vessels after twisting of the microanastomosis. Microsurgery 2006; 26: 524–528. [DOI] [PubMed] [Google Scholar]
- 37.Higueras Suñé MC, López Ojeda A, Narváez García JA, et al. Use of angioscanning in the surgical planning of perforator flaps in the lower extremities. J Plast Reconstr Aesthet Surg 2011; 64: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 38.Teo TC. The propeller flap concept. Clin Plast Surg 2010; 37: 615–626. [DOI] [PubMed] [Google Scholar]
- 39.Wei FC, Chen HC, Chuang CC, et al. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg 1986; 78: 191–200. [DOI] [PubMed] [Google Scholar]
- 40.Ribuffo D, Atzeni M, Saba L, et al. Clinical study of peroneal artery perforators with computed tomographic angiography: implications for fibular flap harvest. Surg Radiol Anat 2010; 32: 329–334. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya E, Saloner D, Leon P, et al. Magnetic resonance angiography to evaluate septocutaneous perforators in free fibula flap transfer. J Plast Reconstr Aesthet Surg 2010; 63: 1099–1104. [DOI] [PubMed] [Google Scholar]