Abstract
Objective
The leading cause of liver injuries in diabetes mellitus may be associated with fatty liver. We aimed to elucidate the relationship between fatty liver and diabetes characteristics.
Methods
Retrospectively, 970 patients with diabetes were analysed. Fatty liver was diagnosed when the liver/spleen Hounsfield unit ratio by computed tomography was below 0.9. Clinical diabetes characteristics were compared between patients with and without fatty liver.
Results
Of 970 patients (717 male and 253 female; mean age 64.4 years), 175 males (24.4%) and 60 females (23.7%) had fatty liver. None of the 28 patients with type 1 diabetes had fatty liver. In male patients with type 2 diabetes, age, visceral adipose tissue (VAT), albumin, alanine amino-transferase (ALT), and triglycerides were independently associated with fatty liver. In females, age and bilirubin were associated with fatty liver.
Conclusions
Fatty liver is associated with type 2 diabetes characteristics, including younger age and elevated VAT, albumin, ALT, and triglycerides in males and younger age and elevated bilirubin levels in females.
Keywords: Fatty liver, diabetes mellitus, visceral adipose tissue
Introduction
The prevalence of diabetes mellitus is rapidly increasing worldwide. In 2011, there were 366 million patients diagnosed with diabetes mellitus globally, and this number is expected to increase to 552 million by 2030.1 The prevalence of diabetes mellitus in the western Pacific region was estimated to be 8.6% in 2013, and there were reported to be approximately 7.2 million patients with diabetes mellitus in Japan according to the International Diabetes Federation Diabetes Atlas.2 The leading cause of death in patients with diabetes mellitus in Japan is malignant neoplasia (34.1%) followed by vascular diseases, including diabetic nephropathy, ischemic heart diseases, and cerebrovascular diseases (26.8%). The most common malignancy was liver cancer (8.6%), and liver cirrhosis was the cause of death in 5.6% of all deaths.3 In Japan, hepatitis viruses are reportedly negative in the majority of patients with chronic liver disease and diabetes mellitus.4 In addition, there is significant evidence demonstrating a high prevalence of fatty liver in patients with diabetes mellitus.5–8 Subsequently, a large proportion of deaths from liver injuries in patients with diabetes mellitus could be associated with fatty liver. In fact, hepatocellular carcinoma related to obesity and diabetes mellitus is rapidly increasing in Japan.9 Thus, it is critical to assess fatty liver and associated factors in patients with diabetes mellitus.
Many researchers have evaluated the modalities used to estimate steatosis. The gold standard for diagnosis of fatty liver is liver biopsy. However, because of the invasiveness of the procedure and sampling variability, liver biopsy is not suitable for a screening examination to detect fatty liver.10 Abdominal echo examination is widely used as a clinical screening test to detect the presence of fatty liver and estimate its severity.11 Recently, researchers have reported the usefulness of the liver/spleen Hounsfield unit (L/S) ratio calculated using computed tomography (CT) in patients with diabetes mellitus.12,13 For example, Yoneda et al.13 reported that serum adiponectin levels are correlated with the L/S ratio. Visceral adipose tissue (VAT) measured by CT is also a reportedly valuable predictor of precancerous lesions, such as colorectal adenoma and Barrett’s oesophagus, and other malignancies, including hepatocellular carcinoma.14–16 However, the association between the L/S ratio and clinical characteristics of diabetes mellitus, such as VAT, subcutaneous adipose tissue (SAT), body mass index (BMI), and alcohol consumption, remains unclear. Thus, in this study, we aimed to elucidate the relationship between fatty liver and diabetes mellitus characteristics.
Methods
Patients
To evaluate the association between fatty liver diagnosed by CT and clinical characteristics of diabetes mellitus, 1264 patients who underwent abdominal CT (for clinical indications, such as abdominal pain and abnormal findings by ultrasound examination) and VAT evaluation at our institution between January 2008 and March 2014 were retrospectively reviewed. We then excluded patients who fulfilled the following criteria: (i) absence or insufficiency of biochemical examinations (n = 21), (ii) positive laboratory confirmation of hepatitis B surface antigen and anti-hepatitis C virus antibodies (n = 26), (iii) history of splenectomy (n = 3), and (iv) not fulfilling the diagnostic criteria of diabetes mellitus according to the 2010 Japan Diabetes Society criteria (n = 244).17 Finally, the remaining 970 patients with diabetes mellitus were analysed. Type 1 diabetes mellitus was diagnosed according to the Japan Diabetes Society criteria, i.e. based on the clinical history and positive detection of anti-glutamic acid decarboxylase antibodies, anti-islet cell cytoplasmic antibodies, anti-insulin autoantibody, and anti-insulinoma-associated antigen-2. Hypertension, hyperlipidaemia, and hyperuricemia diagnoses were based on the need for medical treatment. The study design was approved by the Ethics Committee at the Institute for Adult Diseases, Asahi Life Foundation and conforms to the Declaration of Helsinki. Patient records were anonymized prior to analysis. The decision of the committee was that the requirement for written informed consent was waived unless the patients refused to allow us to use the data for analysis after de-identification. To date, no patient has refused.
Clinical and laboratory evaluation
Demographic parameters, including age, diabetes mellitus duration (defined as duration from patients’ first visit to our hospital to the CT evaluation in this examination), sex, body mass index (BMI), history of smoking [Brinkman index (BI) = daily amounts of tobacco (pieces/day) × period of smoking (years)], and alcohol intake (g/day), and the treatments administered were recorded. Venous blood samples were obtained after overnight fasting. Haemoglobin A1c (HbA1c; levels were converted and expressed by National Glycohemoglobin Standardization Program values), platelet count, albumin, bilirubin, alkaline phosphatase (ALP), gamma-glutamyl transferase (γ-GT), aspartate amino-transferase (AST), alanine amino-transferase (ALT), triglyceride, total cholesterol (total-C), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured using standard laboratory techniques.
CT
Patients were subjected to abdominal plain CT (Asteion Super4 Edition or Alexion/Advance Edition, TOSHIBA Medical Systems, Tochigi, Japan) in the helical mode with 7 mm or thinner slice thickness after overnight fasting. CT numbers (Hounsfield units) were measured at three points respectively in the liver and spleen, avoiding blood vessels and heterogeneous areas. The mean numbers were used to calculate the L/S ratio. We defined the criterion of fatty liver diagnosis as < 0.9 of the L/S ratio. VAT, SAT, and waist circumferences were determined at the umbilical level by Fat Scan software program (Fat Scan, East Japan Institute of Technology, Ibaraki, Japan).
Statistical analysis
All statistical analyses were performed using JMP10 software (SAS Institute, Cary, NC, USA). The Mann–Whitney U test was used to compare means of continuous valuables. Comparisons of nominal variables were conducted by the χ2 test or Fisher’s exact test as appropriate. Odds ratios (OR) with 95% confidence intervals (CI) were used as a measure of association and were adjusted by unconditional logistic regression models. A two-sided p-value of < 0.05 was considered statistically significant. The parameters that had statistical significance (p < 0.05) in the univariate analysis were selected for multivariate analysis. Patients with type 2 diabetes mellitus were divided into an ALT normal or ALT high group, and the ratio of patients with fatty liver in each group was calculated. The patients were also divided according to triglyceride, BMI, VAT, SAT, and alcohol consumption levels, and the ratio of patients with fatty liver was calculated. The normal levels of these factors were defined as follows: ALT < 40 U/ml; triglycerides < 150 mg/dl; BMI < 25 kg/m2; VAT < 100 cm2; SAT < 100 cm2; and alcohol consumption, male, < 30 g/day and female, < 20 g/day. Heavy drinker is defined both in male and female as alcohol consumption ≥ 60 g/day. Heavy smoking was defined as BI ≥ 800.
Results
Characteristics of patients with diabetes mellitus according to sex and fatty liver
The baseline clinical characteristics are summarized in Table 1. A total of 970 patients (717 male and 253 female) were examined. The median age and BMI in male and female patients with diabetes mellitus was 65 and 67 years and 23.9 and 24.5 kg/m2, respectively. Of these, 175 male (24.4%) and 60 female (23.7%) patients with diabetes mellitus were diagnosed with fatty liver by the L/S ratio calculated using CT. There were 17 male and 11 female patients with type 1 diabetes mellitus. None of the patients with type 1 diabetes mellitus had fatty liver.
Table 1.
Baseline characteristics of the 970 patients with diabetes mellitus according to sex and fatty liver.
| Male (n = 717) |
Female (n = 253) |
||||
|---|---|---|---|---|---|
| Total (n = 970) | Non-fatty liver (n = 542) | Fatty liver (n = 175) | Non-fatty liver (n = 193) | Fatty liver (n = 60) | p † |
| Age (years) | 67 (61–72) | 59 (49–67) | 68 (62–75) | 64 (56–71) | 0.0008* |
| Duration of diabetes mellitus (years) | 13 (6–19) | 6 (1–13) | 8 (3–13) | 20 (12–23) | <0.0001* |
| BMI (kg/m2) | 23.3 (21.7–25.3) | 26.0 (24.1–28.2) | 23.5 (21.7–25.3) | 26.9 (24.2–30.6) | 0.1039 |
| Waist (cm) | 86.8 (81.1–92.8) | 93.6 (89.4–98.8) | 88.9 (82.2–96.7) | 96.6 (88.6–105) | 0.0406* |
| L/S ratio | 1.09 (1.02–1.16) | 0.75 (0.59–0.84) | 1.11 (1.02–1.20) | 0.76 (0.59–0.83) | 0.8258 |
| SAT (cm2) | 123 (95.5–164) | 166 (129–203) | 177 (139–238) | 224 (169–304) | <0.0001* |
| VAT (cm2) | 111 (82–151) | 150 (121–187) | 96.3 (60–137) | 132 (101–173) | 0.0668 |
| HbA1c (%) | 7.4 (6.8–8.0) | 7.7 (7.1–8.8) | 7.1 (6.6–8.2) | 7.3 (6.6–8.7) | 0.0539 |
| Platelet (103/µl) | 20.2 (17.6–23.1) | 21.1 (18.1–24.6) | 22.1 (18.9–25.3) | 22.4 (19.1–25.9) | 0.0747 |
| Albumin (g/dl) | 4.3 (4.1–4.5) | 4.5 (4.4–4.6) | 4.3 (4.0–4.5) | 4.3 (4.1–4.5) | <0.0001* |
| Bilirubin (g/ml) | 0.6 (0.5–0.8) | 0.7 (0.6–0.9) | 0.5 (0.4–0.7) | 0.6 (0.5–0.7) | 0.0395* |
| ALP (U/l) | 209 (174–252) | 210 (167–254) | 230 (193–279) | 240 (187–277) | 0.0188* |
| γ-GT (U/l) | 30 (20–47) | 53 (33–100) | 19 (15–31) | 35.5 (24–51) | <0.0001* |
| AST (U/l) | 21 (18–26) | 29 (22–43) | 20 (17–23) | 27.5 (22–36) | 0.3111 |
| ALT (U/l) | 20 (15–27) | 39 (26–63) | 18 (13–24) | 30 (22–47) | 0.0307* |
| Triglycerides (mg/dl) | 112 (84–155) | 164 (116–236) | 113 (82–158) | 129 (106–179) | 0.0369* |
| TC (mg/dl) | 187 (169–205) | 198 (175–223) | 191 (180–211) | 191 (179–213) | 0.5875 |
| HDL-C (mg/dl) | 52 (44–62) | 47 (41–55) | 58 (46–69) | 48.5 (44–59) | 0.1508 |
| LDL-C (mg/dl) | 104 (87.0–123) | 113 (93–138) | 102 (89–119) | 112 (100–127) | 0.8516 |
| Heavy smoker, n (%) | 213 (39.2%) | 50 (28.5%) | 11 (5.69%) | 4 (6.67%) | 0.0002* |
| Heavy drinker, n (%) | 103 (19.0%) | 40 (22.8%) | 1 (0.51%) | 4 (6.67%) | 0.0040* |
| Hypertension, n (%) | 312 (57.6%) | 93 (53.1%) | 118 (61.1%) | 34 (56.6%) | 0.5798 |
| Hyperlipidemia, n (%) | 256 (47.2%) | 76 (39.3%) | 124 (64.2%) | 39 (65.0%) | 0.0039* |
| Hyperuricemia, n (%) | 54 (9.96%) | 15 (8.57%) | 9 (4.66%) | 1 (1.66%) | 0.0780 |
| Type 1 diabetes mellitus, n (%) | 17 (3.13%) | 0 (0%) | 11 (5.69%) | 0 (0%) | - |
| Use of insulin, n (%) | 211 (38.9%) | 48 (27.4%) | 102 (52.8%) | 18 (30.0%) | 0.7021 |
| Use of sulfonylurea, n (%) | 262 (48.3%) | 91 (52.0%) | 77 (39.8%) | 27 (45.0%) | 0.3906 |
| Use of nateglinide, n (%) | 30 (5.53%) | 7 (4.00%) | 2 (1.03%) | 1 (1.66%) | 0.6833 |
| Use of α-GI, n (%) | 93 (17.1%) | 31 (17.7%) | 22 (11.3%) | 12 (20.0%) | 0.6927 |
| Use of biguanide, n (%) | 230 (42.4%) | 100 (57.1%) | 65 (33.6%) | 35 (58.3%) | 0.8721 |
| Use of thiazolidine, n (%) | 44 (8.11%) | 17 (9.71%) | 6 (3.10%) | 3 (5.00%) | 0.2587 |
| Use of DPP-4, GLP-1, n (%) | 36 (6.64%) | 21 (12.0%) | 16 (8.29%) | 12 (20.0%) | 0.0344* |
| Use of ARB, n (%) | 224 (41.3%) | 72 (41.1%) | 88 (45.5%) | 27 (45.0%) | 0.6015 |
| Use of ACE-I, n (%) | 34 (6.27%) | 7 (4.00%) | 8 (4.14%) | 2 (3.33%) | 0.8163 |
| Use of α/β-blocker, n (%) | 39 (7.19%) | 7 (4.00%) | 11 (5.69%) | 0 (0%) | 0.1956 |
| Use of CCB, n (%) | 197 (36.3%) | 64 (36.5%) | 78 (40.4%) | 19 (31.6%) | 0.4927 |
| Use of diuretics, n (%) | 51 (9.40%) | 8 (4.57%) | 17 (8.80%) | 6 (10.0%) | 0.1252 |
| Use of nitrate, n (%) | 20 (3.69%) | 5 (2.85%) | 10 (5.18%) | 3 (5.00%) | 0.4242 |
| Use of aspirin, n (%) | 83 (15.3%) | 15 (8.57%) | 29 (15.0%) | 9 (15.0%) | 0.1558 |
Data shown are median (25th–75th percentile) or n (%).
Heavy smoker, Brinkman Index ≥ 800; Heavy drinker, alcohol consumption ≥ 60 g/day; BMI, body mass index; L/S ratio, liver/spleen Hounsfield unit ratio; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HbA1c, haemoglobin A1c; ALP, alkaline phosphatase; γ-GT, gamma-glutamyl transferase; AST, aspartate amino-transferase; ALT, alanine amino-transferase; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; α-GI, α-glucosidase inhibitor; DPP-4, inhibitors of dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1 receptor agonists; ARB, angiotensin II receptor blocker; ACE-I, angiotensin converting enzyme inhibitor; CCB, calcium channel blocker
p-value for comparison between male and female with fatty liver
statistically significant (p < 0.05)
Most of the parameters, except duration of diabetes mellitus, BMI, albumin, triglycerides, and LDL-C, were significantly different between male and female patients without fatty liver. Age, duration of diabetes mellitus, waist circumference, SAT, and ALP levels were significantly higher in female patients with fatty liver than in male patients with fatty liver. In contrast, albumin, bilirubin, γ-GT, ALT, and triglyceride levels were significantly higher in male patients with fatty liver compared with those in female patients with fatty liver. There were no significant differences in BMI, L/S ratio, platelet count, VAT, or HbA1c, AST, total-C, HDL-C, or LDL-C levels between male and female patients with fatty liver. A total of 50 male (28.5%) and four female (6.67%) heavy smokers (BI ≥ 800) and 40 male (22.8%) and four female (6.67%) heavy drinkers (alcohol intake ≥ 60 g/day) were diagnosed with fatty liver.
Medications for patients with diabetes mellitus
The medications administered to patients with diabetes mellitus are also displayed in Table 1. Insulin was administered to 259 male (36.1%) and 120 female (47.4%) patients. Medications for hypertension, hyperlipidaemia, and hyperuricemia were administered to 557 (57.4%), 495 (51.0%), and 79 (8.1%) patients with diabetes mellitus, respectively. Insulin was more likely to be administered to female than male patients without fatty liver. In contrast, other diabetic medications, except dipeptidyl peptidase-4-inhibitors and glucagon-like peptide-1 agonists, were more likely to be administered to male than female patients without fatty liver. Dipeptidyl peptidase-4-inhibitors, glucagon-like peptide-1 agonists, and hyperlipidaemia medications were more likely to be administered to female than to male patients with fatty liver. The rate of administration of the other medications was not significantly different between male and female patients with fatty liver.
Associated risk factors for fatty liver in patients with diabetes mellitus
Next, we investigated the associated factors for fatty liver in 942 patients with type 2 diabetes mellitus (700 male and 242 female). A univariate analysis was performed to compare male patients with and without fatty liver (Table 2). There was a significant difference in most clinical factors except ALP and alcohol consumption. Using multivariate logistic regression in male patients with diabetes mellitus, age [OR (95% CI), 0.96 (0.94–0.99), p = 0.0359], VAT [1.01 (1.00–1.01), p = 0.0108], albumin [4.23 (1.74–10.2), p = 0.0014], ALT [1.02 (1.00–1.05), p = 0.0255], and triglycerides [1.01 (1.00–1.01) p = 0.0339] were independent associated factors for fatty liver (Table 2). In male patients with diabetes mellitus, no medications examined were associated with fatty liver by multivariate analysis.
Table 2.
Associated factors for fatty liver: Male patients with type 2 diabetes mellitus.
| Univariate |
|
Multivariate |
|
|
|---|---|---|---|---|
| Male (n = 700) | OR (95% CI) | p | OR (95% CI) | p |
| Age (year) | 0.92 (0.90–0.94) | <0.0001* | 0.96 (0.94–0.99) | 0.0359* |
| Duration of diabetes mellitus (year) | 0.92 (0.90–0.94) | <0.0001* | 0.98 (0.95–1.01) | 0.2915 |
| BMI (kg/m2) | 1.40 (1.30–1.50) | <0.0001* | 1.12 (0.97–1.30) | 0.1478 |
| Waist (cm) | 1.12 (1.09–1.14) | <0.0001* | 0.99 (0.91–1.07) | 0.9303 |
| SAT (cm2) | 1.01 (1.01–1.02) | <0.0001* | 1.00 (0.99–1.01) | 0.8384 |
| VAT (cm2) | 1.01 (1.01–1.02) | <0.0001* | 1.01 (1.00–1.01) | 0.0108* |
| HbA1c (%) | 1.31 (1.15–1.50) | <0.0001* | 1.05 (0.85–1.29) | 0.6255 |
| Platelet (103/µl) | 1.04 (1.00–1.07) | 0.0262* | 1.01 (0.96–1.06) | 0.6176 |
| Albumin (g/dl) | 7.72 (3.91–15.2) | <0.0001* | 4.23 (1.74–10.2) | 0.0014* |
| Bilirubin (g/ml) | 2.14 (1.22–3.76) | 0.0023* | 1.56 (0.68–3.57) | 0.2909 |
| ALP (U/l) | 0.99 (0.99–1.00) | 0.8533 | ||
| γ-GT (U/l) | 1.00 (1.00–1.01) | <0.0001* | 0.99 (0.99–1.00) | 0.9997 |
| AST (U/l) | 1.06 (1.04–1.08) | <0.0001* | 1.01 (0.98–1.05) | 0.1850 |
| ALT (U/l) | 1.05 (1.04–1.07) | <0.0001* | 1.02 (1.00–1.05) | 0.0255* |
| Triglycerides (mg/dl) | 1.00 (1.00–1.01) | <0.0001* | 1.01 (1.00–1.01) | 0.0339* |
| TC (mg/dl) | 1.01 (1.01–1.02) | <0.0001* | 0.99 (0.97–1.02) | 0.7055 |
| HDL-C (mg/dl) | 0.97 (0.96–0.98) | 0.0002* | 1.00 (0.97–1.03) | 0.7811 |
| LDL-C (mg/dl) | 1.01 (1.01–1.02) | 0.0001* | 1.01 (0.99–1.04) | 0.1865 |
| Smoking (BI) | 0.99 (0.99–1.00) | 0.0225* | 1.00 (0.99–1.00) | 0.5420 |
| Alcohol (g/day) | 1.00 (0.99–1.01) | 0.8026 | ||
| Hypertension | 0.83 (0.59–1.17) | 0.2340 | ||
| Hyperlipidemia | 0.85 (0.60–1.20) | 0.3144 | ||
| Hyperuricemia | 0.84 (0.46–1.54) | 0.5099 | ||
| Use of insulin | 0.59 (0.40–0.86) | 0.0217* | 1.06 (0.62–1.81) | 0.8047 |
| Use of sulfonylurea | 1.15 (0.82–1.62) | 0.3800 | ||
| Use of nateglinide | 0.71 (0.30–1.64) | 0.4248 | ||
| Use of α-GI | 1.03 (0.66–1.62) | 1.0000 | ||
| Use of biguanide | 1.80 (1.28–2.55) | 0.0022* | 1.17 (0.73–1.88) | 0.4994 |
| Use of thiazolidine | 1.21 (0.67–2.19) | 0.5881 | ||
| Use of DPP-4, GLP-1 | 1.91 (1.08–3.38) | 0.0312* | 1.68 (0.77–3.67) | 0.1864 |
| Use of ARB | 0.99 (0.70–1.40) | 0.8943 | ||
| Use of ACE-I | 0.62 (0.27–1.43) | 0.2270 | ||
| Use of α/β-blocker | 0.53 (0.23–1.22) | 0.1129 | ||
| Use of CCB | 1.01 (0.70–1.43) | 0.9638 | ||
| Use of diuretics | 0.46 (0.21–0.99) | 0.0339* | 0.50 (0.20–1.27) | 0.1472 |
| Use of nitrate | 0.58 (0.19–1.71) | 0.3542 | ||
| Use of aspirin | 0.51 (0.29–0.92) | 0.0194* | 0.77 (0.037–1.61) | 0.4946 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HbA1c, haemoglobin A1c; ALP, alkaline phosphatase; γ-GT, gamma-glutamyl transferase; AST, aspartate amino-transferase; ALT, alanine amino-transferase; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BI, Brinkman Index; α-GI, α-glucosidase inhibitor; DPP-4, inhibitors of dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1 receptor agonists; ARB, angiotensin II receptor blocker; ACE-I, angiotensin converting enzyme inhibitor; CCB, calcium channel blocker
statistically significant (p < 0.05)
Univariate and multivariate analyses were also performed to compare female patients with and without fatty liver (Table 3). Using multivariate logistic regression in female patients with diabetes mellitus, age [OR (95% CI), 0.92 (0.86–0.99) p = 0.0207], diabetes mellitus duration [1.28 (1.18–1.39) p < 0.0001], bilirubin levels [7.36 (1.10–48.9), p = 0.0389], and insulin use [0.27 (0.09–0.75), p = 0.0123] were independent associated factors for fatty liver (Table 3). In female patients with diabetes mellitus, alcohol consumption was not associated with fatty liver by univariate analysis.
Table 3.
Associated factors for fatty liver: Female patients with type 2 diabetes mellitus.
| Female (n = 242) | Univariate |
|
Multivariate |
|
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (year) | 0.96 (0.93–0.99) | 0.0019* | 0.92 (0.86–0.99) | 0.0207* |
| Duration of diabetes mellitus (year) | 1.24 (1.16–1.31) | <0.0001* | 1.28 (1.18–1.39) | <0.0001* |
| BMI (kg/m2) | 1.13 (1.07–1.20) | <0.0001* | 1.26 (0.95–1.67) | 0.1077 |
| Waist (cm) | 1.05 (1.02–1.07) | 0.0001* | 0.99 (0.82–1.18) | 0.9137 |
| SAT (cm2) | 1.00 (1.00–1.01) | 0.0017* | 0.99 (0.98–1.01) | 0.5159 |
| VAT (cm2) | 1.01 (1.01–1.02) | 0.0001* | 0.99 (0.97–1.01) | 0.4879 |
| HbA1c (%) | 1.08 (0.91–1.29) | 0.2869 | ||
| Platelet (103/µl) | 1.01 (0.96–1.07) | 0.4439 | ||
| Albumin (g/dl) | 1.30 (0.54–3.11) | 0.7974 | ||
| Bilirubin (g/ml) | 3.53 (1.09–11.4) | 0.0246* | 7.36 (1.10–48.9) | 0.0389* |
| ALP (U/l) | 1.00 (0.99–1.00) | 0.9162 | ||
| γ-GT (U/l) | 1.00 (1.00–1.01) | <0.0001* | 0.99 (0.98–1.00) | 0.2334 |
| AST (U/l) | 1.06 (1.04–1.09) | <0.0001* | 1.07 (0.97–1.18) | 0.1507 |
| ALT (U/l) | 1.05 (1.03–1.07) | <0.0001* | 1.00 (0.94–1.07) | 0.8473 |
| Triglycerides (mg/dl) | 1.00 (1.00–1.01) | 0.0310* | 1.00 (0.99–1.01) | 0.2129 |
| TC (mg/dl) | 1.00 (0.99–1.01) | 0.5658 | ||
| HDL-C (mg/dl) | 0.96 (0.94–0.98) | 0.0070* | 0.98 (0.93–1.02) | 0.3730 |
| LDL-C (mg/dl) | 1.01 (1.00–1.02) | 0.0105* | 1.01 (0.98–1.03) | 0.4886 |
| Smoking (BI) | 1.00 (0.99–1.00) | 0.1817 | ||
| Alcohol (g/day) | 0.98 (0.96–1.01) | 0.5064 | ||
| Hypertension | 0.83 (0.46–1.49) | 0.4105 | ||
| Hyperlipidemia | 1.03 (0.56–1.89) | 0.9567 | ||
| Hyperuricemia | 0.84 (0.46–1.54) | 0.4583 | ||
| Use of insulin | 0.59 (0.40–0.86) | 0.0085* | 0.27 (0.09–0.75) | 0.0123* |
| Use of sulfonylurea | 1.23 (0.68–2.21) | 0.7148 | ||
| Use of nateglinide | 1.61 (0.14–18.1) | 0.7303 | ||
| Use of α-GI | 1.94 (0.89–4.20) | 0.0976 | ||
| Use of biguanide | 2.75 (1.52–4.99) | 0.0020* | 2.25 (0.80–6.30) | 0.1769 |
| Use of thiazolidine | 1.64 (0.39–6.76) | 0.6937 | ||
| Use of DPP-4, GLP-1 | 2.76 (1.22–6.23) | 0.0185* | 2.24 (0.46–10.8) | 0.3136 |
| Use of ARB | 0.97 (0.54–1.74) | 0.8763 | ||
| Use of ACE-I | 0.79 (0.16–3.86) | 0.7199 | ||
| Use of α/β-blocker | - | 0.0700 | ||
| Use of CCB | 0.68 (0.39–1.26) | 0.1439 | ||
| Use of diuretics | 1.15 (0.43–3.06) | 0.8799 | ||
| Use of nitrate | 0.96 (0.25–3.62) | 0.8828 | ||
| Use of aspirin | 0.99 (0.44–2.24) | 0.9427 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HbA1c, haemoglobin A1c; ALP, alkaline phosphatase; γ-GT, gamma-glutamyl transferase; AST, aspartate amino-transferase; ALT, alanine amino-transferase; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BI, Brinkman Index; α-GI, α-glucosidase inhibitor; DPP-4, inhibitors of dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1 receptor agonists; ARB, angiotensin II receptor blocker; ACE-I, angiotensin converting enzyme inhibitor; CCB, calcium channel blocker
statistically significant (p < 0.05)
The ratio of fatty liver in diabetes mellitus according to ALT, triglyceride, BMI, VAT, SAT, and alcohol consumption levels
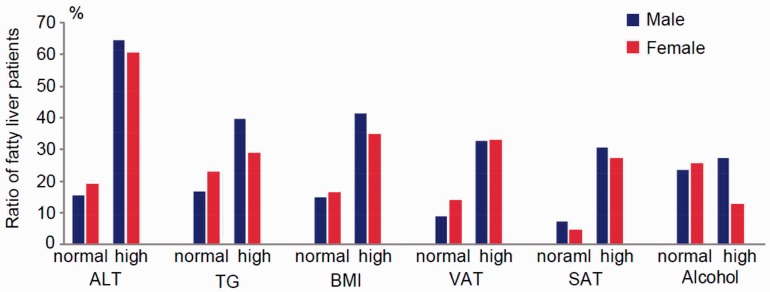
Finally, we examined the incidence of fatty liver in patients with type 2 diabetes mellitus according to ALT, triglyceride, BMI, VAT, SAT, and alcohol consumption levels. Of the patients with type 2 diabetes mellitus, 25.0% (175/700) of male and 24.7% (60/242) of female patients had fatty liver. In the ALT normal group, 15.4% (89/576) of male and 19.1% (40/209) of female patients with type 2 diabetes mellitus were diagnosed with fatty liver. In the ALT high group, fatty liver was diagnosed in 64.5% (86/124) of male and 60.6% (20/33) of female patients. In the normal BMI group, 14.9% (65/434) of male and 16.1% (21/130) of female patients were diagnosed with fatty liver, and in the high BMI group, 41.3% (110/266) of male and 34.8% (39/112) of female patients were diagnosed with fatty liver. In the normal alcohol consumption group, 23.5% (103/437) of male and 25.6% (58/226) of female patients were diagnosed with fatty liver, and in the high alcohol consumption group, 27.3% (72/263) of male and 12.5% (2/16) of female patients were diagnosed with fatty liver. The ratio of patients with fatty liver in each group is shown in Figure 1.
Figure 1.
The incidence of fatty liver in patients with type 2 diabetes mellitus. The incidence of fatty liver in patients with type 2 diabetes mellitus according to indicated group is shown. The normal ranges are defined as follows: ALT < 40 U/ml; triglycerides < 150 mg/dl; BMI < 25 kg/m2; VAT < 100 cm2; SAT < 100 cm2; and alcohol consumption, male, < 30 g/day and female, < 20 g/day. ALT, alanine amino-transferase; TG, triglyceride; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue
Discussion
In this study, we aimed to elucidate the clinical characteristics of patients with diabetes mellitus that were diagnosed with fatty liver by L/S ratio using CT. Our results indicated that, in patients with type 2 diabetes mellitus, younger age, high VAT, and albumin, ALT, and triglyceride levels in males and younger age and high bilirubin levels in females were associated with fatty liver.
The prevalence of fatty liver diagnosed by CT in patients with diabetes mellitus was 24.4% (175/717) in males and 23.7% (60/252) in females. Furthermore, we showed that there were no patients with type 1 diabetes mellitus diagnosed with fatty liver (28 patients). Jimba et al.6 previously reported that the prevalence of patients with fatty liver diagnosed by ultrasonography in patients with newly diagnosed diabetes was as high as 62% (28/45). The prevalence may vary with the diagnosis modality and targeted patients. Our study patients had a relatively long history of diabetes mellitus (median, 6 and 20 years in male and female patients with fatty liver, respectively). The age of the study patients would also be important for steatosis. In this examination, multivariate analysis revealed that the prevalence of fatty liver decreased with advancing age both in males and females in agreement with a previous study.18 The term “burned-out” denotes a significant reduction in hepatic adiposity with the natural course of non-alcoholic steatohepatitis and is a well-known phenomenon.19 Our results may reflect the natural course of patients with fatty liver diagnosed by CT in the same manner. In females, although younger age was associated with increased fatty liver, longer duration of diabetes mellitus was a risk factor for fatty liver. The bidirectional relationship linking type 2 diabetes mellitus and fatty liver as previously reported20 could be a possible explanation, and early onset of diabetes mellitus could be a possible risk factor for fatty liver in female patients with diabetes mellitus.
Interestingly, the associated factors for fatty liver were different between males and females.21 By multivariate analysis, BMI, waist circumference, and SAT were not significant risk factors for fatty liver in males, but high VAT in males was a significant risk factor, suggesting a critical role of VAT in metabolic syndrome, consistent with a previous report.22 In contrast, VAT was not a significant risk factor for fatty liver in female patients with type 2 diabetes mellitus by multivariate analysis, in accordance with a previous report indicating a sex difference of the role of VAT in the pathogenesis of fatty liver.23 Biochemical examinations revealed that high albumin, triglyceride, and ALT levels in males and high bilirubin levels in females were associated risk factors for fatty liver. In this study, the age of female patients was relatively high [median, 67 years (25th–75th percentile, 60–74)]. The progression of liver fibrosis is reportedly accelerated by postmenopausal status.24 The increased risk of fibrosis in postmenopausal women could be a potential explanation for the observed sex differences. In this report, alcohol intake was not related to fatty liver in either males or females, consistent with a previous report on patients with diabetes mellitus.4,25 Alcohol consumption may not be a critical factor in the pathogenesis of fatty liver in patients with diabetes mellitus.
Improvements in steatosis by various medications have previously been reported.26–29 By univariate analysis in the present study, the ratio of administration of several anti-diabetes medications was statistically different between patients with fatty liver and those without. The only significant difference that remained after adjusting for clinical characteristics, including age and BMI, was insulin use in females. Although our multivariate analysis showed low incidence of fatty liver in female insulin users with type 2 diabetes mellitus, prescription selection depends on several factors, including patient history and extent of complications, which were not included in this study. Subsequently, further examinations are required to identify effective medications for fatty liver.
There are limitations to our study. First, it was a cross-sectional study. We could not assess the effect of duration of anti-diabetic treatments. Thus, a future prospective study is required. Second, liver biopsies were not included. Fibrosis and hepatitis were not evaluated in this study. Third, some possibly influential information, such as exercise habits, diet, and obstructive sleep apnoea of the patients,30,31 was not included.
Previous studies have reported that a high percentage (15%–21%) of Asian-Pacific patients with non-alcoholic fatty liver disease are non-obese (with BMI < 25.0 kg/m2).32 In this study, 14.9% and 16.1% of fatty liver cases were diagnosed in male and female patients with type 2 diabetes mellitus with BMI < 25.0 kg/m2, respectively (Figure 1). In addition, the present study revealed that 15.4% of males and 19.1% of females with type 2 diabetes mellitus that were diagnosed with fatty liver had normal ALT levels. This is consistent with a previous report showing that non-alcoholic fatty liver disease can be observed in individuals with normal ALT values.33
Conclusion
In conclusion, this study identified the factors associated with fatty liver diagnosed by L/S ratio using CT in patients with type 2 diabetes mellitus. Fatty liver is associated with clinical characteristics of type 2 diabetes mellitus, such as younger age, increased VAT, and elevated albumin, ALT, and triglyceride levels in males and younger age and elevated bilirubin levels in females.
Acknowledgements
The authors are grateful for constructive comments from Y. Ohnishi and A. Kushiyama and the extensive data collection by all the diabetes specialists (T. Kikuchi, T. Tahara, T. Takao, K. Tanaka, R. Sonoda, and Y. Yoshida) at The Institute for Adult Diseases, Asahi Life Foundation (Tokyo, Japan).
Abbreviations
CT, computed tomography; L/S ratio, liver/spleen ratio; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index; ALP, alkaline phosphatase; γ-GT, gamma-glutamyl transferase; AST, aspartate amino-transferase; ALT, alanine amino-transferase; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Author’s contributions
Conceived and designed the experiments: KS and HK. Performed the experiments: KS, HK, AT, and HA. Analysed the data: KS, KE, HK, SK, and KK. Wrote the paper: KS. All authors approved the final manuscript.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Cho NH, Tajima N, et al. Diabetes in the Western Pacific Region–past, present and future. Diabetes Res Clin Pract 2014; 103: 244–255. [DOI] [PubMed] [Google Scholar]
- 3.Hotta N, Nakamura J, Iwamoto Y, et al. Causes of death in Japanese diabetics: A questionnaire survey of 18,385 diabetics over a 10-year period. J Diabetes Investig 2010; 1: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shima T, Uto H, Ueki K, et al. Clinicopathological features of liver injury in patients with type 2 diabetes mellitus and comparative study of histologically proven nonalcoholic fatty liver diseases with or without type 2 diabetes mellitus. J Gastroenterol 2013; 48: 515–525. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara T, Hyogo H, Yoneda M, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol 2014; 49: 1477–1484. [DOI] [PubMed] [Google Scholar]
- 6.Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med 2005; 22: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 7.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008; 48: 792–798. [DOI] [PubMed] [Google Scholar]
- 8.Non-alcoholic Fatty Liver Disease Study, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis 2015; 47: 997–1006. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol 2015; 50: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 11.Ballestri S, Lonardo A, Romagnoli D, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int 2012; 32: 1242–1252. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DE, McKolanis TM, Hegazi RA, et al. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003; 285: E906–E916. [DOI] [PubMed] [Google Scholar]
- 13.Yoneda M, Iwasaki T, Fujita K, et al. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol Clin Exp Res 2007; 31(1 Suppl): S15–S21. [DOI] [PubMed] [Google Scholar]
- 14.Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009; 58: 839–844. [DOI] [PubMed] [Google Scholar]
- 15.Nagata N, Sakamoto K, Arai T, et al. Visceral abdominal fat measured by computed tomography is associated with an increased risk of colorectal adenoma. Int J Cancer 2014; 135: 2273–2281. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut 2014; 63: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonardo A, Lombardini S, Scaglioni F, et al. Fatty liver, carotid disease and gallstones: a study of age-related associations. World J Gastroenterol 2006; 12: 5826–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 1990; 11: 74–80. [DOI] [PubMed] [Google Scholar]
- 20.Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res 2013; 43: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonardo A, Trande P. Are there any sex differences in fatty liver? A study of glucose metabolism and body fat distribution. J Gastroenterol Hepatol 2000; 15: 775–782. [DOI] [PubMed] [Google Scholar]
- 22.Park BJ, Kim YJ, Kim DH, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol 2008; 23: 900–907. [DOI] [PubMed] [Google Scholar]
- 23.Ayonrinde OT, Olynyk JK, Beilin LJ, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology 2011; 53: 800–809. [DOI] [PubMed] [Google Scholar]
- 24.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014; 59: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000; 132: 112–117. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahady SE, Webster AC, Walker S, et al. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol 2011; 55: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 28.Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012; 35: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017; 66: 180–190. [DOI] [PubMed] [Google Scholar]
- 30.Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013; 58: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Shinjo S, Arai T, et al. Hypoxia and fatty liver. World J Gastroenterol 2014; 20: 15087–15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol 2012; 27: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 33.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003; 37: 1286–1292. [DOI] [PubMed] [Google Scholar]