Abstract
Objective
To assess the application of antibacterial agents, alongside pathogen prevalence and Pseudomonas aeruginosa drug resistance, with the aim of understanding the impact of inappropriate antibacterial use.
Methods
This retrospective study assessed bacteria from wounds, catheters, blood, faeces, urine and sputum of hospitalized patients in burn wards between 2007 and 2014. The intensity of use of antibacterial agents and resistance of P. aeruginosa to common anti-Gram-negative antibiotics were measured.
Results
Annual detection rates of Staphylococcus aureus were significantly decreased, whereas annual detection rates of P. aeruginosa and Klebsiella pneumoniae were significantly increased. Multidrug-resistant strains of P. aeruginosa were increased. The intensity of use of some anti-Gramnegative antibiotics positively correlated with resistance rates of P. aeruginosa to similar antimicrobials.
Conclusion
In burn wards, more attention should be paid to P. aeruginosa and K. pneumoniae. The use of ciprofloxacin, ceftazidime and cefoperazone/sulbactam should be limited to counter the related increase in resistance levels.
Keywords: Burn, Pseudomonas aeruginosa, antibacterial agents, drug resistance, antibiotic, antimicrobial
Introduction
Although treatment for burns has been greatly improved, infection remains one of the main causes of death in burn patients, especially in critically-ill burn patients.1–3 Indeed, compared with other hospitalized individuals, burn patients are characterized by skin deficiency, long hospital stays and multiple invasive operations, and are therefore more prone to infection. In addition, common bacterial species from burn patient wounds are constantly changing during the course of disease: initially, the burn wound is sterile, but it becomes colonized with Gram-positive bacteria such as β-haemolytic Streptococcus after 48 h.4 With the application of surgical debridement and skin grafting in early surgery, as well as extensive use of systemic antibiotics and other treatment interventions, Gram-negative bacteria such as Pseudomonas aeruginosa can be detected.5 During treatment, bacterial resistance also changes with the application of significant amounts of antibacterial agents.6 Furthermore, bacterial prevalence differs between the burn wards of different hospitals: some are dominated by Gram-negative bacteria,7 while others predominantly report Gram-positive organisms.8 Therefore, in the treatment for burns, regular monitoring of bacterial epidemiology in hospital wards is critical for the rational use of antibiotics.9
In our burn ward, P. aeruginosa is the most prevalent bacteria,10 and it is particularly difficult to treat. Indeed, P. aeruginosa harbours many virulence factors, including elastase, exotoxin A, phospholipase and homoserine lactone.11 In addition, this organism possesses a variety of drug resistance mechanisms: inactivation or suppression of enzyme production, increased expression of an active efflux pump system, biofilm formation, and loss or decreased expression of outer membrane proteins.12,13 Therefore, multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains are common. Burn patients infected with P. aeruginosa show a higher mortality rate.14 Therefore, the development of effectively therapeutic strategies to treat P. aeruginosa infection has been the focus of our study group.
The widespread application of antibacterial agents has resulted in increasing levels and severity of bacterial resistance,15,16 which in turn, demands greater use of antibacterial agents, further aggravating bacterial resistance in a vicious cycle.6 Thus, it is essential to select appropriate antibacterial agents, to avoid increased patient mortality17 and the economic burden on patients and society.18 However, in one study, more than 40% of antibacterial agents used in a hospital were reported to be inappropriate.19 Similarly, a report from Tehran indicated that 40% of antibacterial agent use was inappropriate.20 In the United States, irrational application of antibacterial agents has also been observed.21 These deficiencies in the rational use of antibacterial agents are often accompanied by adverse consequences, including high mortality22 and increased medical costs.18 Therefore, it is not only necessary to monitor bacterial prevalence and drug resistance in hospital wards, but also antibacterial agent use. An increasing number of countries and researchers are now attempting to simultaneously monitor antibacterial agent use together with bacterial epidemiological data, with the aim of guiding policy development for the use of antibacterial agents.23 However, many previous studies assessing anti-infective treatments for burns7,8 only monitored the prevalence and drug resistance of common bacteria in wards and neglected antibacterial use, making it difficult to understand the impact of inappropriate use of antibacterial agents in these cases.
In this retrospective study, we statistically analysed the use of antibacterial agents and bacterial epidemiology in wards treating burn patients. In particular, the use of antibacterial agents and drug resistance of P. aeruginosa were simultaneously evaluated, identifying any inappropriate antibacterial use. Through this combined analysis, we aimed to provide reliable data to guide policy development for the rational use of antibacterial agents in burn wards.
Materials & methods
Bacterial sample collection
The study was retrospective. Bacterial samples were collected from hospitalized patients in the burn wards of Ruijin Hospital, Shanghai Jiaotong University School of Medicine, from January 2007 to December 2014. A total of 10276 hospitalized patients were enrolled, including 6935 men and 3341 women, aged 1–111 years (34.13 ± 20.59). Upon admission, the patients received routine preventative treatment which comprised lincomycin, and further treatment was adjusted according to antibiotic susceptibility test results. This study was approved by the institutional review board of Ruijin Hospital, Shanghai Jiaotong University, School of Medicine and written informed consent was obtained from every participant.
Wound secretion specimens were collected for microbial culture at the first dressing change after admission, and subsequently on a weekly basis. Wound specimens were collected by sterile swabs from the wound surface after the removal of the dressing. In patients with central venous catheters, germiculture was also carried out with catheterization specimens and blood samples from ipsilateral/contralateral limbs when the catheter was extracted. In individuals with hyperpyrexia, diarrhoea, pulmonary infection (evidenced by a chest X-ray) and urinary tract infection, germiculture was also performed on blood, faecal, sputum and mid-stream urine samples, respectively.
Bacterial strain isolation and identification
All samples were routinely inoculated onto Mueller–Hinton agar medium (Oxoid, UK) and incubated at 35℃ for 24 h. After bacterial strain isolation and purification, identification was carried our using an API bacterial identification strip on a Vitek-2 fully-automatic germ analysis system (Biomerieux, France). Identical bacterial identification in different samples from the same patient indicated a positive result.
Drug susceptibility test
Drug susceptibility was determined by the Kirby–Bauer disk diffusion method (filter paper purchased from Oxoid), in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines.24 A total of six antibiotics were selected for assessing the drug resistance of P. aeruginosa, including amikacin (30 µg), ceftazidime (30 µg), cefoperazone/sulbactam (75/30 µg), imipenem (10 µg), meropenem (10 µg) and ciprofloxacin (5 µg). Standard strains for quality control were Escherichia coli ATCC 25922, P. aeruginosa ATCC 27853 and Staphylococcus aureus, which were all provided by the Shanghai Centre for Clinical Laboratory. The results were expressed as the rate of resistant strains among all detected P. aeruginosa strains. MDR strains of P. aeruginosa were also calculated annually. The definition of MDR is resistance to three or more antimicrobial classes.
Antibacterial use density analysis
As recommended by the World Health Organisation,25 the annual use densities of common antibiotics from 2007 to 2014 were calculated and expressed in defined daily doses/1000 patient-days (DDDs/1000 PD). The assessed antibiotics were vancomycin, penicillin, teicoplanin, imipenem, meropenem, lincomycin, minocycline, azithromycin, ciprofloxacin, cefradine, cefuroxime, ceftazidime, cefoperazone/sulbactam and amikacin.
Data analysis
The WHONET 5.6 software was used to assess the following parameters: (1) detection of pathogens, especially P. aeruginosa, in the ward during each year; and (2) changes in the P. aeruginosa resistance rates for antibacterial agents. Statistical analysis was performed using the SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). Time-trend analysis through curve estimation was performed for the percentage of specific bacteria among all detected pathogens, antibacterial use density, P. aeruginosa resistance to specific antibiotics and MDR strains of P. aeruginosa. Pearson correlation analyses were performed between indexes when they followed a normal distribution in the Kolmogorov–Smirnov Z test. P < 0.05 was considered statistically significant.
Results
Annual changes in specific bacteria
From 2007 to 2014, a total of 3005 pathogenic strains were isolated, including 2561, 80, 6, 6, 98, 5, 30 and 220 from wound secretions, catheters, drainage fluid, throat swabs, blood, faecal, urine and sputum samples, respectively.
The number of detected strains for each bacterial species, and the percentage of specific bacteria among all detected pathogens, were calculated annually (Table 1 & Figure 1).
Table 1.
Pathogens detected annually from 2007 to 2014.
| Bacteria | 2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | % | Strains | % | Strains | % | Strains | % | Strains | % | Strains | % | Strains | % | Strains | % | |
| Staphylococcus aureus | 107 | 43.67 | 132 | 33.50 | 105 | 28.53 | 142 | 29.04 | 157 | 30.78 | 103 | 30.21 | 98 | 27.53 | 87 | 28.81 |
| Staphylococcus epidermidis | 22 | 8.98 | 58 | 14.72 | 56 | 15.22 | 85 | 17.38 | 44 | 8.63 | 14 | 4.11 | 10 | 2.81 | 12 | 3.97 |
| Enterococcus faecalis | 15 | 6.12 | 35 | 8.88 | 26 | 7.07 | 38 | 7.77 | 26 | 5.10 | 22 | 6.45 | 27 | 7.58 | 24 | 7.95 |
| Faecal Enterococci | 0 | 0.00 | 4 | 1.02 | 12 | 3.26 | 14 | 2.86 | 15 | 2.94 | 7 | 2.05 | 6 | 1.69 | 7 | 2.32 |
| Pseudomonas aeruginosa | 25 | 10.20 | 41 | 10.41 | 45 | 12.23 | 73 | 14.93 | 85 | 16.67 | 67 | 19.65 | 66 | 18.54 | 79 | 26.16 |
| Klebsiella pneumoniae | 9 | 3.67 | 13 | 3.30 | 19 | 5.16 | 21 | 4.29 | 29 | 5.69 | 16 | 4.69 | 39 | 10.96 | 37 | 12.25 |
| Acinetobacter baumannii | 28 | 11.43 | 72 | 18.27 | 61 | 16.58 | 56 | 11.45 | 76 | 14.90 | 65 | 19.06 | 56 | 15.73 | 36 | 11.92 |
| Proteus mirabilis | 6 | 2.45 | 9 | 2.28 | 13 | 3.53 | 10 | 2.04 | 20 | 3.92 | 22 | 6.45 | 10 | 2.81 | 8 | 2.65 |
| Enterobacter cloacae | 8 | 3.27 | 4 | 1.02 | 12 | 3.26 | 11 | 2.25 | 12 | 2.35 | 7 | 2.05 | 13 | 3.65 | 7 | 2.32 |
| Escherichia coli | 18 | 7.35 | 20 | 5.08 | 11 | 2.99 | 25 | 5.11 | 35 | 6.86 | 8 | 2.35 | 17 | 4.78 | 4 | 1.32 |
| Stenotrophomonas maltophilia | 3 | 1.22 | 2 | 0.51 | 4 | 1.09 | 7 | 1.43 | 5 | 0.98 | 4 | 1.17 | 5 | 1.40 | 0 | 0.00 |
| Others | 4 | 1.63 | 4 | 1.02 | 4 | 1.09 | 7 | 1.43 | 6 | 1.18 | 6 | 1.76 | 9 | 2.53 | 1 | 0.33 |
| Sum | 245 | 100 | 394 | 100 | 368 | 100 | 489 | 100 | 510 | 100 | 341 | 100 | 356 | 100 | 302 | 100 |
The number of strains detected annually for each pathogen, and the percentage (%) of specific bacteria among all pathogens detected in the same year were recorded.
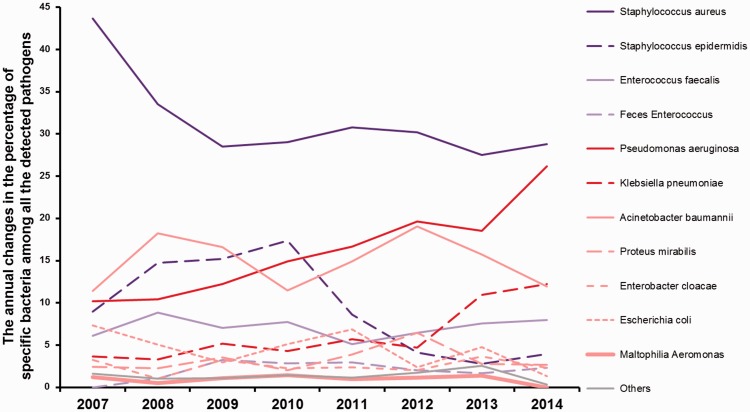
Figure 1.
Annual changes in the percentage of specific bacteria among all detected pathogens from 2007 to 2014.
Purple lines represent Gram-positive bacteria, red lines represent Gram-negative bacteria. Dark colours indicate significant changes in trends, and light colours indicate non-significant changes in trends.
Although S. aureus was the predominant species throughout the years, its percentage detection significantly decreased year by year, from 43.67% in 2007 to 28.81% in 2014 (Logistic, R2 = 0.517, p < 0.05). A similar decreasing trend was found for the detection of Staphylococcus epidermidis, with the rate reducing from 8.98% in 2007 to 3.97% in 2014 (Logistic, R2 = 0.601, p < 0.05). By contrast, annual changes in the detection rates for P. aeruginosa and Klebsiella pneumoniae showed the opposite trend. The detection rate of P. aeruginosa significantly increased from 10.20% in 2007 to 26.16% in 2014 (Logistic, R2 = 0.952, p < 0.01); with this bacterium being the predominant species among Gram-negative bacteria by 2014. The detection rate of K. pneumoniae also significantly increased from 3.67% in 2007 to 12.25% in 2014 (Logistic, R2 = 0.760, p < 0.01), which was the second highest detection rate among Gram-negative bacteria. No significant changes in the detection rates were found for the other bacterial species assessed.
Given that P. aeruginosa showed the most significant increase in the detection rate, our subsequent analyses focused on this bacterium.
Annual changes in the use densities of common antibiotics
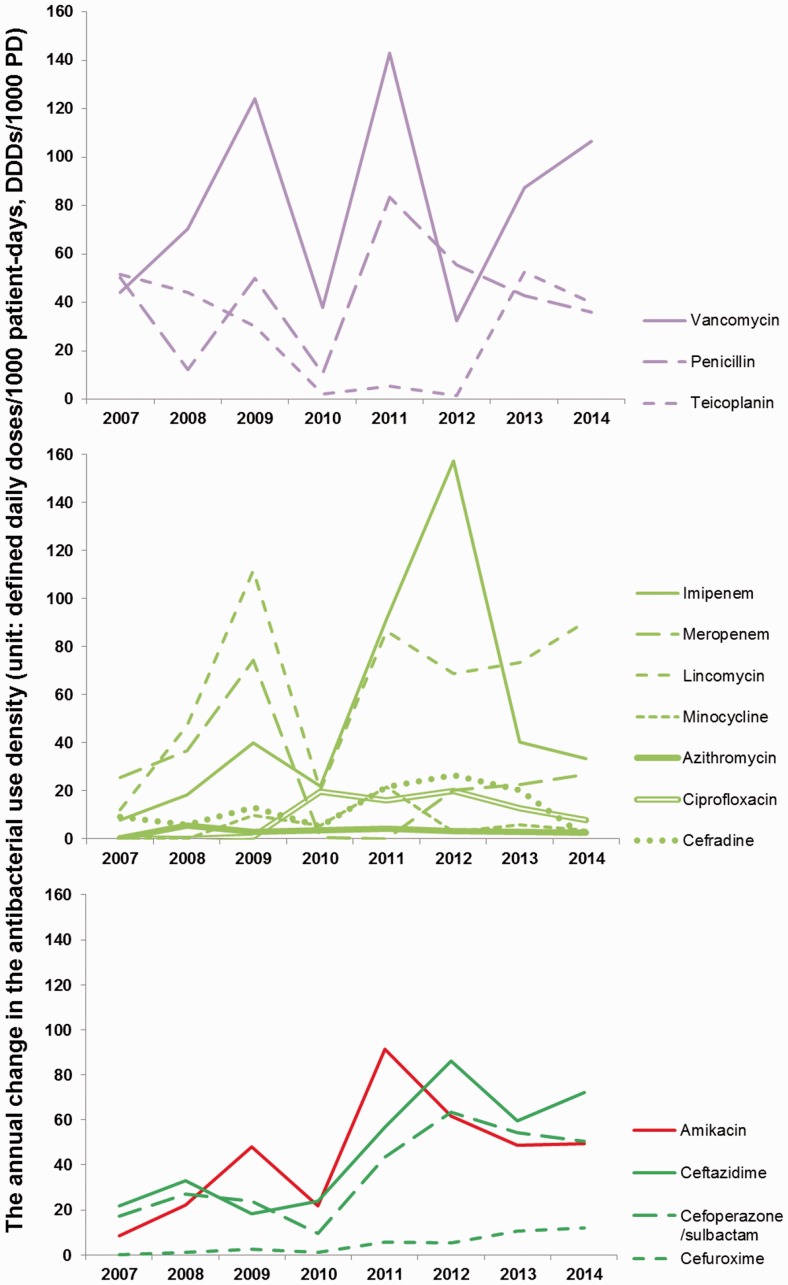
From 2007 to 2014, the use densities of common antibiotics (vancomycin, penicillin, teicoplanin, imipenem, meropenem, lincomycin, minocycline, azithromycin, ciprofloxacin, cefradine, cefuroxime, ceftazidime, cefoperazone/sulbactam and amikacin) in our department varied, as shown in Table 2 and Figure 2. Interestingly, the use densities of amikacin (mainly used against Gram-negative bacteria) and cephalosporins (used for both Gram-negative and Gram-positive bacteria), such as ceftazidime, cefoperazone/sulbactam and cefuroxime, were significantly increased throughout the years. Specifically, use density of amikacin increased from 8.65 in 2007 to 49.41 in 2014 (Logistic, R2 = 0.506, p < 0.05), and that of ceftazidime from 21.84 in 2007 to 72.05 in 2014 (Linear, R2 = 0.673, p < 0.05). The values obtained for cefoperazone/sulbactam were 17.35 and 50.62 in 2007 and 2014, respectively (Linear, R2 = 0.615, p < 0.05), and those of cefuroxime were 0.05 in 2007 and 12.16 in 2014 (Linear, R2 = 0.868, p < 0.01). The increasing use of these antimicrobial agents targeting Gram-negative bacteria corroborated with the observed rise in the detection of Gram-negative bacteria, including P. aeruginosa and K. pneumoniae. The use intensities of the remaining antibiotics showed no significant increasing or decreasing trends.
Table 2.
Annual use densities of common antibiotics (defined daily doses/1000 patient-days, DDDs/1000 PD) from 2007 to 2014.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
|---|---|---|---|---|---|---|---|---|
| Vancomycin | 44.05 | 70.34 | 124.24 | 37.91 | 142.97 | 32.42 | 87.52 | 106.74 |
| Penicillin | 50.17 | 12.12 | 50.00 | 10.55 | 83.67 | 55.45 | 42.88 | 36.02 |
| Teicoplanin | 51.58 | 44.02 | 30.00 | 2.16 | 5.52 | 1.51 | 52.55 | 39.92 |
| Amikacin | 8.65 | 22.23 | 47.93 | 21.90 | 91.44 | 61.65 | 48.90 | 49.41 |
| Cefradine | 9.22 | 5.89 | 12.95 | 5.10 | 21.48 | 26.57 | 20.22 | 0.52 |
| Ceftazidime | 21.84 | 33.08 | 18.39 | 23.90 | 56.65 | 86.11 | 59.64 | 72.05 |
| Cefoperazone/sulbactam | 17.35 | 27.09 | 24.05 | 9.62 | 43.50 | 63.56 | 54.18 | 50.62 |
| Cefuroxime | 0.05 | 1.20 | 2.66 | 1.01 | 5.82 | 5.42 | 10.70 | 12.16 |
| Imipenem | 7.63 | 18.14 | 39.89 | 21.89 | 91.57 | 157.25 | 40.13 | 33.29 |
| Meropenem | 25.64 | 36.71 | 74.53 | 0.48 | 0.01 | 20.28 | 22.48 | 26.87 |
| Lincomycin | 12.03 | 47.12 | 111.67 | 20.10 | 86.63 | 68.81 | 73.31 | 90.53 |
| Minocycline | 0.00 | 0.00 | 9.69 | 5.55 | 21.55 | 2.79 | 5.91 | 3.68 |
| Azithromycin | 0.23 | 5.55 | 2.73 | 3.42 | 4.13 | 3.26 | 2.76 | 2.66 |
| Ciprofloxacin | 0.31 | 0.00 | 1.01 | 19.71 | 15.94 | 19.77 | 12.85 | 7.68 |
Figure 2.
Annual changes in antibacterial use density (defined daily doses/1000 patient-days, DDDs/1000 PD) from 2007 to 2014.
Purple lines represent antibiotics commonly used against Gram-positive bacteria; red lines represent antibiotics used against Gram-negative bacteria; green lines represent antibiotics with antibacterial activity against both Gram-positive and Gram-negative bacteria. Dark colours (bottom panel) indicate significant changes in trends, light colours (upper and middle panels) indicate non-significant changes in trends.
As shown in Table 2, the use density of vancomycin ranked highest throughout the years, except in 2007 and 2012; the use density of lincomycin also ranked highly. The use densities of meropenem, imipenem and teicoplanin ranked moderately, whereas penicillin, azithromycin, minocycline, ciprofloxacin and cefradine showed lower use densities.
Annual changes in P. aeruginosa drug resistance
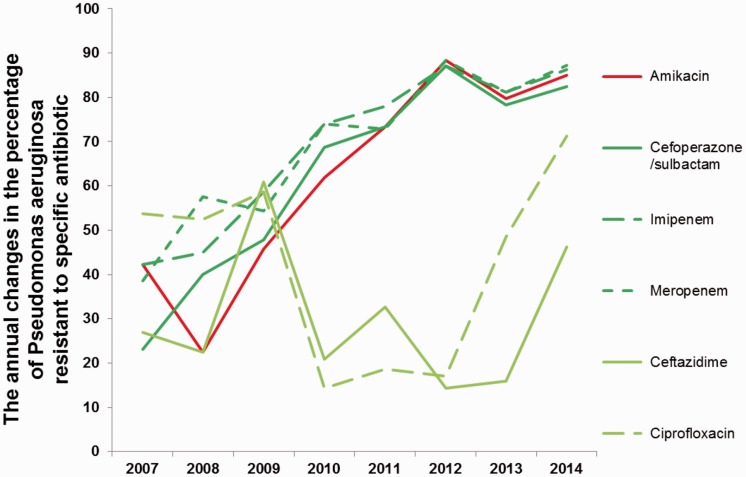
From 2007 to 2014, the resistance rates of P. aeruginosa to six common anti-Gram-negative antibiotics are shown in Table 3 and Figure 3, including amikacin, ceftazidime, cefoperazone/sulbactam, imipenem, meropenem and ciprofloxacin. Interestingly, the resistance rates of P. aeruginosa to amikacin (Linear, R2 = 0.805, p < 0.01), cefoperazone/sulbactam (Linear, R2 = 0.860, p < 0.01), imipenem (Linear, R2 = 0.874, p < 0.01) and meropenem (Linear, R2 = 0.861, p < 0.01) were significantly increased. By contrast, the resistance rates of P. aeruginosa to ceftazidime and ciprofloxacin showed no significant trend from 2007 to 2011, but started to rise from 2012, as shown in Figure 3. These findings indicated that P. aeruginosa resistance to antibiotics targeting Gram-negative bacteria generally increased over the time period assessed.
Table 3.
Annually detected resistance of P. aeruginosa to common anti-Gram-negative antibiotics from 2007–2014.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
|---|---|---|---|---|---|---|---|---|
| Amikacin | 42.3 | 22.5 | 45.7 | 61.8 | 73.3 | 88.4 | 79.7 | 85.0 |
| Ceftazidime | 26.9 | 22.5 | 60.9 | 20.8 | 32.6 | 14.3 | 15.9 | 46.2 |
| Cefoperazone/sulbactam | 23.1 | 40.0 | 47.8 | 68.8 | 73.3 | 87.1 | 78.3 | 82.5 |
| Imipenem | 42.3 | 45.0 | 58.7 | 74.0 | 77.9 | 87.0 | 81.2 | 86.2 |
| Meropenem | 38.5 | 57.5 | 54.3 | 74.0 | 72.9 | 88.4 | 81.2 | 87.3 |
| Ciprofloxacin | 53.8 | 52.5 | 58.7 | 14.3 | 18.6 | 17.1 | 48.5 | 71.2 |
The annual percentage (%) of P. aeruginosa strains resistant to specific antibiotics was measured by the Kirby–Bauer disk diffusion method.
Figure 3.
Annual changes in the percentage of P. aeruginosa resistant to specific antibiotics from 2007 to 2014.
The percentage (%) of P. aeruginosa resistant to specific antibiotics was measured by the Kirby–Bauer disk diffusion method. Red lines represent antibiotics commonly used against Gram-negative bacteria, green lines represent antibiotics with antibacterial activities against both Gram-positive and Gram-negative bacteria. Dark colors indicate significant changes in trends, light colors indicate non-significant changes in trends.
Annual changes in the prevalence of MDR strains of P. aeruginosa
The number and percentage of detected MDR strains of P. aeruginosa were calculated annually (Table 4). The percentage of MDR strains significantly increased (Linear, R2 0.806, P < 0.01) from 64.00% in 2007 to 89.87% in 2014.
Table 4.
Multidrug-resistant (MDR) strains of P. aeruginosa detected annually from 2007 to 2014.
| Strains | % | |
|---|---|---|
| 2007 | 16 | 64.00 |
| 2008 | 24 | 58.54 |
| 2009 | 29 | 64.44 |
| 2010 | 57 | 78.08 |
| 2011 | 68 | 80.00 |
| 2012 | 64 | 95.52 |
| 2013 | 57 | 86.36 |
| 2014 | 71 | 89.87 |
Note: The definition of MDR is resistance to three or more antimicrobial classes.
Correlation analyses
Table 5 shows the results of Pearson correlation analyses between the percentage of P. aeruginosa strains detected and the use densities of common antibiotics. Interestingly, the P. aeruginosa detection rate showed a positive correlation with the use intensities of cefuroxime (r = 0.89, p < 0.01), ceftazidime (r = 0.82, p < 0.05) and cefoperazone/sulbactam (r = 0.73, p < 0.05); however, no significant associations were found for the remaining antibiotics tested.
Table 5.
Correlation analyses of the detected percentages of P. aeruginosa and the use densities of common antibiotics.
| Correlation coefficient (r) | P value | |
|---|---|---|
| Vancomycin | 0.19 | 0.65 |
| Penicillin | 0.16 | 0.71 |
| Teicoplanin | −0.16 | 0.70 |
| Amikacin | 0.50 | 0.20 |
| Imipenem | 0.38 | 0.35 |
| Meropenem | −0.28 | 0.49 |
| Lincomycin | 0.45 | 0.27 |
| Minocycline | 0.11 | 0.79 |
| Azithromycin | 0.00 | 1.00 |
| Ciprofloxacin | 0.49 | 0.21 |
| Cefradine | 0.05 | 0.91 |
| Ceftazidime* | 0.82* | 0.01 |
| Cefoperazone/sulbactam* | 0.73* | 0.04 |
| Cefuroxime** | 0.89** | <0.01 |
:p < 0.05; **: p < 0.01
Table 6 shows the results of Pearson correlation analyses between the percentage of P. aeruginosa strains resistant to specific antibiotics and the use densities of common anti-Gram-negative antibiotics. Interestingly, the intensity of use of ciprofloxacin was positively correlated with the resistance rates of P. aeruginosa to amikacin (r = 0.75, p < 0.05), cefoperazone/sulbactam (r = 0.80, p < 0.05), imipenem (r = 0.80, p < 0.05) and meropenem (r = 0.75, p < 0.05), and negatively correlated with the resistance rate to itself (r = −0.82, p < 0.05). The intensity of use of ceftazidime showed a positive correlation with the resistance rates of P. aeruginosa to amikacin (r = 0.82, p < 0.05), cefoperazone/sulbactam (r = 0.81, p < 0.05), imipenem (r = 0.79, p <0.05) and meropenem (r = 0.84, p < 0.01). Finally, the intensity of use of cefoperazone/sulbactam was positively correlated with the resistance rates of P. aeruginosa to amikacin (r = 0.75, p < 0.05), imipenem (r = 0.71, p = 0.05), meropenem (r = 0.74, p < 0.05) and the resistance rate to cefoperazone/sulbactam itself (r = 0.73, p < 0.05).
Table 6.
Correlation analyses of the percentage of P. aeruginosa resistant strains to specific antibiotics and the use densities of common anti-Gram-negative antibiotics.
| Amikacin | Ceftazidime | Cefoperazone/ sulbactam | Imipenem | Meropenem | Ciprofloxacin | |
|---|---|---|---|---|---|---|
| Amikacin | 0.63 (0.10) | 0.16 (0.71) | 0.67 (0.07) | 0.67 (0.07) | 0.57 (0.14) | −0.33 (0.43) |
| Imipenem | 0.61 (0.11) | −0.25 (0.55) | 0.62 (0.10) | 0.60 (0.12) | 0.57 (0.14) | −0.58 (0.13) |
| Azithromycin | −0.14 (0.74) | −0.15 (0.72) | 0.27 (0.51) | 0.12 (0.77) | 0.33 (0.43) | −0.29 (0.49) |
| Ciprofloxacin | 0.75* (0.03) | −0.49 (0.21) | 0.80* (0.02) | 0.80* (0.02) | 0.75* (0.03) | −0.82* (0.01) |
| Ceftazidime | 0.82* (0.01) | −0.29 (0.48) | 0.81* (0.01) | 0.79* (0.02) | 0.84* (0.01) | −0.17 (0.69) |
| Cefoperazone/ sulbactam | 0.75* (0.03) | −0.20 (0.64) | 0.73* (0.04) | 0.71* (0.05) | 0.74* (0.04) | −0.03 (0.94) |
| Cefuroxime | 0.76* (0.03) | 0.08 (0.86) | 0.74* (0.03) | 0.76* (0.03) | 0.75* (0.03) | 0.27 (0.52) |
The common anti-Gram-negative antibiotics are listed in the rows. The specific antibiotics, to which the resistance rates of P. aeruginosa were measured, are listed in the columns.
:p < 0.05; **: p < 0.01
Discussion
Infection is one of the main causes of death in burn patients.1–3 Simultaneously monitoring the use of antibacterial agents and bacterial epidemiology6,23 can help effectively understand bacterial resistance and the inappropriate use of antibacterial agents, hereby enabling specific application of antibacterial agents. Specific and appropriate antibacterial use would achieve the purposes of fighting infection and mitigating drug resistance, avoiding the harm caused by irrational use of antibacterial agents. However, most reports on burn infections7,8 only monitored the prevalence and drug resistance of common bacteria, not taking into consideration the use of antibacterial agents in burn wards. In this study, we combined the analysis of antibacterial agent use and resistance of P. aeruginosa, an important bacterial species detected in our burn wards.10 Our data provide insight into inappropriate use of antibacterial agents, and may provide guidance for more effective therapeutic strategies that may help alleviate bacterial resistance.
We focused on P. aeruginosa because prevalence rates revealed a significant increasing trend, followed by K. pneumoniae, another Gram-negative bacterium. By contrast, Gram-positive S. aureus and S. epidermidis showed a significant decreasing trend in prevalence. This was in agreement with a recent study of pathogen prevalence and drug resistance in a burn ward, which reported 33.9%, 52.7% and 13.4% Gram-positive, Gram-negative bacteria and fungi, respectively.26 Studies have reported that burn patients infected with Gram-negative bacteria, especially P. aeruginosa,27 have a higher risk of death. Thus, more attention should be paid to Gram-negative bacteria, especially P. aeruginosa and K. pneumoniae, in determining antibiotic use in burn wards.
Our results revealed that the use intensities of amikacin, ceftazidime, cefuroxime and cefoperazone/sulbactam showed increasing trends. This might be due to extensive detection of Gram-negative bacteria, as these agents are commonly employed to treat Gram-negative bacterial infections. Consistent with this, correlation analysis revealed that the detection rate of P. aeruginosa positively correlated with the use intensities of cefuroxime (r = 0.89, p <0.01), ceftazidime (r = 0.82, p < 0.05) and cefoperazone/sulbactam (r = 0.73, p < 0.05). Although the intensity of use of vancomycin did not significantly increase, it remained high and ranked first for all of the years assessed except 2007 and 2012. This might be attributed to the fact that S. aureus always ranked first among the detected pathogens, although its rates of detection decreased over time.
It has been reported that P. aeruginosa strains detected in burn patients are usually MDR, i.e. show resistance to ciprofloxacin, cephalexin, aztreonam and ceftriaxone,28 and are associated with higher mortality,29 longer hospital stays and an increased number of ventilator days.30 Patients with resistant P. aeruginosa infection have a poor prognosis and it is therefore increasingly important that close attention is paid to P. aeruginosa strains displaying severe drug resistance. Unfortunately, our study showed that the percentage of MDR P. aeruginosa strains in our burn ward had increased significantly from 64.00% in 2007 to 89.87% in 2014. In this study, P. aeruginosa presented a significantly increasing trend in resistance rates to amikacin, cefoperazone/sulbactam, imipenem and meropenem. The resistance rates to ceftazidime and ciprofloxacin were also increased from 2012 to 2014. Extensive use of antibacterial agents gradually leads to bacterial resistance.15,16 We speculate that the observed increased resistance rates may result from the continuous and significant overuse of these antibiotics; and for ceftazidime and ciprofloxacin, this overuse appeared to be relatively serious from 2010 to 2012 (Figure 2).
We further assessed the correlation between intensity of use of ceftazidime or ciprofloxacin, and the resistance rates of P. aeruginosa. Our results revealed that the intensity of use of ceftazidime was not significantly correlated with the resistance rates of P. aeruginosa to ceftazidime, and the intensity of use of ciprofloxacin was negatively correlated with the resistance rates of P. aeruginosa to ciprofloxacin. These findings do not contradict the association of drug resistance and use intensities of these two antibiotics because it may take time to increase P. aeruginosa resistance upon antibacterial overuse. For cefoperazone/sulbactam, the intensity of use positively correlated with the cefoperazone/sulbactam resistance rate, indicating that resistance levels to certain antibiotics may increase without delay in P. aeruginosa.
The different timings for the appearance of drug resistance following drug overuse in P. aeruginosa may be attributed to the different antibiotic mechanisms. Drug resistance mechanisms in P. aeruginosa include: inactivating or inhibitory enzymes, increased active efflux pump system expression, changing target of antibacterial agents, biofilm formation, and loss or decreased outer membrane protein expression.12,13 Resistance mechanisms of P. aeruginosa to ciprofloxacin mainly include mutations in DNA gyrase and topoisomerase IV (encoded by the gyrA and parC genes, respectively).31 It takes time for mutations to occur and spread within a bacterial population, which may account for the delayed drug resistance we observed. Exposure to ciprofloxacin may also increase expression of the active efflux pump system in P. aeruginosa, as a rapid stress response. Such resistance mechanisms often induce P. aeruginosa resistance to a variety of antibacterial agents.13,32 In agreement with this, we found that the intensity of use of ciprofloxacin was positively correlated with the resistance rates of bacteria to other antibiotics such as amikacin, cefoperazone/sulbactam, imipenem and meropenem. P. aeruginosa resistance to cefoperazone/sulbactam has likely increased active efflux pump system expression and biofilm formation.33 Consistent with this, the intensity of use of cefoperazone/sulbactam was also found to be positively correlated with the resistance rates of bacteria to amikacin, imipenem, meropenem and cefoperazone/sulbactam itself. Extensive use of ciprofloxacin or cefoperazone/sulbactam may thereby enable transformation of bacteria to MDR or XDR forms.
It is also worth noting that there are other sources of antibacterial agents. For example, antibacterial agents are sometimes added to foods, such as milk,34–36 and it is conceivable that the regular consumption of such foods may contribute to antibiotic resistance. Therefore, in the face of serious levels of antibiotic resistance,37–39 it is important to consider all possible contributory factors.
A few limitations of this study should be mentioned. First, this retrospective study only collected data for bacteria and antibiotic use from one hospital ward, and did not record the clinical characteristics and demographic features of patients, which might have impacted on bacterial resistance. Second, bacterial specimens were not subjected to molecular identification and homology analyses. Third, it was impossible to distinguish nosocomial from community-acquired infections, which might lead to excessive resistance rates. Furthermore, this was only a retrospective descriptive analysis, in which no control group was included, no intervention was applied for antibacterial agent use, and bacterial resistance variations were not analysed after intervention. These limitations should be considered when interpreting the data, and further studies are warranted to clarify these issues.
Based on our findings, we conclude that anti-bacterial treatment strategies in burn departments should focus on Gram-negative bacteria, especially P. aeruginosa and K. pneumoniae, for which the prevalence rates are increasing year by year. The use of ciprofloxacin, ceftazidime and cefoperazone/sulbactam should be limited to counter the increase in resistance of P. aeruginosa to these agents and other common anti-Gram-negative antibiotics. These findings also confirmed that it is insufficient to only monitor bacterial prevalence in burn wards when selecting appropriate therapy. Antibiotic use and the corresponding resistance status of bacteria must also be considered to ensure the rational use of antibacterial agents and the development of effective therapeutic strategies.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Panghal M, Singh K, Kadyan S, et al. The analysis of distribution of multidrug resistant Pseudomonas and Bacillus species from burn patients and burn ward environment. Burns 2015; 41: 812–819. [DOI] [PubMed] [Google Scholar]
- 2.Gomez R, Murray CK, Hospenthal DR, et al. Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J Am Coll Surg 2009; 208: 348–354. [DOI] [PubMed] [Google Scholar]
- 3.Kwei J, Halstead FD, Dretzke J, et al. Protocol for a systematic review of quantitative burn wound microbiology in the management of burns patients. Syst Rev 2015; 4: 150– 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma BR. Infection in patients with severe burns: causes and prevention thereof. Infect Dis Clin North Am 2007; 21: 745–759, ix. [DOI] [PubMed] [Google Scholar]
- 5.Wanis M, Walker SA, Daneman N, et al. Impact of hospital length of stay on the distribution of Gram negative bacteria and likelihood of isolating a resistant organism in a Canadian burn center. Burns 2016; 42: 104–111. [DOI] [PubMed] [Google Scholar]
- 6.Hsu LY, Tan TY, Tam VH, et al. Surveillance and correlation of antibiotic prescription and resistance of Gram-negative bacteria in Singaporean hospitals. Antimicrob Agents Chemother 2010; 54: 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yali G, Jing C, Chunjiang L, et al. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns 2014; 40: 402–407. [DOI] [PubMed] [Google Scholar]
- 8.Alrawi M, Crowley TP, Pape SA. Bacterial colonisation of the burn wound: a UK experience. J Wound Care 2014; 23: 274–277. [DOI] [PubMed] [Google Scholar]
- 9.Keen EF, 3rd, Robinson BJ, Hospenthal DR, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 2010; 36: 819–825. [DOI] [PubMed] [Google Scholar]
- 10.Dou Y, Zhang Q, Liao ZJ. [Investigation on the drug resistance of Pseudomonas aeruginosa in our burn ward in the past 11 years]. Zhonghua Shao Shang Za Zhi 2004; 20: 6–9. [in Chinese, English Abstract]. [PubMed] [Google Scholar]
- 11.McCarthy RR, Mooij MJ, Reen FJ, et al. A new regulator of pathogenicity (bvlR) is required for full virulence and tight microcolony formation in Pseudomonas aeruginosa. Microbiology 2014; 160: 1488–1500. [DOI] [PubMed] [Google Scholar]
- 12.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 2002; 34: 634–640. [DOI] [PubMed] [Google Scholar]
- 13.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 2008; 322: 107–131. [DOI] [PubMed] [Google Scholar]
- 14.Mahar P, Padiglione AA, Cleland H, et al. Pseudomonas aeruginosa bacteraemia in burns patients: Risk factors and outcomes. Burns 2010; 36: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 15.Gallini A, Degris E, Desplas M, et al. Influence of fluoroquinolone consumption in inpatients and outpatients on ciprofloxacin-resistant Escherichia coli in a university hospital. J Antimicrob Chemother 2010; 65: 2650–2657. [DOI] [PubMed] [Google Scholar]
- 16.Vernaz N, Huttner B, Muscionico D, et al. Modelling the impact of antibiotic use on antibiotic-resistant Escherichia coli using population-based data from a large hospital and its surrounding community. J Antimicrob Chemother 2011; 66: 928–935. [DOI] [PubMed] [Google Scholar]
- 17.Zilberberg MD, Shorr AF, Micek ST, et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18: 596– 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsky BA, Napolitano LM, Moran GJ, et al. Economic outcomes of inappropriate initial antibiotic treatment for complicated skin and soft tissue infections: a multicenter prospective observational study. Diagn Microbiol Infect Dis 2014; 79: 266–272. [DOI] [PubMed] [Google Scholar]
- 19.Micek ST, Heard KM, Gowan M, et al. Identifying critically ill patients at risk for inappropriate antibiotic therapy: a pilot study of a point-of-care decision support alert. Crit Care Med 2014; 42: 1832–1838. [DOI] [PubMed] [Google Scholar]
- 20.Gholami A, Barati M, Vahdani M, et al. Pattern of empirical antibiotic administration in emergency department of an educational hospital in Tehran. Razi J Med Sci 2011; 18: 17–23. [Google Scholar]
- 21.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: A randomized clinical trial. JAMA 2016; 315: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquet K, Liesenborgs A, Bergs J, et al. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care 2015; 19: 63– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou YM, Ma Y, Liu JH, et al. Trends and correlation of antibacterial usage and bacterial resistance: time series analysis for antibacterial stewardship in a Chinese teaching hospital (2009–2013). Eur J Clin Microbiol Infect Dis 2015; 34: 795–803. [DOI] [PubMed] [Google Scholar]
- 24.Institute CaLS Performance standards for antimicrobial susceptibility testing: Twenty-fifth Informational Supplement M100–S25. In, CLSI, Wayne, PA, USA: 2015.
- 25.Methodology WCCfDS ATC/DDD Index In, http://www.whocc.no/atc_ddd_index/ (16 December 2015, date last accessed): 2016.
- 26.Cen H, Wu Z, Wang F, et al. Pathogen distribution and drug resistance in a burn ward: a three-year retrospective analysis of a single center in China. Int J Clin Exp Med 2015; 8: 19188–19199. [PMC free article] [PubMed] [Google Scholar]
- 27.Glik J, Kawecki M, Gazdzik T, et al. The impact of the types of microorganisms isolated from blood and wounds on the results of treatment in burn patients with sepsis. Pol Przegl Chir 2012; 84: 6–16. [DOI] [PubMed] [Google Scholar]
- 28.Farshadzadeh Z, Khosravi AD, Alavi SM, et al. Spread of extended-spectrum beta-lactamase genes of blaOXA-10, blaPER-1 and blaCTX-M in Pseudomonas aeruginosa strains isolated from burn patients. Burns 2014; 40: 1575–1580. [DOI] [PubMed] [Google Scholar]
- 29.Ressner RA, Murray CK, Griffith ME, et al. Outcomes of bacteremia in burn patients involved in combat operations overseas. J Am Coll Surg 2008; 206: 439–444. [DOI] [PubMed] [Google Scholar]
- 30.Armour AD, Shankowsky HA, Swanson T, et al. The impact of nosocomially-acquired resistant Pseudomonas aeruginosa infection in a burn unit. J Trauma 2007; 63: 164–171. [DOI] [PubMed] [Google Scholar]
- 31.Guss J, Abuzeid WM, Doghramji L, et al. Fluoroquinolone-resistant Pseudomonas aeruginosa in chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec 2009; 71: 263–267. [DOI] [PubMed] [Google Scholar]
- 32.Bubonja-Sonje M, Matovina M, Skrobonja I, et al. Mechanisms of carbapenem resistance in multidrug-resistant clinical isolates of Pseudomonas aeruginosa from a Croatian hospital. Microb Drug Resist 2015; 21: 261–269. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 2008; 190: 4447–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mardaneh J, Dallal MM. Isolation, identification and antimicrobial susceptibility of Pantoea (Enterobacter) agglomerans isolated from consumed powdered infant formula milk (PIF) in NICU ward: First report from Iran. Iran J Microbiol 2013; 5(3): 263–267. [PMC free article] [PubMed] [Google Scholar]
- 35.Mardaneh J, Soltan-Dallal MM. Isolation and identification of E. cowanii from powdered infant formula in NICU and determination of antimicrobial susceptibility of isolates. Iran J Pediatr 2014; 24(3): 261–266. [PMC free article] [PubMed] [Google Scholar]
- 36.Mardaneh J, Soltan Dallal MM, Taheripoor M, et al. Isolation, identification and antimicrobial susceptibility pattern of Tatumella ptyseos strains isolated from powdered infant formula milk consumed in neonatal intensive care unit: first report from Iran. Jundishapur J Microbiol 2014; 7(6): e10608– e10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anvarinejad M, Japoni A, Rafaatpour N, et al. Burn patients infected with metallo-beta-lactamase-producing Pseudomonas aeruginosa: multidrug-resistant strains. Arch Trauma Res 2014; 3(2): e18182– e18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poorabbas B, Mardaneh J, Rezaei Z, et al. Nosocomial infections: multicenter surveillance of antimicrobial resistance profile of Staphylococcus aureus and Gram negative rods isolated from blood and other sterile body fluids in Iran. Iran J Microbiol 2015; 7(3): 127–135. [PMC free article] [PubMed] [Google Scholar]
- 39.Soltani J, Poorabbas B, Miri N, et al. Health care associated infections, antibiotic resistance and clinical outcome: A surveillance study from Sanandaj, Iran. World J Clin Cases 2016; 4(3): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]