SUMMARY
Setting
A large tuberculosis clinic in Durban, South Africa.
Objective
To determine the association between isoniazid mono-resistant tuberculosis and treatment outcomes.
Design
We performed a retrospective longitudinal study of patients seen from 2000–2012 to compare episodes of isoniazid mono-resistant to drug-susceptible tuberculosis using logistic regression with robust standard errors. Isoniazid mono-resistant tuberculosis was treated with modified regimens.
Results
Among 18,058 TB patients, there were 19,979 TB episodes for which drug susceptibility tests were performed. Of these, 557 were INH mono-resistant and 16,311 were drug-susceptible. Loss to follow-up, transfer, and HIV co-infection (41% had known HIV serostatus) were similar between groups. Isoniazid mono-resistant episodes were more likely to result in treatment failure (4.1% versus 0.6%, P<0.001) and death (3.2% versus 1.8%, P=0.01) than drug-susceptible episodes. After adjusting for age, sex, race, retreatment status, and disease site, isoniazid mono-resistant episodes were more likely to have resulted in treatment failure (odds ratio [OR] 6.84; 95% confidence interval [CI] 4.29–10.89; P<0.001) and death (OR 1.81; 95% CI 1.11–2.95; P=0.02).
Conclusion
Isoniazid mono-resistance was associated with worse clinical outcomes compared to drug-susceptible tuberculosis. Our findings support the need for rapid diagnostic tests for isoniazid resistance and improved treatment regimens for isoniazid mono-resistant tuberculosis.
Keywords: drug-resistant tuberculosis, epidemiology, tuberculosis outcomes
INTRODUCTION
Drug-resistant tuberculosis has high rates of morbidity and mortality globally, and presents a formidable obstacle to tuberculosis elimination.1 Isoniazid and rifampicin are the most important drugs for the treatment of drug-susceptible tuberculosis. Despite considerable global variability, the prevalence of isoniazid resistance is higher than rifampicin resistance; the global population-weighted mean of any resistance to isoniazid is 13.3% and to rifampicin is 6.3%.2–4 Although several studies have determined isoniazid mono-resistance is not associated with poor outcomes,5–7 others have found associations with higher rates of treatment failure and progression to multidrug-resistant tuberculosis (MDR-TB; Mycobacterium tuberculosis resistant to at least isoniazid and rifampicin).8–11
Determining the clinical outcomes of isoniazid mono-resistance is particularly important in countries like South Africa, where screening for drug resistance is focused on rifampicin resistance through the use of Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA); this test does not detect isoniazid resistance.12–15 South Africa’s tuberculosis case detection rate (68%), proportion of HIV co-infection (60%) and burden of MDR-TB (6,200 cases in 2014) present an alarming context for optimizing diagnostic and treatment strategies.1
We performed a large retrospective longitudinal study of tuberculosis patients in South Africa to compare the clinical outcomes of patients with isoniazid mono-resistance to those with drug-susceptible tuberculosis. We hypothesized that isoniazid mono-resistant tuberculosis is associated with worse clinical outcomes than drug-susceptible tuberculosis in this setting. If true, this could have implications for current diagnostic and treatment strategies.
STUDY POPULATION AND METHODS
Study design and definitions
We performed a retrospective longitudinal study of all tuberculosis patients who had drug susceptibility tests (DST) performed and were treated at the Prince Cyril Zulu Communicable Diseases Centre (PCZCDC) in Durban, South Africa from January 2000 through December 2012. We compared all isoniazid mono-resistant tuberculosis episodes to all drug-susceptible tuberculosis episodes based on the initial phenotypic DST of each episode during the study period, and any additional DST performed within 30 days of the initial test. The University of KwaZulu-Natal Biomedical Research Ethics Committee and Vanderbilt University Institutional Review Board approved the study and waived the need for informed consent.
Cultures were routinely performed for all retreatment episodes, and for new episodes if patients did not sputum smear convert by 2 months of treatment, or had known contact with MDR-TB patients. DST for isoniazid and rifampicin were performed for patients with positive cultures for M. tuberculosis using the proportion method or absolute concentration method on solid media,16 though the results of both were not available for every sample (numbers reported in Results). Isoniazid DST results were reported as susceptible or resistant based on a critical concentration of 0.2μg/ml. Ethambutol and streptomycin DST were performed less frequently during the study period.
Isoniazid mono-resistance was defined as tuberculosis episodes with isoniazid resistance and susceptibility to any other anti-tuberculosis drugs tested, regardless of which other drugs were tested. For comparison, drug-susceptible tuberculosis was defined as susceptibility to isoniazid and rifampicin, and any other drug tested. Due to variable availability of DST results of each drug for tuberculosis episodes, we also evaluated isoniazid mono-resistance when the definition additionally included: 1) documented susceptibility to rifampicin, and 2) documented susceptibility to rifampicin, ethambutol, and streptomycin. For comparison to number 2 above, we defined drug-susceptible tuberculosis as documented susceptibility to isoniazid, rifampicin, ethambutol, streptomycin, and any other drug tested.
Data collection
Data were collected from the PCZCDC electronic tuberculosis medical record. Due to gradual integration of HIV and tuberculosis care in Durban, documentation of HIV serostatus was limited in electronic records.
Tuberculosis treatment
Individual pharmacy records were not routinely captured in the database. According to treatment guidelines at the time,17 new drug-susceptible tuberculosis patients were treated with isoniazid (H), rifampicin (R), ethambutol (E), and pyrazinamide (Z) for two months (2HREZ) followed by 4HR. New tuberculosis patients found to have isoniazid mono-resistance were also treated for six months, but with all four drugs (6HREZ). Patients treated previously for tuberculosis received a retreatment regimen: streptomycin (S) added to HREZ for the first two months (2SHREZ), then 1HREZ/5HRE. Previously treated patients found to have isoniazid mono-resistant tuberculosis received a modified retreatment regimen, also for eight months: 2SHREZ/6HREZ.
Study outcomes
We assessed treatment outcomes as recorded in the electronic medical record by clinic health care providers, who used WHO definitions.18 Our primary study outcomes were treatment failure, death, and a combined endpoint of treatment failure and death. Neither cause nor timing of death in relation to tuberculosis treatment initiation was available in patient records. We also assessed treatment success (combined endpoint of cure and treatment completion).
We used the resistance profile of the tuberculosis episode immediately preceding the MDR-TB episode to compare progression to MDR-TB among persons with isoniazid mono-resistant and drug-susceptible tuberculosis.
Statistical analysis
We assessed differences between isoniazid mono-resistant and drug-susceptible tuberculosis episodes using the chi squared test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
We fit logistic regression models to compare outcomes of tuberculosis episodes with isoniazid mono-resistance to episodes with drug-susceptible tuberculosis after controlling for other variables. The logistic regression models used robust (sandwich) variance estimation to account for correlation between possibly multiple episodes from the same patient.19 Models included episodes for which none of the variables had missing data. To avoid potential bias in outcomes introduced by the inclusion of retreatment episodes, we performed sensitivity analyses using only the first tuberculosis episode of each patient.
We performed all analyses using Stata, version 12.1 (Stata Corporation, College Station, TX, USA). All P values were two-sided and P<0.05 was considered statistically significant.
RESULTS
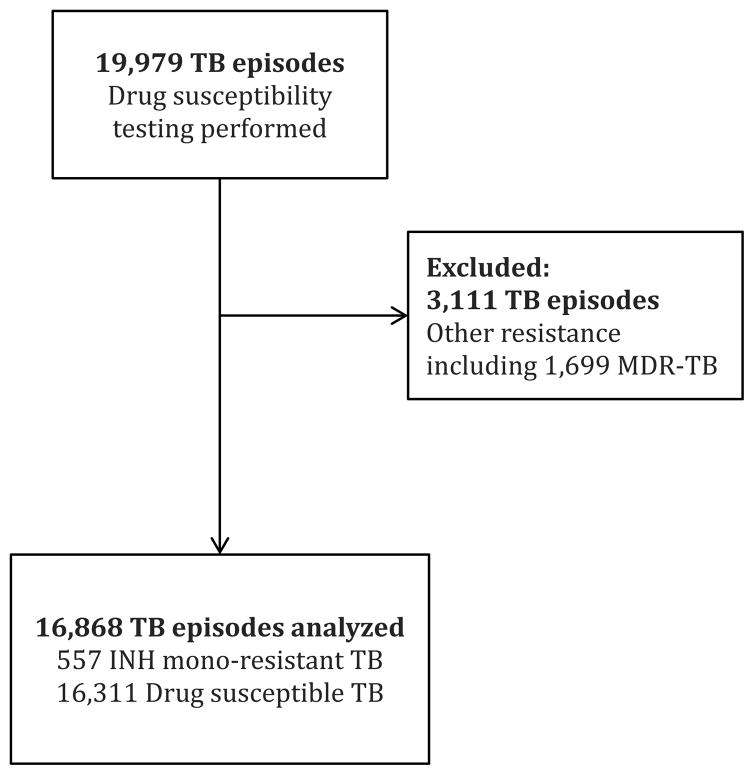
During the study period, DST were performed according to routine clinic practice for 19,979 tuberculosis episodes among 18,058 patients. Of the total tuberculosis episodes, 16,868 (84%) were included in the study, consisting of 557 (3%) isoniazid mono-resistant episodes and 16,311 (82%) drug-susceptible episodes. The remaining 3,111 (16%) episodes had other resistance patterns, including 1,699 (9%) episodes of MDR-TB (Figure 1).
Figure 1.
Flow diagram of study patients.
TB = tuberculosis; MDR-TB = multidrug-resistant tuberculosis; INH = isoniazid.
Tuberculosis episodes for which DST were performed represented 22% of the total tuberculosis episodes at PCZCDC during the study period. Episodes with DST performed were less likely to have treatment success (45% versus 52%; P<0.001) and more likely to have treatment failure (1.1% versus 0.3%; P<0.001) and death (2.5% versus 2.2%; P=0.02) than episodes without DST.
Isoniazid mono-resistant and drug-susceptible tuberculosis episodes did not differ significantly in age, sex, race, or site of disease (Table 1). Isoniazid mono-resistant episodes were more likely to be in the retreatment category than were drug-susceptible episodes (67% versus 63%; P=0.07), but the difference was not significant.
Table 1.
Demographic and clinical characteristics of drug-susceptible compared to isoniazid mono-resistant tuberculosis episodes
| Drug Susceptible N=16,311* |
INH mono-resistant N=557* |
P Value | |
|---|---|---|---|
| Age (median, IQR), years | 34 (28, 42) | 34 (29, 43) | 0.11 |
| Female sex | 5,671 (35) | 193 (35) | 0.95 |
| Race† | 0.69 | ||
| Black | 15,129 (93) | 515 (92) | |
| Asian | 579 (4) | 22 (4) | |
| Coloured | 508 (3) | 15 (3) | |
| White | 95 (1) | 5 (1) | |
| Pulmonary tuberculosis | 15,446/16,309 (95) | 538 (97) | 0.05 |
| Retreatment | 10,207 (63) | 370 (66) | 0.07 |
| HIV infected | 5,245/6,636 (79) | 186/227 (82) | 0.29 |
| Outcome | |||
| Successfully treated‡ | 8,186/16,230 (50) | 235/556 (42) | 0.001 |
| Failed | 101/16,230 (0.6) | 23/556 (4.1) | <0.001 |
| Died | 293/16,230 (1.8) | 18/556 (3.2) | 0.01 |
| Defaulted (LTFU) | 3,436/16,230 (21) | 129/556 (23) | 0.25 |
| Moved | 4,350/16,230 (27) | 150/556 (27) | 0.93 |
Bold values denote P <0.05.
Total unless otherwise indicated due to missing data.
Race categories reported as they were recorded in the clinical database.
Successfully treated is a combined endpoint of cure and treatment completion.
INH = isoniazid; IQR = interquartile range; HIV = human immunodeficiency virus; LTFU = loss to follow-up.
The HIV serostatus of patients was known for 6,863 (41%) tuberculosis episodes included in the study, 5,431 (79%) of which were known to be HIV infected. HIV serostatus was not significantly different between isoniazid mono-resistant and drug-susceptible tuberculosis episodes (Table 1).
Tuberculosis outcomes
Of the 16,868 tuberculosis episodes included in the study, 16,786 (99.5%) had an outcome reported, including 8,072 (48%) episodes that were identified as default, moved, or transferred. Isoniazid mono-resistant episodes were more likely to have treatment failure than drug-susceptible episodes (4.1% versus 0.6%, P<0.001; Table 1). Similarly, isoniazid mono-resistant episodes were more likely to end in death than drug-susceptible episodes (3.2% versus 1.8%, P=0.01). Isoniazid mono-resistant episodes were less likely to achieve treatment success than drug-susceptible episodes (42% versus 50%, P=0.001). However, isoniazid mono-resistant and drug-susceptible episodes did not differ regarding loss to follow up (default) or transfer or move out of the clinic.
In a logistic regression model adjusting for age, sex, race, retreatment status, and site of disease, isoniazid mono-resistance was associated with increased odds of treatment failure (odds ratio [OR] 6.84; 95% confidence interval [CI] 4.29, 10.89; P<0.001; Table 2), increased odds of death (OR 1.81; 95% CI 1.11, 2.95; P=0.02), and an increased odds of the combined endpoint of death and treatment failure (OR 3.19; 95% CI 2.28, 4.46; P<0.001). Furthermore, isoniazid mono-resistant episodes were less likely to have treatment success than drug-susceptible episodes (OR 0.74; 95% CI 0.62, 0.88; P=0.001). Sensitivity analyses using only the first episode of tuberculosis for patients who had multiple tuberculosis episodes demonstrated similar results.
Table 2.
Adjusted logistic regression models for four treatment outcomes, n=16,784* for each model
| Failed | Died | Failed + Died | Success† | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Isoniazid mono-resistance | 6.84 (4.29, 10.89) | <0.001 | 1.81 (1.11, 2.95) | 0.02 | 3.19 (2.28, 4.46) | <0.001 | 0.74 (0.62, 0.88) | 0.001 |
| Age (per year) | 1.00 (0.98, 1.01) | 0.60 | 1.03 (1.02, 1.04) | <0.001 | 1.02 (1.01, 1.03) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| Female sex | 0.68 (0.45, 1.04) | 0.08 | 1.05 (0.82, 1.34) | 0.71 | 0.93 (0.75, 1.15) | 0.52 | 1.42 (1.33, 1.51) | <0.001 |
| Race‡ | ||||||||
| Asian vs Black | 4.38 (2.57, 7.47) | <0.001 | 1.01 (0.57, 1.80) | 0.96 | 1.84 (1.25, 2.72) | 0.002 | 0.99 (0.84, 1.16) | 0.87 |
| Coloured vs Black | 1.80 (0.78, 4.18) | 0.17 | 0.98 (0.52, 1.86) | 0.95 | 1.19 (0.71, 1.99) | 0.50 | 0.76 (0.64, 0.91) | 0.003 |
| White vs Black | 1.40 (0.18, 10.66) | 0.74 | 0.36 (0.05, 2.64) | 0.32 | 0.58 (0.14, 2.42) | 0.46 | 0.66 (0.44, 0.97) | 0.04 |
| Pulmonary tuberculosis | 1.88 (0.59, 5.92) | 0.28 | 0.59 (0.39, 0.88) | 0.01 | 0.72 (0.49, 1.06) | 0.10 | 1.49 (1.29, 1.72) | <0.001 |
| Retreatment | 1.08 (0.73, 1.60) | 0.68 | 0.93 (0.74, 1.18) | 0.56 | 0.97 (0.79, 1.19) | 0.79 | 0.56 (0.52, 0.60) | <0.001 |
Bold values denote P <0.05.
Episodes missing outcome (n=82) and site of disease (n=2) excluded from regression models.
Success is a combined endpoint of cure and treatment completion.
Race categories reported as they were recorded in the clinical database.
OR = odds ratio; CI = confidence interval.
Among the 557 episodes with isoniazid mono-resistance, 520 had documented rifampicin susceptibility, 410 ethambutol susceptibility, and 387 streptomycin susceptibility (311 had documented susceptibility to all three drugs concurrently). Among the 16,311 drug-susceptible episodes, all had documented rifampicin susceptibility, 12,016 ethambutol susceptibility and 5,325 streptomycin susceptibility (5,065 had documented susceptibility to all three drugs concurrently).
Comparison of the 520 episodes with documented rifampicin susceptibility to the 16,311 drug-susceptible tuberculosis episodes demonstrated that isoniazid mono-resistant episodes had higher odds of death than drug-susceptible episodes, but the association was no longer statistically significant (OR 1.61; 95% CI 0.95, 2.74; P=0.08). These isoniazid mono-resistant episodes were still significantly more likely to have treatment failure (OR 7.06; 95% CI 4.39, 11.34; P<0.001) and a combined endpoint of treatment failure and death (OR 3.08; 95% CI 2.16, 4.37; P<0.001), and less likely to have treatment success (OR 0.74; 95% CI 0.62, 0.89; P=0.001) than drug-susceptible episodes. Similarly, the analysis comparing the 311 isoniazid-resistant episodes with documented rifampicin, ethambutol, and streptomycin susceptibility to 5,065 episodes with documented isoniazid, rifampicin, ethambutol, and streptomycin susceptibility demonstrated that isoniazid mono-resistance was significantly more likely to have treatment failure (OR 8.09; 95% CI 4.43, 14.76; P<0.001), a combined endpoint of treatment failure and death (OR 3.15; 95% CI 2.04, 4.88; P<0.001), and less likely to have treatment success (OR 0.63; 95% CI 0.49, 0.80; P<0.001) than drug-susceptible episodes.
In a similar model that included HIV status and was limited to patients with known HIV serostatus (41% of study population), the association of isoniazid mono-resistance with mortality was no longer statistically significant (OR 1.75; 95% CI 0.81, 3.78; P=0.16; Table 3), though the OR was similar to that in the model without HIV status. Isoniazid mono-resistance was associated with treatment failure and the combined endpoint of death and treatment failure.
Table 3.
Adjusted logistic regression models for four treatment outcomes, including adjustment for HIV serostatus, n=6,801* for each model.
| Failed | Died | Failed + Died | Success† | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Isoniazid mono-resistance | 6.87 (3.39, 13.95) | <0.001 | 1.75 (0.81, 3.78) | 0.16 | 3.20 (1.90, 5.39) | <0.001 | 0.82 (0.63, 1.07) | 0.14 |
| Age (per year) | 0.98 (0.94, 1.01) | 0.21 | 1.03 (1.01, 1.05) | 0.002 | 1.02 (1.00, 1.04) | 0.07 | 1.02 (1.01, 1.02) | <0.001 |
| Female sex | 0.55 (0.31, 0.99) | 0.05 | 0.93 (0.65, 1.35) | 0.72 | 0.81 (0.59, 1.10) | 0.18 | 1.48 (1.34, 1.64) | <0.001 |
| Race‡ | ||||||||
| Asian vs Black | 1.67 (0.23, 11.96) | 0.61 | 0.75 (0.10, 5.76) | 0.79 | 1.02 (0.24, 4.33) | 0.98 | 0.88 (0.57, 1.34) | 0.54 |
| Coloured vs Black | 1.24 (0.17, 9.23) | 0.83 | 0.93 (0.22, 3.98) | 0.93 | 1.02 (0.31, 3.36) | 0.97 | 0.54 (0.38, 0.78) | 0.001 |
| White vs Black§ | - | - | - | - | - | - | 0.69 (0.31, 1.54) | 0.37 |
| Pulmonary tuberculosis | 3.13 (0.43, 22.66) | 0.26 | 0.66 (0.36, 1.21) | 0.17 | 0.85 (0.48, 1.51) | 0.58 | 1.39 (1.13, 1.72) | 0.002 |
| Retreatment | 1.76 (0.96, 3.15) | 0.06 | 0.72 (0.50, 1.03) | 0.07 | 0.93 (0.69, 1.26) | 0.63 | 0.67 (0.61, 0.74) | <0.001 |
| HIV infection | 1.64 (0.79, 3.41) | 0.19 | 3.16 (1.61, 6.20) | 0.001 | 2.45 (1.49, 4.04) | <0.001 | 0.86 (0.76, 0.98) | 0.02 |
Bold values denote P <0.05.
Episodes missing HIV serostatus (n=9,985), outcome (n=62), HIV serostatus and outcome (n=20), and HIV serostatus and site of disease (n=2) excluded from regression models.
Success is a combined endpoint of cure and treatment completion.
Race categories reported as they were recorded in the clinical database.
Among the 6,801 episodes, those with white race (n=26) did not have any outcomes of failed or died, therefore the analyses with outcomes of failed, died, or failed + died excluded those 26 episodes.
OR = odds ratio; CI = confidence interval.
Progression to MDR-TB
The 16,868 tuberculosis episodes included in the study occurred among 15,442 tuberculosis patients. Of these, 162 patients developed MDR-TB after previously having isoniazid mono-resistant or drug-susceptible episodes. Among 507 patients with isoniazid mono-resistance, 14 (3%) developed MDR-TB, and among 14,935 patients with drug-susceptible tuberculosis, 148 (1%) developed MDR-TB (P<0.001).
DISCUSSION
In this study, isoniazid mono-resistant tuberculosis was associated with worse clinical outcomes than drug-susceptible tuberculosis. Although several other studies have assessed the outcomes of isoniazid mono-resistant tuberculosis,5–11,20–22 to our knowledge this study is the largest to date from a high-burden setting with a direct comparison between tuberculosis episodes with isoniazid mono-resistance and drug-susceptible disease.
The varying outcomes reported in different studies may be related to several factors, including differences in study populations and treatment programs, incidence of tuberculosis and resistance to different drugs, and treatment regimens. Previously reported isoniazid mono-resistant treatment regimens are highly variable, both in terms of drugs used and duration of treatment.10,21,23,24 The 1994 American Thoracic Society tuberculosis treatment guidelines recommended treating patients with isoniazid mono-resistant disease with four-drug therapy for six months, which was the regimen used for new tuberculosis patients in our study.25,26 Current WHO recommendations for isoniazid-resistant tuberculosis (with or without streptomycin resistance) include six to nine months of rifampicin, pyrazinamide, and ethambutol, with or without a fluoroquinolone.27 The most recent American Thoracic Society tuberculosis treatment guidelines recommend the same regimen for six months.28 Other reported treatment strategies include the use of high dose isoniazid,9 and more recently the use of fluoroquinolones.21,29 Two systematic reviews emphasized the benefit of longer rifampicin duration and more effective drugs early in the treatment of isoniazid mono-resistant tuberculosis.10,24 These findings, together with our study results, suggest that more effective treatment regimens are needed for isoniazid mono-resistant tuberculosis.
Even if optimal treatment regimens are identified, isoniazid mono-resistance is frequently undiagnosed. Programs with treatment algorithms based on Xpert MTB/RIF often do not perform routine DST on rifampicin-susceptible isolates. Thus, patients with isoniazid-resistant, rifampicin-susceptible tuberculosis go undetected and are treated as drug-susceptible tuberculosis. Our findings suggest that isoniazid resistance is important to identify so that treatment can be adjusted to a more effective treatment regimen. Further assessment regarding implementation of rapid diagnostic tests that include isoniazid DST such as line probe assays may prove beneficial in high burden settings.30
Our study was retrospective and observational, with a substantial proportion of outcomes (48%) recorded as default, moved, or transferred out. This might explain the lower than expected mortality and treatment failure for this setting. However, we did not find any significant differences in the proportion of patients who “defaulted” or “moved” between isoniazid mono-resistant and drug-susceptible tuberculosis episodes. Furthermore, according to WHO definitions, the outcome “cured” was based on smear conversion rather than culture conversion, and “treatment completion” did not require bacteriological confirmation, leaving the possibility of underestimating “treatment failure” and progression to MDR-TB. The high proportion of patients recorded as having defaulted, moved, or transferred out underscores the critical need for tuberculosis programs to improve the monitoring of patient movements to ensure optimal tuberculosis treatment.
We used an electronic medical record not specifically designed for research purposes. Furthermore, our study was limited by absence of individual treatment records. However, available data were captured in an operational context, which provides important insight into clinical practice in this high-burden setting. The incomplete HIV-related data available in the study period limit our ability to account for the effect of HIV status on outcomes associated with isoniazid mono-resistance. However, the proportion of those with known HIV infection did not significantly differ between isoniazid mono-resistant and drug-susceptible tuberculosis episodes.
Our definition of isoniazid mono-resistance was limited by the restricted panel of DST recorded in the database. Confirmation of rifampicin susceptibility was not available for 37 (7%) of the isoniazid mono-resistant episodes and ethambutol and pyrazinamide DST were not routinely performed (which is the case for many resource limited settings27). Patients with limited DST could have had resistance to drugs that were not tested, and therefore poor outcomes could have been related to treatment regimens that had very few effective drugs. However, additional analyses in which isoniazid mono-resistance included 1) those with confirmed rifampicin susceptibility, and 2) those with confirmed rifampicin, ethambutol, and streptocmycin susceptibility demonstrated that although isoniazid mono-resistant tuberculosis was no longer significantly associated with death, it remained significantly associated with other unfavorable outcomes.
The study only included episodes for which DST had been performed (retreatment cases, patients who failed to convert, and contacts of MDR patients). These were associated with worse clinical outcomes than those not tested, suggesting our study population was not representative of all tuberculosis episodes. However, selection of isolates for DST was based on the same criteria for both isoniazid mono-resistant and susceptible groups, and most patients in both groups were retreatment cases. Requests for DST were dictated by standard clinical practice for the area. In addition, the drug-susceptible comparison group was large relative to the isoniazid mono-resistance group. Our findings highlight the need for more accessible diagnostic tools in resource-limited settings.
CONCLUSIONS
We found that isoniazid mono-resistance is associated with worse clinical outcomes when compared to drug-susceptible tuberculosis. Our findings indicate that isoniazid mono-resistant tuberculosis needs to be diagnosed more rapidly and treated more effectively than current tests and treatment allow.
Acknowledgments
The authors thank Noluthando Ngomane and the staff at the eThekwini Municipality and Prince Cyril Zulu Communicable Diseases Centre for their assistance. The authors also thank Gary Parker for his assistance with data collection.
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, grant numbers K08 AI106420 (Y.F.v.d.H.), K24 AI065298 (T.R.S.), U01 AI069924 (T.R.S. and A.S.P.), P30 AI110527 (B.E.S.).
Conflicts of interest: T.R.S. is on the data safety monitoring board of a clinical trial of delamanid (Otsuka). M.S.M. reports personal fees from Mylan Pharmaceuticals, Merck/MSD/Mylan, Abbott Laboratories, ViiV, and Aspen Pharmaceuticals. All other authors: none declared.
Author’s Contributions: Study concept and design: Y.F.v.d.H., F.K., T.R.S., and A.S.P. Acquisition of data: Y.F.v.d.H., F.K., G.M., L.Z., and T.C. Analysis and interpretation of data: Y.F.v.d.H., B.E.S., F.M., M.S.M., T.R.S., and A.S.P. Drafting of the manuscript: Y.F.v.d.H. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Y.F.v.d.H. and B.E.S. Study supervision: T.R.S. and A.S.P.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. WHO/HTM/TB/2015.22. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
- 2.Jenkins HE, Zignol M, Cohen T. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. PLoS One. 2011;6(7):e22927. doi: 10.1371/journal.pone.0022927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16(2):203–205. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Anti-Tuberculosis Drug Resistance in the World: Fourth Global Report. WHO/HTM/TB/2008.394. Available from: http://apps.who.int/iris/bitstream/10665/43889/1/WHO_HTM_TB_2008.394_eng.pdf?ua=1.
- 5.Cattamanchi A, Dantes RB, Metcalfe JZ, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48(2):179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang D, Andersen PH, Andersen AB, Thomsen VO. Isoniazid-resistant tuberculosis in Denmark: mutations, transmission and treatment outcome. J Infect. 2010;60(6):452–457. doi: 10.1016/j.jinf.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Wang TY, Lin SM, Shie SS, et al. Clinical characteristics and treatment outcomes of patients with low- and high-concentration isoniazid-monoresistant tuberculosis. PLoS One. 2014;9(1):e86316. doi: 10.1371/journal.pone.0086316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gegia M, Cohen T, Kalandadze I, Vashakidze L, Furin J. Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007–2009. Int J Tuberc Lung Dis. 2012;16(6):812–816. doi: 10.5588/ijtld.11.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin Infect Dis. 2011;53(4):369–372. doi: 10.1093/cid/cir406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzies D, Benedetti A, Paydar A, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009;6(9):e1000150. doi: 10.1371/journal.pmed.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 12.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Xpert MTB/RIF implementation manual; Technical and operational ‘how-to’: practical considerations. WHO/HTM/TB/2014.1. Available from: http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf. [PubMed]
- 15.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 16.Canetti G, Fox W, Khomenko A, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41(1):21–43. [PMC free article] [PubMed] [Google Scholar]
- 17.South African Department of Health. The South African Tuberculosis Control Programme Practical Guidelines. 2000 Available from: https://www.westerncape.gov.za/text/2003/tb_guidelines2000.pdf.
- 18.World Health Organization. Global Tuberculosis Report 2013. WHO/HTM/TB/2013.11. Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf.
- 19.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics; 1967; Berkeley, Calif: University of California Press; 1967. [Google Scholar]
- 20.Babu Swai O, Aluoch JA, Githui WA, et al. Controlled clinical trial of a regimen of two durations for the treatment of isoniazid resistant pulmonary tuberculosis. Tubercle. 1988;69(1):5–14. doi: 10.1016/0041-3879(88)90035-9. [DOI] [PubMed] [Google Scholar]
- 21.Chien JY, Chen YT, Wu SG, Lee JJ, Wang JY, Yu CJ. Treatment outcome of patients with isoniazid mono-resistant tuberculosis. Clin Microbiol Infect. 2015;21(1):59–68. doi: 10.1016/j.cmi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Fox L, Kramer MR, Haim I, Priess R, Metvachuk A, Shitrit D. Comparison of isoniazid monoresistant tuberculosis with drug-susceptible tuberculosis and multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2011;30(7):863–867. doi: 10.1007/s10096-011-1167-4. [DOI] [PubMed] [Google Scholar]
- 23.Reves R, Heilig CM, Tapy JM, et al. Intermittent tuberculosis treatment for patients with isoniazid intolerance or drug resistance. Int J Tuberc Lung Dis. 2014;18(5):571–580. doi: 10.5588/ijtld.13.0304. [DOI] [PubMed] [Google Scholar]
- 24.Stagg HR, Harris RJ, Hatherell HA, et al. What are the most efficacious treatment regimens for isoniazid-resistant tuberculosis? A systematic review and network meta-analysis. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-208262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bass JB, Jr, Farer LS, Hopewell PC, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149(5):1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 26.Nolan CM, Goldberg SV. Treatment of isoniazid-resistant tuberculosis with isoniazid, rifampin, ethambutol, and pyrazinamide for 6 months. Int J Tuberc Lung Dis. 2002;6(11):952–958. [PubMed] [Google Scholar]
- 27.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2014.11. Available from: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1. [PubMed]
- 28.American Thoracic Society, CDC, Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 29.Lee H, Jeong BH, Park HY, et al. Treatment Outcomes with Fluoroquinolone-Containing Regimens for Isoniazid-Resistant Pulmonary Tuberculosis. Antimicrob Agents Chemother. 2015;60(1):471–477. doi: 10.1128/AAC.01377-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. The use of molecular line probe assays for the detection of resistance to isoniazid and rifampicin: policy update. WHO/HTM/TB/2016.12. Available from: http://apps.who.int/iris/bitstream/10665/250586/1/9789241511261-eng.pdf?ua=1.