Abstract
Objective
To evaluate levels of the calcium-binding proteins S100A8 and S100A9 in the skin of patients with psoriatic arthritis.
Methods
Skin punch biopsies were obtained from patients with psoriatic arthritis and healthy control subjects. S100A8/A9 were semiquantified via immunohistochemistry and semiquantitative polymerase chain reaction.
Results
The study included biopsies from nine patients with psoriatic arthritis and nine control subjects. S100A8 and S100A9 were present at visibly higher levels in psoriatic plaques compared with normal skin samples. S100A8 and S100A9 RNA levels were significantly higher in the peripheral region of plaques compared with the central region.
Conclusion
Both S100A8 and S100A9 may represent good therapeutic targets in psoriasis and psoriatic arthritis.
Keywords: Inflammation, psoriatic arthritis, psoriatic plaques, S100A8, S100A9, skin lesions
Introduction
Psoriatic arthritis is a chronic inflammatory disease characterized by an association between psoriatic skin lesions and arthritis. Striking features of the synovium in psoriatic arthritis are the marked tortuosity of blood vessels at the macroscopic level, and plasma cell and mononuclear cell infiltration.1 These histological findings are associated with monocyte-derived cytokines, which are important mediators in synovitis.2
Myeloid-related protein 8 (MRP8; S100A8) and MRP14 (S100A9) are calcium-binding proteins belonging to the S100 protein family. They are involved in both intracellular functions (cell differentiation and cell-cycle progression, regulation of kinase activities and cytoskeleton–membrane interactions) and extracellular functions (neutrophil extension, chemoattraction and induction of adhesion molecule expression).3 Elevated S100A8 and S100A9 levels are found in fluid samples from inflamed tissues in rheumatoid arthritis and psoriatic arthritis, strengthening the hypothesis that these molecules are important inflammatory factors.4 The gene-expression profile of psoriatic arthritis includes increased expression of genes involved in downregulation or suppression of innate and acquired immune responses, such as S100A8.5 In addition, a mouse model of psoriasis that targeted deletion of jun-b and c-jun in keratinocytes resulted in a psoriasiform phenotype, with both erosive inflammatory arthritis and periostitis, reminiscent of human psoriatic arthritis. In this model, epidermal expression of S100A8/A9 was significantly increased in prediseased skin,6 suggesting that a selective defect of keratinocytes could induce an immune reaction that leads to the psoriatic phenotype and also to erosive arthritis.
The aim of our study was to evaluate the presence of S100A8/A9 in skin samples from patients with psoriatic arthritis.
Patients and methods
Study population
The study recruited patients with untreated psoriatic arthritis attending the combined outpatient unit of Dermatology and Rheumatology, University of Rome ‘‘Tor Vergata’’, Rome, Italy, between January 2012 and February 2013. Age- and sex-matched healthy control subjects were recruited through advertisements placed by the University of Rome “Tor Vergata”, Rome, Italy.
All patients and control subjects provided written informed consent prior to enrolment. The study was approved by the local ethics committee of the University of Rome “Tor Vergata”, Rome, Italy.
Immunohistochemistry
Skin punch biopsies were obtained from healthy controls (two samples per subject), and from the centre and periphery of psoriatic lesions from patients. From each donor, one sample was snap-frozen in liquid nitrogen for RNA extraction and the other was fixed in 10% buffered formalin solution (pH, 5.5) at 4°C overnight, processed and embedded in paraffin wax. After deparaffinizing and rehydration, 5 µm-thick skin sections were incubated at 80°C overnight in citrate buffer (pH 6.0), incubated with 3% hydrogen peroxide for 10 min, then incubated with SuperBlock™ (ScyTek, Logan, UT, USA) for 5 min. Sections were then incubated for 1 h at room temperature with polyclonal rabbit anti-human S100A8 (HPA 024372; Sigma Aldrich, St Louis, MO USA), polyclonal rabbit anti-human S100A9 (OPA 004193 Sigma Aldrich; both at 2 µg/ml in antibody diluent with background reducing components [S3022; Dako, Carpinteria, CA USA]), or rabbit immunoglobulin G, polyclonal isotype control (ab 27472; Abcam, Cambridge, MA USA). Slides were washed three times with phosphate buffered saline (PBS; pH, 7.4), then incubated with Dako REAL Detection system (Dako) according to the manufacturer’s instructions.
Staining intensity was semiquantified using digital image analysis. Briefly, stained tissue sections were viewed under a light microscope. Digital images were amplified to 1280 × 960 pixels, rasterized and three fields per section were analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA; available at: http://imagej.nih.gov/ij/).
RT–PCR
For semiquantitative polymerase chain reaction (PCR), total RNA was extracted from snap-frozen skin samples using TRIzol® (Thermo Fisher Scientific; Waltham, MA, USA) according to the manufacturer’s instructions. Total RNA (1 µg) was reverse transcribed using oligo(dT) primers and MuLV reverse transcriptase (Applied Biosystems Italy, Milan, Italy). The resulting cDNA was used in PCR analysis with AmpliTaq™ Gold 360 DNA polymerase (Applied Biosystems) and an iCycler® thermal cycler (Bio-Rad, Hercules, CA USA). Primer sequences were: S100A8 forward primer, 5′-ATTTCCATGCCGTCTACAGG-3′ and reverse primer, 5′-TGGCTTTCTTCATGGCTTTT-3′; S100A9 forward primer, 5′-CAGCTGGAACGCAACATAGA-3′ and reverse primer, 5′-CCACAGCCAAGACAGTTTGA-3′: glyceraldehyde-3-phosphate dehydrogenase (GAPDH; internal control) forward primer, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse primer, 5′-TCCACCACCCTGTTGCT -3′. PCR cycling conditions were an initial denaturation step at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 45 s, elongation at 72°C for 45 s, and a final extension step at 72°C for 10 min.
The PCR products were separated on 1.5% agarose gels and visualized using ethidium bromide staining and UV light with UV trasilluminator 2000, (Biorad Hercules, CA, USA). Images were acquired using Gel Logic 100 imaging system (Kodak®, Rochester, NY, USA), and optical density was calculated and expressed relative to GAPDH.
Statistical analyses
Data were presented as mean ± SD and analysed using Pearson’s parametric test for univariate analysis. Statistical analyses were performed using SPSS® version 10.0 (SPSS Inc., Chicago, IL, USA) for Windows®; P-values < 0.05 were considered statistically significant.
Results
The study included skin biopsies from nine patients with psoriatic arthritis (5 male/4 female; mean age 48.7 ± 12.8 years; age range 20–62 years; mean disease duration 6.5 ± 3.8 years; mean disease activity score [DAS]44 4.6 ± 1.2; mean psoriasis area severity index score7 7.6 ± 1.5) and nine healthy control subjects (6 male/3 female; mean age 41.3 ± 11.6 years; age range 30–55 years).
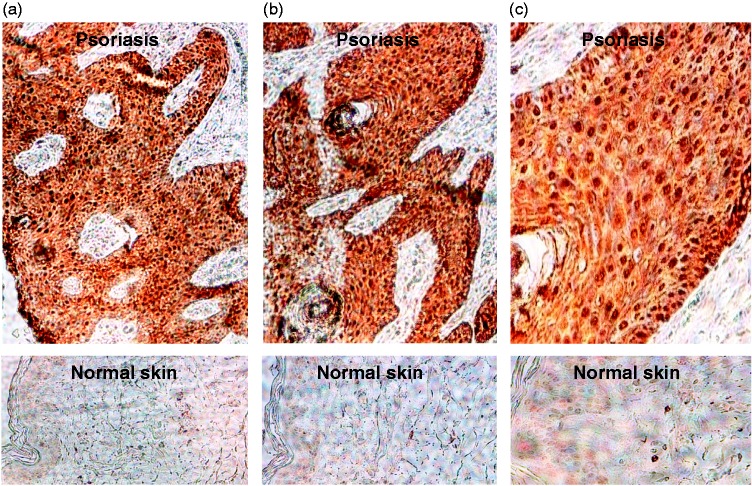
Levels of S100A8 staining were visibly higher in psoriasis plaques compared with normal skin samples (Figure 1), with staining being concentrated in the nucleus. Similar results were obtained for S100A9 (data not shown). Levels of S100A8 and S100A9 RNA were significantly higher in patients than in controls (S100A8, 10.6 ± 7.5 vs 6.9 ± 5.1, P < 0.01; S100A9, 2.7 ± 1.7 vs 1.0 ± 0.5, P < 0.01).
Figure 1.
Representative light photomicrographs of immunohistochemical staining for S100A8 in (top panels) psoriatic skin lesions from patients with psoriatic arthritis and (lower panels) normal skin biopsies from healthy control subjects. Original magnification (a, b) × 20, (c) × 40. The colour version of this figure is available at: http://imr.sagepub.com
Levels of S100A8 and S100A9 RNA were significantly higher in the peripheral region of plaques compared with the central region (peripheral S100A8 10.69.8 vs central S100A8 2.9 ± 1.9, P < 0.01; peripheral S100A9 10.5 ± 9.1 vs central S100A9 5.1 ± 4.1, P < 0.01).
Discussion
Clinical and experimental observations have indicated that monocyte–macrophage mediated inflammation is a key factor in stimulating keratinocyte and synoviocyte hyperproliferation.4 Inflammatory cytokines such as tumour necrosis factor-α are overexpressed in psoriatic synovium and plaques.8 The strong nuclear staining for S100A8/A9 seen in the present study is consistent with the idea that these proteins are involved in psoriasis plaque initiation and amplification through their direct binding and transcriptional activation of genes of the complement system.9 Our finding that levels of S100A8/A9 are higher at the periphery than the centre of psoriatic lesions confirms the active role of these proteins as inflammation amplifiers. It is likely that S100A8/A9 act by orchestrating the recruitment of inflammatory cells and the expansion of the psoriatic plaque via secreted cytokines. S100A8 and S100A9 may represent good therapeutic targets in psoriasis and psoriatic arthritis.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Editorial assistance was provided by Gayle Robins on behalf of HPS–Health Publishing and Services Srl and funded by Pfizer Italia.
References
- 1.Veale D, Yanni G, Rogers S, et al. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum 1993; 36: 893–900. [DOI] [PubMed] [Google Scholar]
- 2.Chimenti MS, Ballanti E, Perricone C, et al. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev 2013; 12: 599–606. [DOI] [PubMed] [Google Scholar]
- 3.Vandal K, Rouleau P, Boivin A, et al. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol 2003; 171: 2602–2609. [DOI] [PubMed] [Google Scholar]
- 4.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 2003; 24: 155–158. [DOI] [PubMed] [Google Scholar]
- 5.Batliwalla FM, Li W, Ritchlin CT, et al. Microarray analyses of peripheral blood cells identifies unique gene expression signature in psoriatic arthritis. Mol Med 2005; 11: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 2005; 437: 369–375. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244. [DOI] [PubMed] [Google Scholar]
- 8.Brunner PM, Glitzner E, Reininger B, et al. CCL7 contributes to the TNF-alpha-dependent inflammation of lesional psoriatic skin. Exp Dermatol 2015; 24: 522–528. [DOI] [PubMed] [Google Scholar]
- 9.Schonthaler HB, Guinea-Viniegra J, Wculek SK, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013; 39: 1171–1181. [DOI] [PubMed] [Google Scholar]