Abstract
Objectives
An observational study to evaluate the relationship between serum concentrations of adalimumab and disease activity in patients receiving long-term adalimumab treatment for psoriatic arthritis.
Methods
Serum adalimumab and adalimumab antidrug antibodies were quantified by enzyme linked immunosorbent assay. Disease activity was assessed using Disease Activity Score (44 joint measures). Serum C-reactive protein was quantified using standard methods.
Results
A total of 30 patients were recruited. There were significant inverse correlations between serum adalimumab concentration and serum C-reactive protein (CRP) concentration [r = −0.43], the number of tender joints (r = −0.4), and Disease Activity Score (DAS44)-CRP (r = −0.36). Mean serum adalimumab levels were significantly higher in patients with DAS44-CRP <1.6 than in patients with DAS44-CRP ≥1.6.
Conclusions
Serum adalimumab could be an important tool that may improve the management of psoriatic arthritis in patients responding to long-term treatment.
Keywords: Adalimumab, anti-drug antibodies, disease activity, immunogenicity, psoriatic arthritis, tumour necrosis factor-α
Introduction
Psoriatic arthritis is a chronic inflammatory joint disease closely associated with psoriasis. Elevated tumour necrosis factor (TNF)-α levels have been detected in both the blood and the synovial fluid from patients with psoriatic arthritis,1 and treatment with TNF-α inhibitors represents a significant advance in the management of this condition.
Adalimumab (ADL) is a fully humanized monoclonal antibody directed against TNF-α that is approved for the treatment of psoriatic arthritis. ADL treatment has both a good clinical response and a good safety profile.1,2 It has been reported to improve joint and skin manifestations of disease, reduce disability and inhibit radiographic progression.1,2
Nonresponse to ADL cannot be completely explained, although it is thought that the development of antidrug antibodies may influence serum ADL levels and therefore clinical response.3,4 Since TNF-α inhibitors are high-cost therapies, the detection of nonresponding patients would be useful in adjusting dosage and determining treatment efficacy.4
The aims of this study were to evaluate serum levels of ADL and antidrug antibodies in patients with psoriatic arthritis with long-term response to ADL treatment, and to explore correlations with disease activity.
Patients and methods
Study population
This observational study included sequential patients with psoriatic arthritis attending the Department of Systemic medicine, University of Rome “Tor Vergata”, Rome, Italy between November 2012 and December 2014.
Inclusion criteria were: diagnosis of psoriatic arthritis made by using the Classification Criteria for Psoriatic Arthritis5 by an experienced rheumatologist (M.S.C.); and ≥8 weeks’ ADL treatment, with or without concomitant steroids or/and disease-modifying antirheumatic drugs (DMARDs).
Disease activity was assessed using the Disease Activity Score (44 joint measures)-C-Reactive Protein (DAS44-CRP),6 where a higher score (i.e., ≥1.6) represents more severe disease. Clinical data including duration of disease, duration of treatment, and the use of concomitant DMARDs or steroids were recorded.
Patients included in the study provided oral informed consent. This study was approved by the local ethics committee, and was conducted in accordance with the ethical principles of the Declaration of Helsinki, consistent with the guidelines for good clinical practice.
Laboratory analyses
Peripheral venous blood (10 ml) samples were taken from each patient, using standard methods between 2 and 4 days before the next ADL dose. Blood samples were centrifuged at 1500 g for 15 min at 4°C, and the resulting serum was stored at −20°C until used. Erythrocyte sedimentation rate (ESR; normal range <20 mm/h) was determined with the standard Westergren method. Serum C-reactive protein (CRP; normal range <0.5 mg/dl) was quantified using standard methodology.
Serum ADL and antidrug antibody levels were quantified by enzyme linked immunosorbent assay (ELISA; Promonitor® ADL/Promonitor® ADA, Proteomika SLU, Derio, Spain), according to the manufacturer’s instructions. The adalimumab assay detected free drug, present in serum. Results were plotted on a titration curve and expressed in µg/ml.
Statistical analyses
Data were expressed as mean ± SD or n, and normality of distribution was tested using D’Agostino and Pearson omnibus test. Between-group differences were analysed with Mann–Whitney U-test. Pearson’s correlation coefficient was used to test correlations between two variables. Data were analysed using Prism version 6 (GraphPad™ software, San Diego, CA, USA). P-values < 0.05 were considered statistically significant.
Results
The study included 30 patients with psoriatic arthritis (20 male/10 female; mean age 55.4 ± 10.4 years; age range 30–77 years). Demographic and clinical characteristics of the study population are summarized in Table 1. When patients were stratified according to DAS (<1.6 or ≥1.6), there were no differences between groups in mean age, psoriatic arthritis duration, duration of ADL therapy, ESR or serum CRP (data not shown).
Table 1.
Demographic and clinical characteristics of patients with psoriatic arthritis receiving long-term treatment with ADL, included in a study evaluating the relationship between serum ADL concentration and clinical response (n = 30).
| Parameter | N |
|---|---|
| Age, years | 55.4 ± 10.4 (30–77) |
| Sex, male/female | 20/10 |
| Disease duration, years | 12.4 ± 9.7 (4–44) |
| Treatment duration, months | 56.4 ± 29.6 (2.8–105.4) |
| Concomitant DMARDs | 20 |
| ESR, mm/h | 13.9 ± 10.4 (2–38) |
| CRP, mg/l | 3 ± 6.9 (0–29) |
| DAS6 | 1.4 ± 0.5 (0.6–2.3) |
| Serum ADL, µg/ml | 9.1 ± 4.9 (0–20) |
Data presented as mean ± SD (range) or n.
DMARDs, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS, disease activity score.
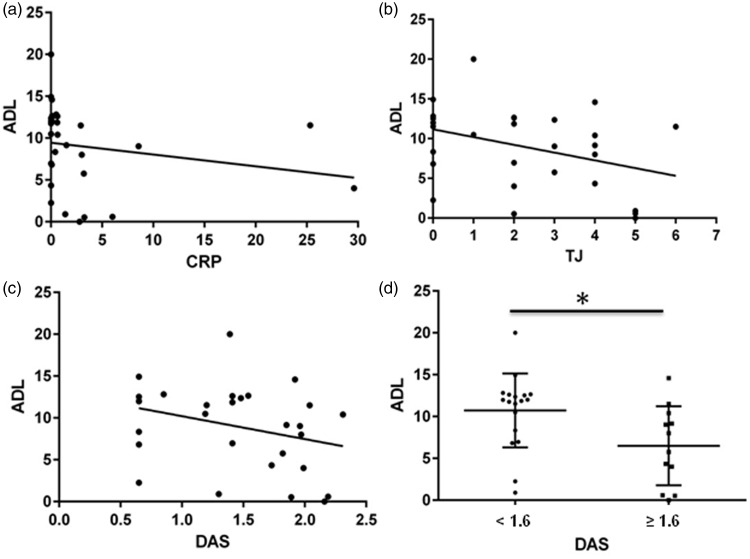
In the total study population, there were significant inverse correlations between serum ADL concentration and CRP (r = −0.43, P < 0.05, Figure 1a), the number of tender joints (r = −0.4, P < 0.05, Figure 1b), and the DAS44-CRP score (r = −0.36, P < 0.05, Figure 1c). There were no significant correlations between serum ADL concentration and sex, age, psoriatic arthritis duration, duration of ADL therapy, concomitant administration of DMARDs and ESR. The mean serum ADL concentration was significantly higher in patients with DAS44-CRP < 1.6 (n = 18, mean 10.7 µg/ml ± 4.4) than in those with DAS44-CRP ≥ 1.6 (n = 12, mean 6.5 µg/ml ± 4.7; P = 0.018) (Figure 1d). None of the enrolled patients tested positive for antidrug antibodies.
Figure 1.
Pearson’s correlation coefficient analysis of the association between serum adalimumab concentration (ADL, µg/ml) and (a) C-reactive protein (CRP, mg/l; Pearson’s r = −0.43, P < 0.05); (b) number of tender joints (TJ; Pearson’s r = −0.4, P < 0.05); and (c) disease activity score (DAS6; Pearson’s r = −0.36, P < 0.05), in patients with psoriatic arthritis receiving long-term adalimumab treatment(n = 30). (d) Serum adalimumab concentration in patients with psoriatic arthritis stratified according to DAS (<1.6 or ≥1.6; horizontal lines indicate mean values; *P < 0.05).
Discussion
Serum ADL concentrations were inversely related to serum CRP, DAS44 score and the number of tender joints in patients with psoriatic arthritis in the present study. In addition, when patients were stratified according to disease activity, those with lower scores (i.e. <1.6) showed significantly higher serum ADL concentrations than those with higher disease activity scores. This finding suggests the possibility of a nonresponder condition or the presence of anti-adalimumab antibodies in patients with more severe disease, suggesting that ADL immunogenicity may be associated with patients’ predisposition or disease-related factors.
We did not detect antidrug antibodies in any patient in the present study. This may be due to the fact that the ELISA kit used cannot detect adalimumab –antidrug antibody immunocomplexes, which may affect serum concentrations of both drug and antibody.3,4 We did not perform analyses related to route of drug administration or differences in dosing regimen, further investigation of which may improve our understanding of ADL immunogenicity. Serum ADL concentrations varied widely among our patient group, and neither disease duration nor ADL treatment duration influenced the serum ADL concentration.
In conclusion, although the detection of antidrug antibodies may be useful in the identification of nonresponder patients, serum ADL can be considered an important tool improving the management of psoriatic arthritis in patients responding to long-term treatment.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Editorial assistance was provided by Gayle Robins on behalf of HPS–Health Publishing and Services Srl and funded by Pfizer Italia.
References
- 1.Mease PJ. Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors. Ann Rheum Dis 2002; 61: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito M, Giunta A, Mazzotta A, et al. Efficacy and safety of subcutaneous anti-tumor necrosis factor-alpha agents, etanercept and adalimumab, in elderly patients affected by psoriasis and psoriatic arthritis: an observational long-term study. Dermatology 2012; 225: 312–319. [DOI] [PubMed] [Google Scholar]
- 3.Zisapel M, Zisman D, Madar-Balakirski N, et al. Prevalence of TNF-α blocker immunogenicity in psoriatic arthritis. J Rheumatol 2015; 42: 73–78. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang EH, Kneepkens EL, Nurmohamed MT, et al. Anti-adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow-up. Ann Rheum Dis 2014; 73: 2178–2182. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WJ, Helliwell PS. Development of diagnostic criteria for psoriatic arthritis: methods and process. Curr Rheumatol Rep 2004; 6: 299–305. [DOI] [PubMed] [Google Scholar]
- 6.Helliwell PS, Fitzgerald O, Mease PJ. Development of composite measures for psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J Rheumatol 2012; 39: 398–403. [DOI] [PubMed] [Google Scholar]