Abstract
Coronary heart disease is the main cause of mortality in patients with rheumatoid arthritis (RA), a disease known to be associated with accelerated atherosclerosis. The role of inflammation and immunity in atherosclerotic process offers possible explanations for the increased cardiovascular risk in patients with RA. The immune response to citrullinated peptides has been extensively studied in RA; antibodies directed to citrullinated peptides are now a cornerstone for RA diagnosis. However, few studies have investigated the response to citrullinated peptides and the development of atherosclerotic plaque. Antibodies to carbamylated proteins can be detected before the clinical onset of RA, suggesting a potential predictive role for these antibodies; on the other hand, carbamylation of lipoproteins has been described in patients with cardiovascular disease. This review examines the role of citrullination and carbamylation, two post-translational protein modifications that appear to be involved in the pathogenesis of both RA and atherosclerosis, expanding the similarities between these two diseases. Further investigation on the role of the immune response to modified proteins may contribute to a better comprehension of cardiovascular disease in patients with RA.
Keywords: Atherosclerosis, carbamylation, citrullination, post-translational modifications, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease that has been shown to be associated with accelerated atherosclerosis.1 Coronary heart disease (CHD) represents a major cause of mortality in patients with RA.1 The role of inflammation and immunity in the atherosclerotic process offers possible explanations for the increased cardiovascular risk observed in RA patients.
Post-translational modifications encompass a group of reactions that modify the structure and extend the functions of proteins. Two of these modifications, citrullination and carbamylation, seem to be involved in the pathogenesis of both RA and atherosclerosis, extending the links between the two diseases.
Citrullination and RA
Citrullination is an enzymatic post-translational modification that is mediated by peptidylarginine deiminases (PAD), which transform peptide-bound arginine residues into citrulline, a non-natural amino acid.2 Over the past decade, the immune response to citrullinated peptides has been extensively studied in RA, and anticitrullinated protein antibodies (ACPA) are now a cornerstone for the diagnosis of RA, with a specificity of 85–95% and a sensitivity of 68–79%.3 These antibodies have a predictive role (as they can be detected before RA onset), and a prognostic role, being associated with a particularly severe and erosive arthritis.3
Anticitrullinated protein antibodies are implicated in the pathogenesis of RA. Citrullinated peptides bind human leukocyte antigen DRB1, the so-called shared epitope, and a strong correlation between ACPA positivity and shared epitope expression was demonstrated.4 Moreover, smoking may induce an immune response to citrullinated peptides, with generation of ACPA and the onset of RA in shared-epitope carriers.5 Published data support a link between RA and chronic periodontitis; the oral pathogen Porphyromonas gingivalis has the unique ability to produce a PAD enzyme that citrullinates proteins and may induce the development of RA in genetically predisposed individuals.6 Interestingly, both cigarette smoking and P. gingivalis are also risk factors for cardiovascular disease (Figure 1). The effect of therapy on ACPA status remains controversial.4
Figure 1.
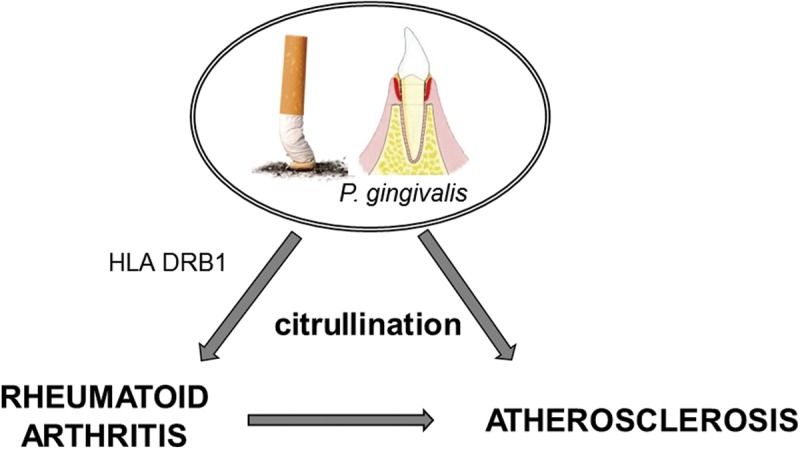
Schematic representation of the shared pathogenic pathway linking rheumatoid arthritis and atherosclerosis.
Citrullination and atherosclerosis
Few studies have investigated the response to citrullinated peptides and the development of atherosclerotic plaque. Sokolove et al.7 demonstrated that both citrullinated fibrinogen and vimentin were correlated with the coronary artery calcium score in 134 female patients with RA. Moreover, citrullinated proteins and PAD-4 enzyme were detected within atherosclerotic plaques obtained from non-RA patients, and ACPA isolated from patients with RA were able to target these proteins.7 Citrullinated proteins and PAD enzymes have also been detected in the perivascular myocardial interstitium, especially in RA patients.8 Cambridge et al.9 investigated the possible association between ACPA and CHD in 432 healthy subjects who were followed up for 5 years. In this study,9 a significantly higher percentage of participants who developed CHD were ACPA-positive compared with those who did not develop CHD; the association remained significant even after adjustment for traditional atherosclerotic risk factors.9
Carbamylation and RA
Carbamylation is a chemical post-translational modification consisting of the addition of a cyanate group on self peptides, leading to the production of homocitrulline. Among other factors, tobacco smoke seems to induce protein carbamylation.10 The immunogenicity of homocitrulline has been studied in RA patients. Shi et al.11 detected antibodies to carbamylated proteins in both ACPA-positive and -negative patients; in the latter group, carbamylated protein antibody positivity was strongly associated with more erosive forms of RA compared with antibody negativity.11 Moreover, cross-reactivity between antibodies to citrullinated and homocitrullinated proteins seems to be low.11 Similar to ACPA and rheumatoid factor, antibodies to carbamylated proteins can be detected before the clinical onset of RA, suggesting a potential predictive role for these antibodies.12 The exact pathogenic role of carbamylated proteins and the effect of RA treatment on antibodies to these proteins remain unaddressed.
Carbamylation and atherosclerosis
Carbamylation of various lipoproteins has been described in patients with cardiovascular disease. Carbamylated high-density lipoprotein may promote atherogenesis by impairing the balance between macrophage-mediated cholesterol uptake and efflux.13 Carbamylation of low-density lipoprotein (LDL) might induce endothelial dysfunction, acting via the lectin-type oxidized LDL receptor 1,14 a scavenger receptor for oxidized LDL that has been proposed as a biomarker of RA.15 Carbamylated LDL may uncouple endothelial nitric oxide synthase, reducing nitric oxide bioavailability and impairing endothelium vasodilatation.14 Moreover, carbamylated LDL seems to promote monocyte adhesion to endothelial cells, damage endothelial cells and progenitor endothelial cells, and induce vascular smooth muscle-cell proliferation.16 Carbamylation of other proteins that are not yet clearly elucidated, may also contribute to the pathogenesis of atherosclerosis.
Conclusions
Citrullination and carbamylation are two post-translational modifications that seem to link RA and atherosclerosis, expanding the similarities between these two inflammatory, immune-mediated chronic diseases. Further investigation into the role of the immune response against citrulline and homocitrulline may contribute to a better cardiovascular outcome in patients with RA.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Editorial assistance was provided by Gayle Robins on behalf of HPS–Health Publishing and Services Srl and funded by Pfizer Italia.
References
- 1.Nurmohamed MT, Heslinga M and Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 2015; doi: 10.1038/nrrheum.2015.112. [DOI] [PubMed]
- 2.György B, Tóth E, Tarcsa E, et al. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006; 38: 1662–1677. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal R, Liao K, Nair R, et al. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum 2009; 61: 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi S, Soejima M, Levesque MC. The effect of targeted rheumatoid arthritis therapies on anti-citrullinated protein autoantibody levels and B cell responses. Clin Exp Immunol 2013; 173: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006; 54: 38–46. [DOI] [PubMed] [Google Scholar]
- 6.Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 2014; 16: 408–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokolove J, Brennan MJ, Sharpe O, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum 2013; 65: 1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles JT, Fert-Bober J, Park JK, et al. Myocardial citrullination in rheumatoid arthritis: a correlative histopathologic study. Arthritis Res Ther 2012; 14: R39–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambridge G, Acharya J, Cooper JA, et al. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis 2013; 228: 243–246. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Veres PR, Cochran AK, et al. Isocyanic acid in the atmosphere and its possible link to smoke-related health effects. Proc Natl Acad Sci USA 2011; 108: 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA 2011; 108: 17372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, van de Stadt LA, Levarht EW, et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013; 65: 911–915. [DOI] [PubMed] [Google Scholar]
- 13.Holzer M, Gauster M, Pfeifer T, et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal 2011; 14: 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speer T, Owala FO, Holy EW, et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur Heart J 2014; 35: 3021–3032. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa M, Ito H, Akiyoshi M, et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis Rheum 2012; 64: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 16.Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem 2011; 57: 1499–1505. [DOI] [PubMed] [Google Scholar]


