Abstract
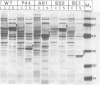
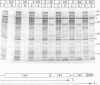
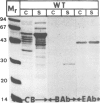
The possible involvement of topogenic export sequences within the colicin A polypeptide chain has been investigated. Different constructs have been made using various techniques to introduce deletions in the central and NH2-terminal regions of colicin A. Together, these deletions span the region from amino acid 15 to the end of the protein. None of these regions was found to be required for extracellular release or had any effect on the efficiency of this process. By inserting a termination codon, a Shine-Dalgarno sequence and an initiation codon into the gene for colicin A, the NH2-terminal and central plus COOH-terminal domains could be demonstrated to be released to the same extent when produced as separate polypeptides as when produced as linked ones. The introduction into the COOH-terminal domain of mutations promoting cytoplasmic aggregation had no effect on the secretion of the NH2-terminal polypeptide. These results demonstrated that no specific interaction between the NH2- and COOH-terminal regions of the colicin A polypeptide chain is involved in the release of colicin A. We are led to conclude that there is no topogenic export signal in the polypeptide chain of colicin A involved in the release mechanism. Thus the process is non-specific with respect to the colicin itself and depends solely on the expression of the colicin A lysis protein (Cavard et al., 1985, 1987). The expression of the protein causes the release of not only the colicin but also many other cellular proteins, including beta-lactamase, EF-Tu, and chloramphenicol acetyltransferase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Knibiehler M., Verheij H., Pattus F., Shire D., Bernadac A., Lazdunski C. Site-directed mutagenesis of the COOH-terminal region of colicin A: effect on secretion and voltage-dependent channel activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1152–1156. doi: 10.1073/pnas.84.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Baty D., Howard S. P., Verheij H. M., Lazdunski C. Lipoprotein nature of the colicin A lysis protein: effect of amino acid substitutions at the site of modification and processing. J Bacteriol. 1987 May;169(5):2187–2194. doi: 10.1128/jb.169.5.2187-2194.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Crozel V., Gorvel J. P., Pattus F., Baty D., Lazdunski C. A molecular, genetic and immunological approach to the functioning of colicin A, a pore-forming protein. J Mol Biol. 1986 Feb 5;187(3):449–459. doi: 10.1016/0022-2836(86)90445-6. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C. Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export. Mol Gen Genet. 1985;199(1):95–100. doi: 10.1007/BF00327516. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. II. Functions and regulation of synthesis of the gene H product. Mol Gen Genet. 1981;183(2):326–332. doi: 10.1007/BF00270636. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., van der Sande C., Tommassen J., Veltkamp E., De Graaf F. K., Oudega B. Effects of divalent cations and of phospholipase A activity on excretion of cloacin DF13 and lysis of host cells. J Gen Microbiol. 1986 Mar;132(3):825–834. doi: 10.1099/00221287-132-3-825. [DOI] [PubMed] [Google Scholar]
- Mock M., Schwartz M. Mechanism of colicin E3 production in strains harboring wild-type or mutant plasmids. J Bacteriol. 1978 Nov;136(2):700–707. doi: 10.1128/jb.136.2.700-707.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol. 1983 Oct 25;170(2):271–285. doi: 10.1016/s0022-2836(83)80148-x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Cole S. T. beta-Galactosidase and alkaline phosphatase do not become extracellular when fused to the amino-terminal part of colicin N. J Gen Microbiol. 1986 Aug;132(8):2297–2307. doi: 10.1099/00221287-132-8-2297. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Lau P. C., Vernet T., Visentin L. P. Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Gene. 1984 Jul-Aug;29(1-2):175–184. doi: 10.1016/0378-1119(84)90178-1. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Walters H. H., Veltkamp E., Nijkamp H. J. Molecular structure and function of the bacteriocin gene and bacteriocin protein of plasmid Clo DF13. Nucleic Acids Res. 1983 Apr 25;11(8):2465–2477. doi: 10.1093/nar/11.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]