Abstract
Objective
To evaluate prospectively serological markers at baseline and during treatment in patients with rheumatoid arthritis (RA) initiating rituximab treatment, following failure of antitumour necrosis factor (TNF)-α therapy.
Methods
Patients with RA and healthy control subjects were recruited. Plasma complement (C)3, C4, rheumatoid factor (RF), anticitrullinated protein antibody (ACPA), immunoglobulin (Ig)M, A and G, disease activity scores (DAS) and therapeutic response were recorded at baseline and at 6, 12 and 18 months.
Results
Patients (n = 35) had significantly higher C3 and C4 levels than controls (n = 30). At 12 months after initiation of rituximab, C3 and C4 levels were significantly lower in patients who responded to treatment, compared with nonresponders. There were direct correlations between C3 levels and DAS at 12 months in the study population as a whole, and between IgM levels and DAS in responding patients after 6, 12 and 18 months’ treatment.
Conclusions
C3 and IgM levels may represent potentially useful serological markers of disease activity during rituximab treatment in patients with RA.
Keywords: CD20, complement, immunoglobulins, rheumatoid arthritis, rituximab, serological markers
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is characterized by the activation of innate and adaptive immunity.1,2 The complement system plays a key role in the pathological processes of RA, and evidence indicates that B cells may act at multiple levels of the inflammatory cascade.3
Biological drugs targeting inflammatory mediators and cells have changed the prognosis of RA.4 It is therefore important to identify biomarkers that are potentially associated with disease activity, in order to monitor effectively the disease progression and treatment efficacy.
Rituximab is a genetically engineered chimeric monoclonal antibody targeted to CD20+ B cells that is approved for the treatment of RA patients not responding to disease-modifying antirheumatic drugs (DMARDs), including anti-tumour necrosis factor (TNF)-α drugs.5 The aim of this study was to evaluate serological markers in patients with RA, before and during rituximab treatment.
Patients and methods
Study population
The study prospectively enrolled consecutive patients with RA who were unresponsive to anti-TNF drug therapy, attending the Rheumatology Unit, University of Rome “Tor Vergata”, Rome, Italy, between January 2008 and December 2014. All patients began treatment with 1000 mg rituximab i.v. infusion on days 0 and 15, followed by the same two doses of rituximab every 6 months. Each treatment was preceded by 100 mg methylprednisolone i.v. Inclusion criteria were: aged >18 years; RA diagnosed according to American College of Rheumatology (ACR) criteria6; failed treatment with DMARDs and one anti-TNFα drug; active disease based on 28-joint disease activity score (DAS28; four variables, erythrocyte sedimentation rate-based7); weekly 15 mg methotrexate intramuscular injection. Age- and sex-matched healthy control subjects were recruited from the staff of the University of Rome “Tor Vergata”. Exclusion criteria for all participants were recent infection, HIV infection, history of cancer, major organ dysfunction or pregnancy.
The study was performed in accordance with the Declaration of Helsinki. All patients and controls provided written informed consent and the ethics committee of the University of Rome “Tor Vergata” approved the study.
Data collection
Venous blood samples were obtained from each participant’s antecubital vein using standard techniques. Data included plasma concentrations of C3 and C4, and serum concentrations of immunoglobulin (Ig)M, A and G, rheumatoid factor (RF) and anticitrullinated protein antibody (ACPA). C4 was quantified by radial immunodiffusion (Meloy Laboratories, Springfield, VA, USA) and C3 by nephelometry according to standard methods.8 RF, IgM and Ig were quantified by nephelometry using Immage 800® (Beckman Coulter, Fullerton, CA, USA) according to the manufacturer’s guidelines. ACPA was quantified by second generation commercial enzyme-linked immunosorbent assay (ELISA) kit (QUANTA Lite® CCP IgG, Medical Technology Promedt Consulting, St. Ingbert, Germany). DAS28, therapy response (European League Against Rheumatism [EULAR] response criteria9) and concomitant prednisone and/or conventional synthetic (cs) DMARD use were recorded. Laboratory assessments and clinical data were recorded at baseline (T0) and at 6, 12 and 18 months.
Statistical analyses
Data were presented as mean ± SD or n (%). Between-group comparisons were made using Mann–Whitney U-test, and Pearson’s correlation coefficient was used to evaluate correlations between two variables. P values < 0.05 were considered statistically significant. Statistical analyses were performed using Prism® version 6 (GraphPad Software, San Diego, CA, USA).
Results
The study included 35 patients with RA (six males/29 female; mean age 58.3 ± 13.5 years; age range 21–78 years) and 30 healthy control subjects (five male/25 female; mean age 50.0 ± 10.3 years; age range 26–75 years). Baseline demographic and clinical data for study participants are shown in Table 1. The majority of patients received prednisone and/or csDMARDs. Plasma C3 and C4 concentrations were significantly higher in patients than controls (P = 0.01 and P = 0.03, respectively).
Table 1.
Baseline demographic and clinical data for patients with rheumatoid arthritis included in a study investigating serological markers associated with disease activity during treatment with rituximab, and healthy control subjects.
| Characteristic | Rheumatoid arthritis group n = 35 | Control group n = 30 |
|---|---|---|
| Sex, male/female | 6/29 | 5/25 |
| Age, years | 58.3 ± 13.5 | 50.0 ± 10.3 |
| Disease duration, years | 13.6 ± 6.8 | NA |
| DAS28 | 5.4 ± 1.6 | NA |
| RF positive | 21 (60) | NA |
| ACPA positive | 20 (57) | NA |
| C3, mg/dl | 128.7 ± 21.3 a | 110.0 ± 25.0 |
| C4, mg/dl | 29.7 ± 10.2 b | 22.7 ± 8.3 |
| Concomitant treatment | ||
| csDMARDs | 26 (74.3) | NA |
| Prednisone | 28 (80.0) | NA |
Data presented as mean ± SD or n (%).
NA, not applicable; DAS28, 28-joint disease activity score; RF, rheumatoid factor; ACPA, anticitrullinated protein antibody; C3, complement 3; C4, complement 4; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs.
P = 0.01 and bP = 0.03 versus control group; Mann–Whitney U-test.
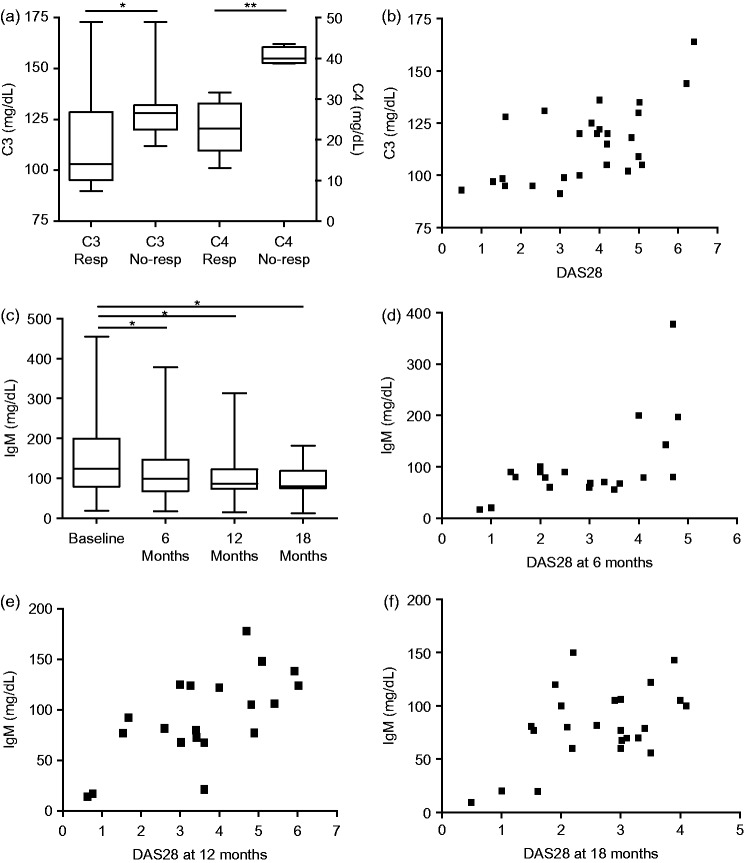
At each time point after initiation of rituximab, patients were classified as treatment responders (6 months n = 21, 12 months n = 20, 18 months n = 25) and nonresponders (6 months n = 14, 12 months n = 15, 18 months n = 10). Plasma concentrations of C3 and C4 were significantly lower in responders than in nonresponders at 12 months (P = 0.03 and P = 0.001, respectively; Figure 1A). There was a direct correlation between C3 and DAS28 at 12 months after treatment initiation in the study population as a whole (P = 0.03, r = 0.5; Figure 1B). There was no corresponding correlation between C4 concentration and DAS28. In the study population as a whole, serum IgM concentrations were significantly lower than baseline after 6, 12 and 18 months’ treatment (P = 0.04, P = 0.03, and P = 0.02, respectively; Figure 1C). Furthermore, a direct correlation was observed between IgM levels and DAS28 in responding patients, after 6, 12 and 18 months’ treatment (6 months P = 0.01, r = 0.5; 12 months P = 0.03, r = 0.7; 18 months P = 0.01, r = 0.5; Figure 1D–F). There were no treatment-associated changes in serum IgG, IgA, RF and ACPA concentrations (data not shown).
Figure 1.
(A) Plasma complement (C)3 and C4 levels in patients with rheumatoid arthritis (RA) classified as treatment responders (Resp) and nonresponders (No-resp) after 12 months’ rituximab therapy. (B) Correlation between plasma C3 level and disease activity score on 28 joints (DAS28) in patients with RA after 12 months’ rituximab treatment. (C) Immunoglobulin (Ig) M levels in patients with RA at baseline and during rituximab treatment. (D) Correlation between IgM levels and DAS28 in responding RA patients after 6, (E) 12 and (F) 18 months’ rituximab treatment. *P < 0.05; Mann–Whitney U-test.
Discussion
Anti-TNF-α drugs have been shown to affect components of both the innate and adaptive immune system.10,11 Modifications of the complement system seem to be associated with disease activity in patients with RA treated with anti-TNF-α therapies.8 Rituximab has good efficacy and safety profiles in RA.5 The present study found that C3 and C4 levels were reduced in patients with a good response to rituximab, compared with nonresponders. Others have shown that IgM levels in patients with RA are reduced by rituximab treatment.12 Our finding suggests that IgM levels correlated with disease activity in treatment responders and was in accordance with rituximab acting via modulation of B lymphocytes.12
In conclusion, C3 and IgM levels may represent potentially useful serological markers of disease activity during rituximab treatment in patients with RA.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Editorial assistance was provided by Gayle Robins on behalf of HPS–Health Publishing and Services Srl and funded by Pfizer Italia.
References
- 1.Nickdel MB, Conigliaro P, Valesini G, et al. Dissecting the contribution of innate and antigen-specific pathways to the breach of self-tolerance in a murine model of arthritis. Ann Rheum Dis 2009; 68: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 2.Conigliaro P, Perricone C, Benson RA, et al. The type I IFN system in rheumatoid arthritis. Autoimmunity 2010; 43: 220–225. [DOI] [PubMed] [Google Scholar]
- 3.Maseda D, Bonami RH, Crofford LJ. Regulation of B lymphocytes and plasma cells by innate immune mechanisms and stromal cells in rheumatoid arthritis. Expert Rev Clin Immunol 2014; 10: 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam JL, Ramiro S, Gaujoux-Viala C, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2014; 73: 516–528. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006; 54: 2793–2806. [DOI] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 7.Prevoo ML, van't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 8.Di Muzio G, Perricone C, Ballanti E, et al. Complement system and rheumatoid arthritis: relationships with autoantibodies, serological, clinical features, and anti-TNF treatment. Int J Immunopathol Pharmacol 2011; 24: 357–366. [DOI] [PubMed] [Google Scholar]
- 9.Fransen J, van Riel PL. The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 2005; 23: S93–S99. [PubMed] [Google Scholar]
- 10.Scrivo R, Conigliaro P, Riccieri V, et al. Distribution of interleukin-10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin Exp Immunol 2015; 179: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conigliaro P, Triggianese P, Perricone C, et al. Restoration of peripheral blood natural killer and B cell levels in patients affected by rheumatoid and psoriatic arthritis during etanercept treatment. Clin Exp Immunol 2014; 177: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De La Torre I, Leandro MJ, Valor L, et al. Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: relationship with B-cell Kinetics. Rheumatology 2012; 51: 833–840. [DOI] [PubMed] [Google Scholar]