Abstract
Objective
To describe the characteristics of hepatitis E virus (HEV) infection in a cohort of children from Upper Egypt using data from a large multicentre prospective study of acute viral hepatitis (AVH).
Methods
Data from subjects aged 2–18 years with AVH or close contacts of those with AVH found to have asymptomatic AVH were included in the analysis. Information concerning medical history, clinical examination, liver function tests and screening for hepatotropic viruses was recorded and analysed.
Results
A total of 123 patients (73 boys, 50 girls) were included in the analysis. Of these, 33 (26.8%) had HEV infection, 17 (13.8%) had hepatitis A virus infection, 10 (8.1%) had hepatitis B virus infection, 14 (11.4%) had cytomegalovirus hepatitis, five (4.1%) had autoimmune hepatitis, 11 (8.9%) had hepatitis due to mixed viral infections and 33 (26.8%) had non A–E hepatitis. Overall, 38 (30.9%) had infection with HEV. HEV infection was significantly higher among those using underground wells as a water source compared with tap water. Liver enzymes were significantly raised in patients with non-HEV infection compared with those with HEV infection.
Conclusions
HEV is a significant cause of AVH among children in Upper Egypt. Contamination of drinking water appears to be a major source of infection. Screening for HEV should be considered in all Egyptian children with AVH.
Keywords: Children, acute viral hepatitis, hepatitis E, Upper Egypt, waterborne outbreak
Introduction
Acute viral hepatitis (AVH) is a common health problem throughout tropical countries in low socioeconomic groups.1 Hepatitis A virus (HAV) and hepatitis E virus (HEV) are common causes of AVH and both are mainly transmitted through the faeco-oral route.1 Egypt is known to have a high seroprevalence for HEV antibodies (anti-HEV); anti-HEV prevalence has been reported to be 84% in pregnant women and up to 60% in children aged 5–10 years.2 Among Egyptians, the incidence of asymptomatic HEV seroconversion has been reported to be 42 per 1000 person-years, while the incidence of symptomatic AVH due to HEV infection was only 3 per 100 000 person-years.2,3 However, HEV infection was reported to account for 12–42% of all cases of AVH in Egypt.4
Infection with HEV is endemic in many developing countries and in recent years autochthonous and sporadic HEV infections have also been described in some developed countries.5 HEV usually causes an acute self-limiting hepatitis, but fulminant hepatic failure resulting in morbidity and mortality may occur.6 The purpose of the present study was to describe the epidemiological, clinical and laboratory data of hepatitis E virus (HEV) infection in a cohort of children from Assiut, Upper Egypt, who were part of a large multicentre prospective study of AVH.7–9
Patients and methods
Data from a large multicentre prospective study of AVH7–9 conducted at Assiut University Children’s Hospital and Assiut Fever Hospital, Assiut, Egypt, from January 2007 to July 2008 were analysed. Subjects included in the present analysis were aged 2 to 18 years and had either been diagnosed with AVH based on symptoms of less than 1 month’s duration that were compatible with acute hepatitis after exclusion of other potential non-viral causes of hepatocellular injury10,11 or were close contacts of those with AVH who were found to have asymptomatic AVH. Patients with chronic liver disease and/or decompensated liver cirrhosis were excluded from the original study.
Data recorded for all study participants included a complete medical history, including possible routes and risks of hepatitis exposure in the previous 6 months, clinical examination, liver function tests and screening for hepatotropic viruses. Serological screening for HAV, hepatitis B virus (HBV) and hepatitis C virus (HCV) was performed using rapid tests for anti-HAV immunoglobulin M (IgM) (CTK Biotech, San Diego, CA, USA), anti-HBV core IgM (IND Diagnostic, Delta, Canada) and anti-HCV immunoglobulin G (IgG) (Clinpro International, Union City, CA, USA), respectively. HEV screening was performed using anti-HEV IgM and IgG kits (Adaltis, Guidonia Montecelio, Italy, and MP Diagnostics, Singapore) according to the manufacturers’ instructions. A diagnosis of autoimmune hepatitis (AIH) was based on a clinical history and symptoms suggestive of an underlying autoimmune aetiology, together with a serological assay for antinuclear and anti-smooth muscle antibodies. Human Anti-nuclear antibodies (ANA) Screen ELISA test kit; Diagnostic Automation/ Cortez Diagnostics INC. Woodland Hills, California, USA. Human Anti-smooth muscle antibody, ASMA ELISA Kit; MyBioSource, Inc. San Diego, USA. Cytomegalovirus (CMV) infection was diagnosed in the presence of CMV IgM Human Anti-cytomegalovirus IgM antibody (anti-CMV-IgM) ELISA Kit; MyBioSource, Inc. San Diego, USA. The presence of Epstein–Barr virus (EBV) was not investigated because none of the patients showed any signs or symptoms of this type of infection. The presence of human hepatitis delta virus (HDV) was not investigated because this cohort of patients presented with symptoms of acute hepatitis in the absence of any known underlying chronic hepatitis infection, whereas HDV can only propagate in the presence of co-infecting HBV.12 Moreover, patients with known chronic liver disease were excluded from the study.
The study was approved by the Ethics Committee of the National Hepatology and Tropical Medicine Research Institution, USA, and the Ethics Committee at the Faculty of Medicine, Assiut University, Egypt. Parents or legal guardians of eligible patients provided written informed consent on enrolment to the study.
Statistical analyses
Quantitative variables were expressed as the mean ± SD and compared between groups using the Student’s t-test. Qualitative variables were compared between groups using the χ2 test or Fisher’s exact test as appropriate. A P-value < 0.05 was considered to be statistically significant. Data were analysed using IBM SPSS software version 20 for Windows® (IBM Corp, Armonk, NY, USA).
Results
From the records for patients or close contacts with AVH involved in a large multicentre study,7–9 data for 123 children (73 boys, 50 girls) aged 2–18 years were analysed. Patient characteristics are given in Table 1. In total, 110 (89.4%) children were symptomatic and 13 (10.6%) were asymptomatic.
Table 1.
Characteristics of children aged 2–18 years (n = 123) with acute viral hepatitis.
| Age, years | 6.4 ± 0.4 |
| Sex | |
| Male | 73 (59.3) |
| Female | 50 (40.7) |
| Residence | |
| Rural | 75 (61.0) |
| Urban | 48 (39.0) |
| Animal contact | |
| Yes | 27 (22.0) |
| No | 96 (78.0) |
| Water source | |
| Tap water | 96 (78.0) |
| Underground well | 27 (22.0) |
| Blood transfusion | |
| Yes | 2 (1.6) |
| No | 121 (98.4) |
| Clinical presentation | |
| Symptomatic | 110 (89.4) |
| Asymptomatic | 13 (10.6) |
| Fulminant disease | |
| Yes | 5 (4.1) |
| No | 118 (95.9) |
| Outcome | |
| Survived | 118 (95.9) |
| Died | 5 (4.1) |
| Type of infection | |
| Isolated infectiona | 79 (64.2) |
| Mixed infectionb | 11 (8.9) |
| Non hepatitis A–E viral infection | 33 (26.8) |
Data presented as mean ± SD or n (%).
Hepatitis due to a single hepatotropic virus.
Hepatitis due to more than one hepatotropic virus.
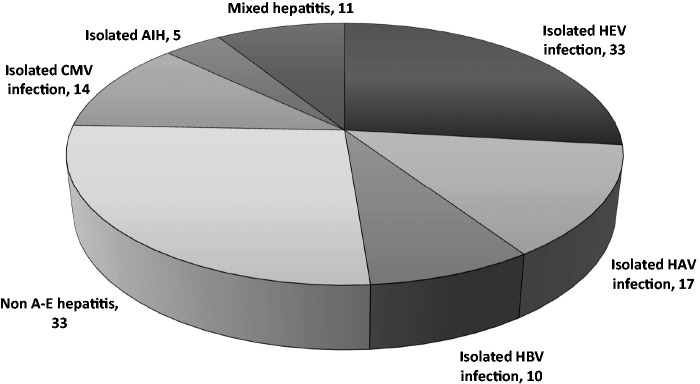
Of the 123 children, 33 (26.8%) had isolated acute HEV infection, 17 (13.8%) had isolated HAV infection, 10 (8.1%) had isolated HBV infection, 14 (11.4%) had isolated CMV hepatitis, five (4.1%) had isolated AIH, 11 (8.9%) had hepatitis due to mixed viral infections and 33 (26.8%) were diagnosed with non A–E hepatitis (Figure 1).
Figure 1.
Prevalence of different types of hepatitis among 123 children with acute viral hepatitis. HEV, hepatitis E virus; HAV, hepatitis A virus; HBV, hepatitis B virus; CMV, cytomegalovirus; AIH, autoimmune hepatitis.
Of the 11 children with mixed viral hepatitis, co-infection with HEV and HAV was found in three patients, HEV and HBV in two patients, HAV and HBV in three patients, HAV and acute HCV in one patient, and CMV with AIH in two patients. Therefore, overall 38 (30.9%) children were infected with HEV.
Of those with HEV infection, statistically significantly more patients used an underground well as a water source compared with a tap water source (P < 0.001) (Table 2). Although not statistically significant, animal contact was greater among children with HEV infection compared with those with non-HEV infection (Table 2).
Table 2.
Characteristics and liver function test results in children aged 2–18 years (n = 123) with hepatitis E virus (HEV) hepatitis and non-HEV hepatitis.
| HEV infection n = 38 | Non-HEV infection n = 85 | Statistical significance | |
|---|---|---|---|
| Age, years | NS | ||
| Mean ± SD | 6.2 ± 0.5 | 6.5 ± 0.5 | |
| Range | 2–17 | 2–18 | |
| Sex | NS | ||
| Male | 19 (50.0) | 54 (63.5) | |
| Female | 19 (50.0) | 31 (36.5) | |
| Residence | NS | ||
| Rural | 22 (57.9) | 53 (62.4) | |
| Urban | 16 (42.1) | 32 (37.6) | |
| Animal contact | NS | ||
| Yes | 12 (31.6) | 15 (17.6) | |
| No | 26 (68.4) | 70 (82.4) | |
| Water source | P < 0.001 | ||
| Tap water | 12 (31.6) | 84 (98.8) | |
| Underground well | 26 (68.4) | 1 (1.2) | |
| Blood transfusion | NS | ||
| Yes | 1 (2.6) | 1 (1.2) | |
| No | 37 (97.4) | 84 (98.8) | |
| Fulminant disease | NS | ||
| Yes | 1 (2.6) | 4 (4.7) | |
| No | 37 (97.4) | 81 (95.3) | |
| Outcome | NS | ||
| Survived | 37 (97.4) | 81 (95.3) | |
| Died | 1 (2.6) | 4 (4.7) | |
| Liver function tests | |||
| Total bilirubin, mg/dl | 6.0 ± 1.2 | 5.6 ± 0.6 | NS |
| Direct bilirubin, mg/dl | 4.7 ± 1.0 | 4.6 ± 0.5 | NS |
| ALT, IU/l | 55 ± 7 | 80 ± 3 | P < 0.001 |
| AST, IU/l | 41 ± 5 | 71 ± 3 | P < 0.001 |
Data presented as mean ± SD or n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
NS, no statistically significant between-group differences (P ≥ 0.05) using Student’s t-test for quantitative variables and χ2 test or Fisher’s exact test for qualitative variables.
No statistically significant differences were found between those with HEV infection and those with non-HEV infection with regard to age, sex, residence, history of blood transfusion, disease severity or outcome. While no differences were detected between the two groups in terms of serum total or direct bilirubin, significantly higher levels of alanine aminotransferase and aspartate aminotransferase were observed in those with non-HEV infection compared with those with HEV infection (P < 0.001) (Table 2).
Discussion
Hepatitis E virus is an enterically transmitted pathogen that usually results in a self-limiting infection.13 It is known to be endemic in many developing countries, and is responsible for both sporadic and epidemic outbreaks in developed countries.5
In the present study, the prevalence rates of the different causes of AVH were comparable with other studies from Egypt, which have reported rates of 0.7–44% for HAV and 12–42% for HEV, with fewer cases being caused by HDV, CMV or EBV.14 In a study from the Nile Delta, of the patients with AVH across all age groups, 8.5% had anti-HAV IgM, 4.3% had anti-hepatitis B surface antigen (HBsAg) antibodies, 78.7% had anti-HCV antibodies, 70.2% had HCV RNA, 2.1% had anti-HEV IgM and 85.1% had anti-HEV IgG; all patients were negative for anti-hepatitis B core IgM and HEV RNA.3
In the present study, 38 (30.9%) out of 123 children with AVH had HEV infection, and of all the hepatotropic viruses it was the most common cause of AVH. Soliman14 reported that 29.2% of patients with AVH in nine villages in the Giza Governorate of Eygpt were seropositive for anti-HEV IgG. In addition, HEV was found to account for 15–40% of adult and paediatric cases of AVH requiring hospitalization to Eygptian fever hospitals.13 In a study conducted in the Nile Delta the seroprevalence rate for HEV in children below the age of 10 years was 57%,15 and in a further study from a fever hospital in Alexandria, 78% of patients with AVH between 15 and 40 years of age were anti-HEV IgM positive.16 In contrast, HEV infection is relatively rare in paediatric age groups in Turkey and Morocco, where rates of 5% have been reported in children under the age of 10 years.17,18 Differences between studies in the prevalence of acute HEV infection may be due to variability in the sociodemographic and socioeconomic characteristics of populations, sanitary conditions, sewage disposal and diagnostic procedural methods.9
The finding in the present study that 8.9% of the children with AVH had mixed hepatitis infection was comparable with the results of a study involving 162 children from Mansoura, Egypt.19 In that study, the HEV co-infection rate among patients with non A–C hepatitis was 7.1%, and anti-HEV IgM antibodies were detected in 4.5% of patients with HAV and in 3.3% of patients with HBV. Similarly, in a study based in a fever hospital in Suez, of the 14.9% of patients who were positive for anti-HEV IgM, only 2.8% showed positivity for anti-HEV IgM alone, with 5.0% also being positive for anti-HAV, 0.7% also being positive for HBsAg, 1.4% also being positive for anti-HCV and 5.0% also being positive for anti-CMV.20
In the present study, there were no differences between children with AVH with HEV infection and those with non-HEV infection in terms of age, sex, type of residence area, animal contact, blood transfusion, disease severity or outcome. These findings are in agreement with other studies that have shown that HEV infection is independent of sex and residence area.18,21–23 However, a higher prevalence for HEV infection in males has been reported in Japan24 and Pakistan,25 while in Italy more women than men were demonstrated to have HEV antibodies.26 A study of the prevalence of HEV antibodies among apparently healthy Egyptians in different age groups found that HEV was endemic in Egypt, especially in rural areas, and there was no significant relationship between HEV and sex but there was a significant relationship with type of residence area, crowded living conditions and poor sanitation, which are important factors in the transmission of the virus.27
In the present study, significantly more individuals with AVH used underground wells as a water source (68.4%) compared with a tap-water source (31.6%). Water from underground wells is not chlorinated and so is thought to be at higher risk of contamination with the virus. In a study from Northern Egypt, contaminated drinking water was found to be the main source of HEV infection.28 Interestingly, in the present study, most of the children who used water from an underground well were from the same village and used the same well. However, the virus could not be isolated from the underground well water or from the sewage of that geographical area. This may have been due to the time lag between the outbreak of the HEV infection and when the samples were taken a few months later.
While in the present study animal contact did not appear to be associated with HEV infection, virological evidence of mammalian HEV has been found in domestic pigs, wild boar, deer, mongoose, cats, dogs, cattle, sheep, goats, horses, macaques, donkeys, rats and mice.29 Similarly, although no association was found in the present study between a history of blood transfusion and HEV infection, blood-borne HEV infection has been reported following blood transfusion.30
As in other studies,4,31 the clinical presentation of acute HEV infection was indistinguishable from AVH due to other viral causes. Disease severity at the time of presentation, evaluated by the presence of fulminant disease, showed no significant difference between patients with HEV infection and those with non-HEV infection (2.6% and 4.7%, respectively). These findings are similar to other reports that have shown that, for most patients, HEV infection is a benign and self-limiting disease, although its severity may be affected by its genotype.31 The low mortality observed in the present study is also consistent with other reports that have estimated overall mortality rates from HEV infection to be between 0.5% and 4%.1 Nevertheless, one study among Egyptian children under the age of 10 years with HEV infection reported a higher mortality rate.32
In the present study, the non-significant differences in serum total and direct bilirubin levels between children with HEV infection and those with non-HEV infection are similar to the findings of other studies.33,34 However, liver enzymes were significantly higher among those with non-HEV infection compared with those with HEV infection.
One limitation of the study was the failure to isolate HEV from the underground well water and sewage from the geographical area that showed a high prevalence of infection with HEV. Another limitation was the lack of polymerase chain reaction testing for CMV or EBV, although CMV IgM was assessed in patients who were negative for the common hepatotropic viruses.
In Upper Egypt, HEV infection is a significant cause of AVH among children and is difficult to distinguish clinically from other acute hepatotropic viral infections. Screening for HEV should be considered among all Egyptian children with AVH. More health education programmes and the development of new vaccines against HEV will also help to protect children from this infection.
Acknowledgements
We would like to express our thanks to our colleagues and nursing staff at Assiut University Children Hospital and Assiut Fever Hospital for their kind help throughout this study. Moreover, we appreciate and acknowledge the team members at the National Hepatology and Tropical Medicine Research Institution, USA for their great help and support for the multicentre project. In addition, we acknowledge the financial support provided by King Saud University, through the Vice Deanship of Research Chairs.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was supported by National Institute of Health grants R21AI067868 (Dr M.T. Shata) and K24DK070528 (Dr K.E. Sherman).
References
- 1.Teshale EH, Grytdal SP, Howard C, et al. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis 2010; 50: 1006–1010. [DOI] [PubMed] [Google Scholar]
- 2.Stoszek SK, Abdel-Hamid M, Saleh DA, et al. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg 2006; 100: 95–101. [DOI] [PubMed] [Google Scholar]
- 3.Meky FA, Stoszek SK, Abdel-Hamid M, et al. Active surveillance for acute viral hepatitis in rural villages in the Nile Delta. Clin Infect Dis 2006; 42: 628–633. [DOI] [PubMed] [Google Scholar]
- 4.Zakaria S, Fouad R, Shaker O, et al. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis 2007; 44: e30–e36. [DOI] [PubMed] [Google Scholar]
- 5.Romanò L, Paladini S, Tagliacarne C, et al. Hepatitis E in Italy: a long-term prospective study. J Hepatol 2011; 54: 34–40. [DOI] [PubMed] [Google Scholar]
- 6.Arends JE, Ghisetti V, Irving W, et al. Hepatitis E: an emerging infection in high income countries. J Clin Virol 2014; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 7.Shata MT, Daef EA, Zaki ME, et al. Protective role of humoral immune responses during an outbreak of hepatitis E in Egypt. Trans R Soc Trop Med Hyg 2012; 106: 613–618. [DOI] [PubMed] [Google Scholar]
- 8.Eldin SS, Seddik I, Daef EA, et al. Risk factors and immune response to hepatitis E viral infection among acute hepatitis patients in Assiut, Ehypt. Egypt J Immunol 2010; 17: 73–86. [PMC free article] [PubMed] [Google Scholar]
- 9.Blackard JT, Rouster SD, Nady S, et al. Genotypic characteristics of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol 2009; 46: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Guidelines for Viral Hepatitis Surveillance and Case Management. Atlanta, GA 2005.
- 11.Ghabrah TW, Strickland GT, Tsarev S, et al. Acute viral hepatitis in Saudi Arabia: seroepidemiological analysis, risk factors, clinical manifestations, and evidence for a sixth hepatitis agent. Clin Infect Dis 1995; 21: 621–627. [DOI] [PubMed] [Google Scholar]
- 12.Makino S, Chang MF, Shieh CK, et al. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature 1987; 329: 343–346. [DOI] [PubMed] [Google Scholar]
- 13.el-Zimaity DM, Hymas KC, Imam IZ, et al. Acute sporadic hepatitis E in an Egyptian pediatric population. Am J Trop Med Hyg 1993; 48: 372–376. [DOI] [PubMed] [Google Scholar]
- 14.Soliman M. Serologic survey for the prevalence of hepatitis E virus in rural community in Giza Governorate. MSc Thesis, Department of Tropical Medicine, University of Cairo, Eygpt, 2000.
- 15.Darwish MA, Faris R, Clemens JD, et al. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in the Nile delta: a pilot study. Am J Trop Med Hyg 1996; 54: 554–558. [DOI] [PubMed] [Google Scholar]
- 16.Anwar AH, El-Hosseiny MM, Khalil KA, et al. Role of polymerase chain reaction and enzyme linked immunosorbent assay in detection of hepatitis E virus. Egypt J Med Microbiol 2005; 14: 1–1. [Google Scholar]
- 17.Cesur S, Akin K, Doğaroğlu I, et al. Hepatitis A and hepatitis E seroprevalence in adults in the Ankara area. Mikrobiyol Bul 2002; 36: 79–83. [in Turkish, English abstract]. [PubMed] [Google Scholar]
- 18.Cevahir N, Demir M, Bozkurt AI, et al. Seroprevalence of hepatitis E virus among primary school children. Pak J Med Sci 2013; 29: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki Mel S, Salama OS, Mansour FA, et al. Hepatitis E virus coinfection with hepatotropic viruses in Egyptian children. J. Microbiol Immunol Infect 2008; 41: 254–258. [PubMed] [Google Scholar]
- 20.El-Gohary A, Hassan A, Uchida T, et al. Prevalence of hepatitis E virus among adult sporadic hepatitis patients in Suez. Int Hepatol Commun 1994; 2: 218–222. [Google Scholar]
- 21.Bashir K, Hussain N, Hasnain S, et al. Seroprevalence of hepatitis E virus immunoglobulin G and M antibodies in adults: a hospital-based study. Indian J Med Microbiol 2009; 27: 139–141. [DOI] [PubMed] [Google Scholar]
- 22.Saffar MJ, Farhadi R, Ajami A, et al. Seroprevalence of hepatitis E virus infection in 2–25-year-olds in Sari district, Islamic Republic of Iran. East Mediterr Health J 2009; 15: 136–142. [PubMed] [Google Scholar]
- 23.Houcine N, Jacques R, Salma F, et al. Seroprevalence of hepatitis E virus infection in rural and urban populations, Tunisia. Clin Microbiol Infect 2012; 18: E119–E121. [DOI] [PubMed] [Google Scholar]
- 24.Agrwal U. Hepatitis E virus. Gastroenterology Jpn 1992; 27: 687–696. [Google Scholar]
- 25.Rab MA, Bile MK, Mubarik MM, et al. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg 1997; 57: 151–157. [DOI] [PubMed] [Google Scholar]
- 26.Stroffolini T, Menchinelli M, Dambruoso V. Prevalence of hepatitis E in a central Italian town at high endemicity for hepatitis C virus. Ital J Gastroenterol 1996; 28: 523–525. [PubMed] [Google Scholar]
- 27.Abd-Al Aziz MS, El-Sherif A, Saad El-Din K. Prevalence of hepatitis E virus antibodies among different Egyptian age groups. Gut 1999; 45(suppl v): A145–A145. [Google Scholar]
- 28.El-Farrash MA, Taher S, Abou-Elela M. Hepatitis type E virus (HEV) epidemiology in Northern Egypt is modified by the predominant hepatitis type C virus (HCV) infection. Egyp J Med Microbiol 2005; 14: 101–109. [Google Scholar]
- 29.Nakamura M, Takahashi K, Taira K, et al. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol Res 2006; 34: 137–140. [DOI] [PubMed] [Google Scholar]
- 30.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a ‘non-hyperendemic’ country. Transfus Med 2006; 16: 79–83. [DOI] [PubMed] [Google Scholar]
- 31.Mizuo H, Yazaki Y, Sugawara K, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 2005; 76: 341–349. [DOI] [PubMed] [Google Scholar]
- 32.Stoszek SK, Engle RE, Abdel-Hamid M, et al. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg 2006; 100: 89–94. [DOI] [PubMed] [Google Scholar]
- 33.Kumar RM, Uduman S, Rana S, et al. Seroprevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol 2001; 100: 9–15. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal R. Hepatitis E: clinical presentation in disease endemic areas and diagnosis. Semin Liver Dis 2013; 33: 30–40. [DOI] [PubMed] [Google Scholar]