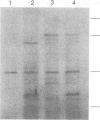
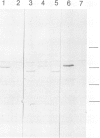
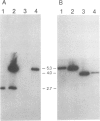
Abstract
The role of tRNA nucleotidyltransferase in Escherichia coli has been uncertain because all tRNA genes studied in this organism already encode the -C-C-A sequence. Examination of a cca mutant, originally thought to contain 1-2% enzyme activity, indicated that it actually produces an inactive fragment of 40 kd compared to 47 kd for the wild-type enzyme due to a nonsense mutation in its cca gene. To confirm that the residual activity in extracts of this strain is due to another enzyme, and that tRNA nucleotidyltransferase is non-essential, we have interrupted the cca gene in vitro, and transferred this mutant gene to a variety of strains. In all cases mutant strains are viable, although as much as 15% of the tRNA population contains defective 3' termini, and no tRNA nucleotidyltransferase is detectable. Mutant strains grow slowly, but can be restored to more normal growth by a relA mutation or by a decrease in RNase T activity. In the latter case the amount of defective tRNA decreases dramatically. These findings indicate that tRNA nucleotidyltransferase is not essential for E. coli viability, and therefore, that all essential tRNA genes in this organism encode the -C-C-A sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. High-level overexpression, rapid purification, and properties of Escherichia coli tRNA nucleotidyltransferase. J Biol Chem. 1986 May 15;261(14):6450–6453. [PubMed] [Google Scholar]
- Cudny H., Lupski J. R., Godson G. N., Deutscher M. P. Cloning, sequencing, and species relatedness of the Escherichia coli cca gene encoding the enzyme tRNA nucleotidyltransferase. J Biol Chem. 1986 May 15;261(14):6444–6449. [PubMed] [Google Scholar]
- Deutscher M. P., Foulds J., McClain W. H. Transfer ribonucleic acid nucleotidyl-transferase plays an essential role in the normal growth of Escherichia coli and in the biosynthesis of some bacteriophage T4 transfer ribonucleic acids. J Biol Chem. 1974 Oct 25;249(20):6696–6699. [PubMed] [Google Scholar]
- Deutscher M. P., Hilderman R. H. Isolation and partial characterization of Escherichia coli mutants with low levels of transfer ribonucleic acid nucleotidyltransferase. J Bacteriol. 1974 May;118(2):621–627. doi: 10.1128/jb.118.2.621-627.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Lin J. J., Evans J. A. Transfer RNA metabolism in Escherichia coli cells deficient in tRNA nucleotidyltransferase. J Mol Biol. 1977 Dec 25;117(4):1081–1094. doi: 10.1016/s0022-2836(77)80014-4. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. RNase T is responsible for the end-turnover of tRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6427–6430. doi: 10.1073/pnas.82.19.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Setlow P., Foulds J. relA overcomes the slow growth of cca mutants. J Mol Biol. 1977 Dec 25;117(4):1095–1100. doi: 10.1016/s0022-2836(77)80015-6. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R. K., Deutscher M. P. Identification of an Escherichia coli nuclease acting on structurally altered transfer RNA molecules. J Biol Chem. 1978 Feb 25;253(4):997–1000. [PubMed] [Google Scholar]
- McClain W. H., Seidman J. G., Schmidt F. J. Evolution of the biosynthesis of 3'-terminal C-C-A residues in T-even bacteriophage transfer RNAs. J Mol Biol. 1978 Mar 15;119(4):519–536. doi: 10.1016/0022-2836(78)90200-0. [DOI] [PubMed] [Google Scholar]
- Moen T. L., Seidman J. G., McClain W. H. A catalogue of transfer RNA-like molecules synthesized following infection of Escherichia coli by T-even bacteriophages. J Biol Chem. 1978 Nov 10;253(21):7910–7917. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. Q., Cudny H., Deutscher M. P. A mutation in Escherichia coli tRNA nucleotidyltransferase that affects only AMP incorporation is in a sequence often associated with nucleotide-binding proteins. J Biol Chem. 1986 Nov 15;261(32):14875–14877. [PubMed] [Google Scholar]