Abstract
Objective
To investigate attentional bias toward happy and sad faces in remitted depressed (RD) patients compared with healthy control (HC) subjects.
Methods
This cross-sectional study enrolled RD patients and sex- and age-matched HC subjects. Eye movement data were acquired for all study participants while free viewing a 2 × 2 matrix of emotional faces. The attentional bias toward different emotional faces and whether the attention maintenance components generated attentional bias in the RD patients were analysed by comparing the attentional modes of the RD group with the HC group.
Results
A total of 27 RD patients and 27 HC subjects were analysed in this study. The RD and HC groups exhibited no significant differences toward first fixation location and initial attentional maintenance. In later attentional maintenance, the RD group showed significantly less attentional bias toward happy faces, but there were no significant differences in their attentional bias toward sad faces, compared with the HC group.
Conclusions
This present study showed that the negative attentional bias of RD patients was successfully eased, but their positive attentional bias was still insufficient.
Keywords: Remitted depression, attentional bias, emotion, eye tracking
Introduction
Numerous studies have shown that patients with depression exhibit attentional bias for negative information and that this negative attentional bias exhibits two important attributes: (i) one is a primary presence for mood-congruent information, i.e. only the depression-related negative information (sadness or dysphoria) causes the excessive attentional processing in depressed patients;1 and (ii) another is that this negative attentional bias occurs at the later attentional stage rather than the early attentional stage.2–5 Patients with depression also lack attentional bias for positive information,6 most likely because their positive emotional processing defects lead to reduced sensitivity of the exogenous system to reward stimuli and thus attentional orientation defects for positive stimuli.7 In other words, the attentional biases of patients with depression involve excessive negative attentional bias but insufficient positive attentional bias. In contrast, the attentional bias of mentally healthy individuals involves an attentional bias toward positive information and the attentional avoidance of negative information.6,8,9 The greater attentional processing bias toward positive information and the lesser attentional processing of negative information in mentally healthy and nondepressed individuals increases their potential pleasure, generating a positive ‘protective bias’ for cognitive psychology that is absent in depressed individuals.10 Furthermore, the extended accumulation of this absence ultimately causes negative cognitive schemata and yields a self-attribution of negative events, leading to the occurrence and maintenance of a depressive mood.11
The remission of depression is an important stage in the depression recovery process. Results from studies of the attentional biases of patients with remitted depression (RD) have been inconsistent and unassertive, which has led to confusion and adverse impacts on further treatment and rehabilitation.3 In general, there are two different viewpoints. One thought is that the negative attentional bias in RD patients is improved and eased. For example, a study that used the emotional Stroop colour-naming task, found that patients suffering depressive episodes showed negative attentional bias, whereas RD patients did not.12 Two other studies both demonstrated that RD patients showed no cognitive differences from the healthy control group, indicating that the attentional functioning of the patients with depression had already recovered.13,14 Another viewpoint is that the negative attentional bias of RD patients is not immediately eased and improved. For example, a study revealed that the characteristics of RD patients and current depressed patients were the same, and both groups exhibited attentional bias toward sad faces and were found to have no attentional bias toward positive information.3 Some researchers have found that depressed patients with impaired attentional functioning continue to show impairments even after their depressive symptoms have been alleviated or recovered, the reasons for which remain unclear.15–17 Other studies also showed that the cognitive functioning of depressed patients was independent of their depressive symptoms, which was the main reason that these patients had not recovered normal social functioning even in remission.18,19 Furthermore, behavioural, event-related potential and neuroimaging studies have revealed that negative attentional bias lingers in RD patients.20–23
A free-viewing study using a 2 × 2 expression matrix task with dysphoric, disgusting, positive, and neutral pictures as stimuli found that for the initial attentional orientation toward negative information, depressed patients spent a longer time gazing at the dysphoric pictures but less time on the positive pictures, showing no attentional orientation biases toward negative information.8 Another study used five types of emotional facial expressions (sad, angry, fearful, disgusted, and happy) and a 2 × 2 expression matrix free-viewing tasks (with a rendering duration of 10 s) and did not observe an initial attentional orientation bias toward negative information in current or former depressed patients.24 Another study used pictures of happy, angry, sad, and neutral faces as stimuli in their free-viewing tasks of paired pictures with one emotional face and one neutral face; the authors did not find an initial attentional orientation bias toward negative information.5 However, another eye-tracking study observed an initial orienting bias using depression-related pictures as stimuli.25
Multiple factors account for the inconsistency in these previous findings, which include (but are not limited to) the experimental stimuli, the experimental paradigms, and, in particular, the different alleviation levels of the depression symptoms. For example, in early studies, emotional words were often used as stimuli,26,27 because pictures used as stimuli were more likely to induce attentional bias in depressed patients than emotional words.28 In particular, the emotional faces of real people are more capable of generating attentional bias than abstract emotional faces,29 because the former contain more interpersonal communication information, which is lacking in the latter. However, it is worth noting that depression-related stimuli could well induce negative attentional bias in depressed patients, while dysphoria-related stimuli such as threat stimuli might fail to induce this bias in the same patients.1 Frequently used experimental paradigms included the reaction time-based emotional Stroop colour-naming task, the dot-probes detecting task, the cue-target mode, and the free-viewing task based on eye movement tracking.
In studies of attentional bias using a reaction time-based technique,30,31 the attention of the subjects is examined through pressing keys, in which there is a relationship between key pressing and attention to a ‘snapshot’ picture and reaction times are recorded at the ‘distal’ time point of the attention. The ‘snapshot’ function can only implicitly evaluate the time course of attention because it cannot accurately capture the fixation time and spatial position of the eye movement.1,32,33 Therefore, it does not reveal dynamic attentional processes or whether the subject had generated attention in the time between the ‘snapshot’ and the key press. Therefore, reaction-time measurement cannot explicitly reflect the real-time course and spatial location of attention, and these deficiencies in temporal and spatial characteristics have restrained investigations of attentional processes and attentional components (attention orienting and attention maintenance). Taking into account the limitations of reaction-time measurements alone, eye-tracking techniques have been increasingly applied. Unlike the camera-style reaction-time technique, eye-tracking is more like ‘videotaping’, providing a relatively continuous and direct measurement of the temporal and spatial processes of attention, and it is more conducive to investigations of the various attentional components of this bias.
The free-viewing paradigm is a common method employed in attentional bias studies using eye tracking.34 It simultaneously provides synchronized and multi-perspective measurements of distinct attentional modes, such as the initial orientation components and the attention maintenance components. One advantage of the free-viewing paradigm is that it more naturally acquires eye movements and is not affected by ‘utilitarian’ requirements such as ‘pressing the key as soon as possible’, as in the reaction-time technique, thereby more truly reflecting the subject’s attentional bias.
Based upon the existing research,5 the present investigation used pictures of a real person’s emotional expressions, which contain more ecological effects and interpersonal relationship information, to design a 2 × 2 facial expression matrix, and happy, sad, and neutral faces were rendered via the free-viewing paradigm. To simultaneously determine the two components of attentional bias in RD patients, the measurement methods for orientation and maintenance of attention were similar to those of previous research.5 However, gaze count rather than the initial fixation duration was used to examine the maintenance components of initial attention, and the later fixation count rather than the total fixation time was used to measure the later-stage attentional maintenance components. Specifically, the initial fixation location was used to determine the attention orientation components, while the gaze count and later fixation count were used to determine the attention maintenance components in the early and later stages. The reason for using the gaze count rather than the fixation duration as the indicator to investigate the attentional components is that the accuracy of calculation based on the gaze count within the region of interest is generally much higher than that based on the fixation duration.
This present study’s first hypothesis was that compared with a healthy control (HC) group, the RD group would not show the initial orienting bias in attention, which would be consistent with the results of most previous research in current depressed or RD patients.24 The second hypothesis was that consistent with the results of previous research,25 relative to the HC group, RD patients would not show the initial orienting bias in attention toward emotional faces, and that attentional bias would occur at the later stage of attention maintenance. The third hypothesis was that at the later stage of attentional maintenance, the attentional bias of RD patients would be eased in a ‘bi-directional’ manner, in which compared with the HC group, RD patients would improve their lack of positive attentional bias while successfully alleviating their excessive negative attention bias.
Patients and methods
Study participants
This cross-sectional study included RD patients and sex- and age-matched HC subjects between January 2014 and December 2015. All RD patients were depressed patients who were treated in the Mood Disorders Centre & China Clinical Research Centre for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China and whose depressive symptoms were successfully alleviated and they were discharged in the remission stage <1 week previously. Inclusion criteria for depression to be successfully alleviated in RD patients were as follows: (i) score on the Clinical Global Impression – severity of illness = 1;35 (ii) score on the 17-item Hamilton Depression Rating Scale < 7;36 (iii) score on the Beck Depression Inventory (BDI) < 10;37 (iv) aged 18–60 years, right-handed; (v) normal vision or corrected-to-normal vision, without colour blindness or other eye diseases; and (vi) capable of performing the eye-tracking test.
The HC subjects were recruited by advertisement from the local population of the Xicheng District of Beijing. The inclusion criteria for the HC subjects were as follows: (i) assessed by a simple interview to exclude past or current mental disorder;24 (ii) BDI score < 4; (iii) aged 18–60 years of age, right-handed; (iv) normal vision or corrected-to-normal vision, without colour blindness and other eye diseases; and (v) capable of completing the eye-tracking test. According to BDI standards, a score of 0–13 represents no depression.37 To ensure the mental health of the HC group, only those with a BDI score < 4 were enrolled.
G*Power 3.1.9.2 software was used to calculate to the sample size needed for the study, in which the medium effect quantity was set to ρ2 = 0.3, with a significance level alpha = 0.05, and statistical test force of 1-beta = 0.95.38 Based on the calculation, the sample size required was at least 27 participants in each group.
During the interview, none of the subjects showed signs of disorders of verbal communication or facial expression recognition. All of the participants provided written informed consent and were remunerated after the test was completed. The study was approved by the Ethics Committee of Beijing Anding Hospital, Capital Medical University, China (no. 2014[65]).
Experimental materials
All facial expression pictures were from the NimStim set of facial expressions.39 Twenty pictures each representing happy, neutral, and sad facial expressions (with each face having three different expressions) were used in the test. After pretreatment, the size, brightness, and resolution of the images were unified.
Experimental paradigm
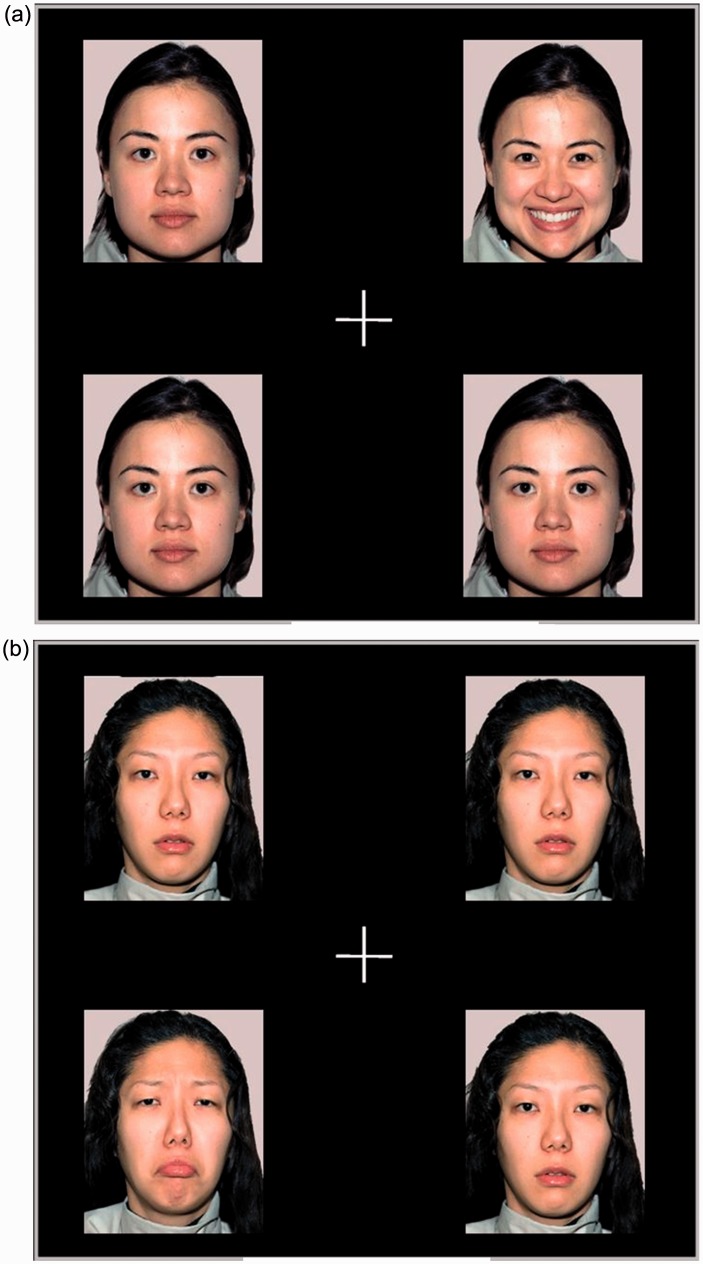
This study used a free-viewing paradigm. The test tasks included the viewing of two types of facial expression (happy and sad faces). The task for each type consisted of four facial expression pictures, placed at four different quadrant locations (upper left, upper right, lower left, and lower right), as shown in Figure 1. To ensure that the facial expression rather than other features of the face would cause the attentional shift, in each task, images of different facial expressions from the same person (3 neutral and 1 happy, or 3 neutral and 1 sad) were used. There were 40 trials in the study, in which the duration of each trial was 13 s, and the overall test duration was 520 s. The number of happy or sad faces appearing at different positions was balanced to reduce position effects.
Figure 1.
Examples of the facial expression stimulus tasks used in the eye-tracking trials. (a) Happy expression task; (b) sad expression task.
Experimental procedure
The study used a Tobii T120 Eye Tracker (Tobii Pro, Stockholm, Sweden) with a sampling rate of 120 Hz. The test materials were rendered on a 17-inch colour liquid crystal display with a resolution of 1024 × 768. The distance between the subject and the display was approximately 60 cm. The tests were conducted in the Eye Movement Laboratory of Beijing Anding Hospital.
The experimental procedure was as follows. First, at the centre of the screen, a white cross was presented for a duration of 1000 ms, and the colour of the sign sometimes changed. When the colour changed, the subjects were asked to respond (the purpose of which was to ensure that the subjects’ gaze was directed at the centre of the screen).5,40 Then, stimuli were displayed on the screen for 10 000 ms,24 while the subject was asked to free-view the faces. Next, a black screen was rendered for 2000 ms, and after the disappearance of the black screen, the next stimuli task proceeded. During the test, subjects were asked to keep their heads as still as possible.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 20.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Sex differences between the RD and HC groups were tested using the χ2-test and differences in age and education were analysed using independent sample t-test. A P-value <0.05 was considered statistically significant.
The screen was divided into four different regions of interest each corresponding to one face. In this study, three eye movement indices were used: first fixation location, gaze count, and later fixation count. The first fixation location was the participant’s initial fixation location during each stimuli task, which reflected their initial fixation orientation or their initial gaze direction. The gaze count for each type of stimuli, was defined as the gaze count of the participant gazing at certain region of interest from the time of the initial gazing to the time when the gaze left the current region of interest, which represents the maintenance component of initial attention. The later fixation count for each stimuli type was the difference between the total fixation count at a certain region of interest and the gaze count. With respect to the gaze count, the difference between the total fixation count and the gaze count was defined as the fixation count of the attentional later period (later fixation count), which represented the maintenance component of attentional later stage. A mixed-model analysis of variance (ANOVA) with 2 (group: RD, HC) ×2 (emotional category: happy, sad) repeated measures was conducted to analyse the eye movement indices. A paired t-test was conducted on the emotion categories.
The bias score was calculated as follows. Compared with the absolute indicators, the relative attentional bias score can better reflect attentional bias in emotional information processing,10 in accordance with which the relative attentional bias scores of three attentional indices of happy and sad faces were calculated, respectively. For the first fixation location score, the percentage of the count of the participant’s fixation on happy faces (or sad faces) rather than on neutral faces was calculated. For the scores for gaze count and later fixation count, the count was acquired from the region of interest in which the emotional faces (happy or sad faces) resided was subtracted from the mean of the counts that were acquired from the region of interest occupied by the three neutral faces. The significance of the bias scores was based on the differences that had been used in a previous study,5 in which, when the score of the first fixation location was >25%, it denoted the presence of an attentional bias for emotional faces, and when the score was ≤25%, it denoted the absence of an attentional bias for emotional faces. When the scores for the gaze and later fixation counts were >0, they denoted the presence of attentional bias for emotional faces, and when the scores were ≤0, they denoted the absence of an attentional bias for emotional faces.
Results
This study included 27 RD patients and 27 sex- and age-matched HC subjects. The RD patients had different histories of depression, in which the mean ± SD duration of episodes was 7.31 ± 6.93 years (the duration from the first episode to the last episode), the mean ± SD number of episodes was 2.41 ± 0.91, and the mean ± SD time since the last episode was 22.26 ± 8.37 days. The demographic and clinical data for the two groups are shown in Table 1.
Table 1.
Demographic and clinical characteristics for patients with remitted depression (RD) and healthy control (HC) subjects who participated in this study.
| Characteristic | RD group n = 27 | HC group n = 27 |
|---|---|---|
| Sex, male:female | 8:19 | 8:19 |
| Age, years | 49.37 ± 12.39 | 49.26 ± 12.49 |
| Educational level, years | 12.04 ± 3.50 | 11.93 ± 3.40 |
| BDI score | 8.96 ± 2.30 | 2.10 ± 0.93 |
| HAMD score | 6.12 ± 1.68 |
Data presented as n of patients or mean ± SD.
No significant between-group differences (P ≥ 0.05); sex differences were tested using χ2-test and differences in age, education, BDI score and HAMD score were analysed using independent sample t-test.
BDI, Beck Depression Inventory; HAMD, Hamilton Depression Rating Scale.
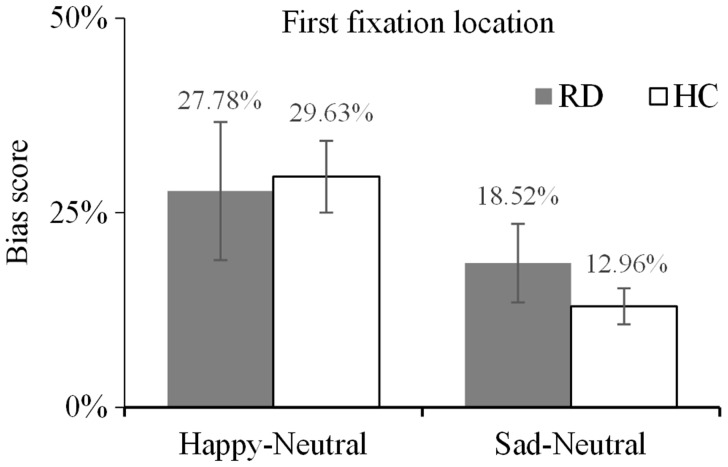
Using first fixation location as the dependent variable and the types of subjects and emotions as the independent variables, a 2 (group: RD, HC) × 2 (emotional category: happy, sad) two-factor mixed-model ANOVA was performed to investigate the initial orientation bias toward different emotional types in the two groups. No significant interaction of group × type of emotion was observed (F [1, 52] = 0.64, P = 0.43, η2 = 0.01). The main effect of group was not significant (F [1, 52] = 0.13, P = 0.72, η2 = 0.00), indicating there were no significant differences in the initial gaze direction toward the facial expressions between the two groups (Figure 2).
Figure 2.
Mean bias scores for the first fixation location for happy and sad facial expressions. Comparison between depressed patients in the remission stage (RD group) and healthy control subjects (HC group). Scores >25% indicate a bias toward emotional facial expressions, while scores ≤25% indicate a bias toward neutral faces. The error bars represent the standard error.
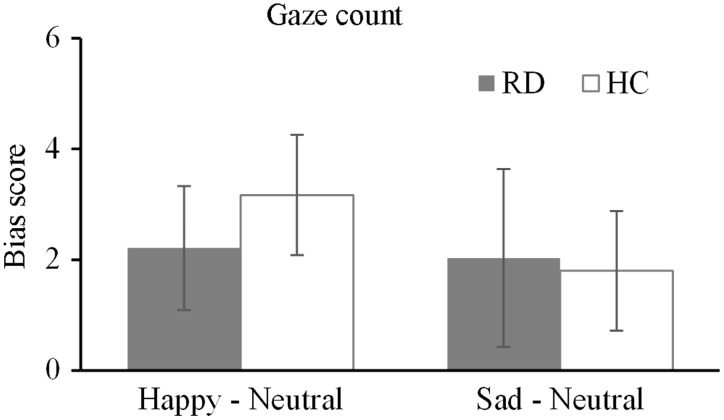
The results for the gaze count are shown in Figure 3. The 2 (group: RD, HC) × 2 (emotional category: happy, sad) two-factor mixed-model ANOVA revealed no significant interaction of group × type of emotion (F [1, 52] = 0.77, P = 0.38, η2 = 0.02). The main effect of group was not significant (F [1, 52] = 0.51, P = 0.57, η2 = 0.01), indicating there were no significant differences in the gaze count toward the emotional faces between the two groups. The gaze count analyses revealed no significant difference in the initial attention maintenance bias toward different emotional faces.
Figure 3.
Mean bias scores for the gaze count for happy and sad facial expressions. Comparison between depressed patients in the remission stage (RD group) and healthy control subjects (HC group). Scores >0 indicate a bias toward emotional facial expressions, while scores ≤0 indicate a bias toward neutral faces. The error bars represent the standard error.
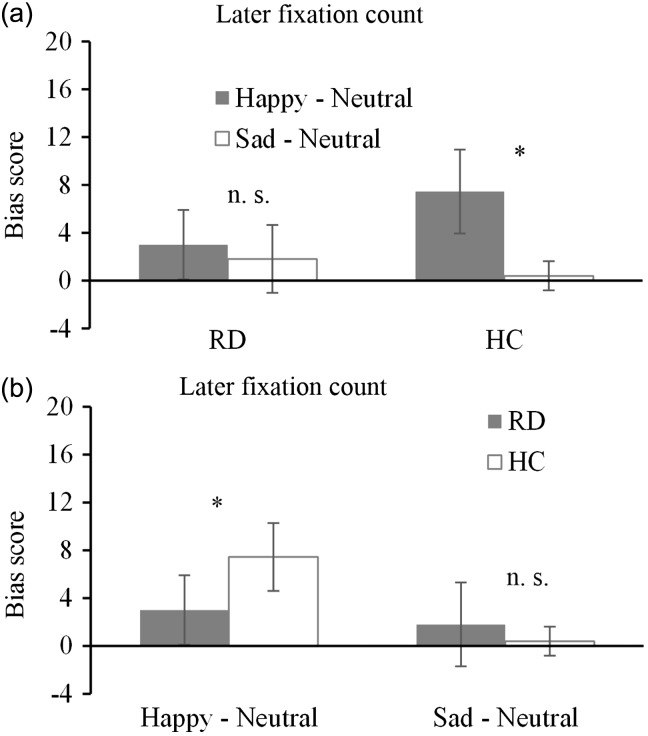
The 2 (group: RD, HC) × 2 (emotional category: happy, sad) two-factor mixed-model ANOVA revealed a significant interaction of group × type of emotion (F [1, 52] = 6.89, P = 0.01, η2 = 0.12). The main effect of group was not significant (F [1, 52] = 0.54, P = 0.47, η2 = 0.12), indicating that there were no significant differences between the two groups with respect to the later fixation count toward the emotional faces. The main effect of emotional category was significant (F [1, 52] = 15.25, P < 0.01, η2 = 0.23), indicating that the later fixation count of the subjects toward happy faces was significantly higher than that toward sad faces.
To determine which group viewed the happy faces more frequently in the later stage of attention, a paired t-test was conducted on the emotion types and the results are shown in Figure 4a. The subjects in the HC group viewed the happy faces significantly more frequently than the sad faces (t [26] = 3.72, P < 0.01, d = 1.13), while the RD group showed no significant difference in viewing the two types of emotional faces (t [26] = 1.34, P = 0.20, d = 0.24), despite viewing the happy faces slightly more during the later stage of attention.
Figure 4.
Mean bias scores for later fixation count for happy and sad facial expressions. (a) Comparison between happy and sad facial expressions in depressed patients in the remission stage (RD group) and healthy control subjects (HC group); (b) Comparison between the RD group and HC group for happy and sad facial expressions. Scores >0 indicate a bias toward emotional facial expressions while score ≤0 indicate a bias toward neutral faces. *Significant difference (P < 0.05); n.s., no significant difference (P ≥ 0.05); error bars represent standard error.
Although a main effect of group was absent, because of the presence of the interaction between the group and the emotional type, the inter-group difference was analysed. As shown in Figure 4b, the RD group had significantly less bias toward happy faces than the HC group did (t [52] = –2.05, P < 0.05, d = 0.57), indicating that the RD group’s attentional bias for happy faces was less than that of the HC group. Regarding the bias toward sad faces, although the RD group viewed the sad faces slightly more than the HC group did, there were no significant differences between the two groups (t [52] = 1.28, P = 0.21, d = 0.36), indicating that the late stage negative attentional bias in the RD group was effectively eased.
Discussion
In this present study, fixation location patterns were obtained using a free-viewing strategy for the attentional bias of the RD and HC groups toward emotional faces. The initial orientation bias, the initial attention maintenance bias and the later-stage attention maintenance bias were investigated.
The first hypothesis for this present study was that compared with the HC group, the RD group would show no initial orientation bias toward emotional faces. With respect to initial orientation, the main effect between groups was not significant, which left us unable to verify the first hypothesis. A large number of previous studies have shown that depressed patients exhibit no initial orientation bias.5,8,24,41,42 Thus, there is no reason to believe that the depressed patients in this study would show attentional bias while in remission. However, inconsistent with these current findings as well as those of most of the previous studies,5,8,24,41,42 a previous study found that formerly depressed patients had an initial orientation bias toward negative information but showed no such bias toward positive information.25 This discrepancy might be mostly caused by the sex difference in the subjects as the previous study focused on female RD patients,25 while in this present study, both males and females were enrolled, although the number of male patients was only 1/3 of the female patients. In fact, multiple studies have shown that women in puberty, after childbirth, and during menopause have greater mood swings and are more prone to anxiety and depression compared with women at other stages in their reproduction lives.43–45 Compared with men, women also have longer depressive episodes and are also more prone to relapse.46 These findings imply that the attentional bias of RD patients may exhibit a sex difference, which requires further investigation.
The second hypothesis of this present study was that compared with the HC group, the RD patients would show no initial attention maintenance bias toward emotional faces and that their attentional bias would occur in the later stage of attention maintenance, which was supported by the current results that there was no significant main effect of group on gaze counts. These current results are consistent with those of previous studies on current depressed patients as well as RD patients, all indicating that attentional bias occurs at the later stage of attention rather than the early stages of attention.2–5,41 The characteristics of the bias sensitivity toward the later stage of attention in depressed patients revealed that these patients exhibited detailed and careful attentional processing of depression-related information, and the depression-related stimuli were more likely to cause contemplation (one of the core features of depression) in these patients, thereby deepening the attentional maintenance of the mood-congruent depression-related information.4
The third hypothesis of this present study was that in the later stage of attention maintenance, the attentional bias of the RD patients would exhibit ‘bi-directional’ alleviation, i.e., relative to the HC group, the lack of positive attention bias of the RD patients would be improved while the excessive negative attention bias would also be successfully eased. The current results only partially validated this hypothesis, i.e., the negative attentional bias in the RD patients did not significantly differ from that in the HC group and was successfully mitigated. However, compared with the HC group, the RD group still lagged behind in their positive attentional bias, which did not reach the levels of the HC group. The BDI score of the RD patients recruited in this study was <10, which indicated that the depressive mood of the RD patients was effectively alleviated; however, the current finding that the RD patients’ attentional bias did not achieve ‘bi-directional’ alleviation was unanticipated. This result regarding an insufficient positive stimuli bias in the RD patients is consistent with the results of a previous study in depressed patients,6 but in conflict with other research that showed that the lack of positive attentional bias in RD patients was also successfully mitigated.24 These current results illustrate that the depressed mood of the RD patients was eased and improved, but they still exhibited deficits in their attentional maintenance and processing motivation for positive stimuli; furthermore, their ‘protective’ attentional bias was far from being established, with inadequate sensitivity toward the external reward stimuli likely being one of the critical reasons for the relapse tendency of depressive symptoms in these patients. It has been demonstrated that attention training toward positive stimuli could improve attentional bias toward positive stimuli;47 therefore, in addition to medical treatment, RD patients in remission should also be offered attentional training toward positive stimuli.48
It has been found in previous research that when performing the free-viewing task, depressed patients lowered their attentional maintenance of positive stimuli while enhancing the attentional maintenance viewing of depression-related negative stimuli.1 The results obtained in this present study indicate that although the RD patients had a slightly higher attentional bias toward sad faces than the HC group did, there were no significant differences between the two groups, indicating that the excessive processing of negative information by the RD patients disappeared with the easing of their depressive symptoms. However, the most significant discrepancy between these present results and those of previous studies lies in the negative attentional bias of the RD patients. For example, some researchers believe that RD patients’ negative attentional bias does not ease and continues to lingers,3,17 mainly because the cognitive functioning of depressed patients is independent of their depressive symptoms, and thus the attentional functioning of RD patients cannot consciously be recovered.19,22 However, other investigators have presented evidence supporting the notion that the negative attentional bias of RD patients can be successfully eased with the disappearance of depressive symptoms,12–14 which supports these current results.
With the prolongation of the remission stage, negative events and chronic stress will be increased, which will increase the risk of recurrence of depression. Therefore, differences in the duration of remission may be one of the key reasons for the current conflicting results compared with previous studies of the attentional biases of RD patients. Accordingly, the RD patients included in this present study were recommended by their doctor and had only recently entered the remission stage (within 1 week). It is our opinion that the attention bias of RD patients at the beginning stage of the remission period more realistically reflects the cognitive functioning of depressed patients at the initial successful alleviation of their condition.
In addition, Beck’s theory of depression reflects that cognitive biases are a trait-like feature of depressed patients,49 but there are contradictions in current studies. Attentional functioning is an important basis and a component of cognitive functioning. The present study on the attentional bias of RD patients investigated whether attention dysfunction was present at the immediate cessation of a depressive episode. If it is, the findings suggest that attentional bias is a trait-like feature; if not, it is a state-like feature. These current results show that, compared with HC subjects, there was still a lack of positive attentional bias in RD individuals, and there was no negative attentional bias, which may suggest that the lack of positive attentional bias is a trait-like feature, while negative attentional bias is a state-like feature.
The present study had several limitations: First, only a cross-sectional comparison study was conducted; so the lack of a longitudinal comparison was unfavourable for the causal interpretation of the attentional bias. Secondly, although the sample size in this study was similar to that used in a previous similar eye-tracking study,5 it would be easier to identify the inter-group differences with a larger sample size. The effect size (the η2 value of the main effect and the d value of the paired t-test) that is independent of the sample size was provided to help elucidate any significant differences. Therefore, in future studies, a longitudinal investigation should be undertaken and the sample size should be appropriately increased so that the characteristics of attentional bias in RD patients can be better understood. Thirdly, this present study enrolled RD patients who had just entered into remission (<1 week). The inclusion of another RD group with a longer period of remission (e.g., >6 months and without recurrence) is warranted to investigate the effects of the remission period itself on attentional bias. Moreover, there were also no comparisons between currently depressed patients and RD patients. Although this did not affect the current investigation of the attentional bias of RD patients, it was not conducive to investigating the improvement of attentional bias.
In conclusion, this present study shows that the negative attentional bias of depressed patients in remission was successfully eased, while their defects in attentional bias toward positive information were only partially relieved, which may suggest that the lack of positive attentional bias is a trait-like feature, while negative attentional bias is a state-like feature. If these results can be confirmed in future studies, they would be very helpful for relief treatment and attention training in depressed patients in remission.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding acknowledgement
This work is supported by the National Basic Research Programme of China (2014CB744600), the National Natural Science Foundation of China (61602017), the Beijing Natural Science Foundation (4164080), the Beijing Outstanding Talent Training Foundation (2014000020124G039), the National Natural Science Foundation of China (61420106005), the Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (26350994), the Beijing Municipal Science and Technology Project (D12100005012003), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (ZY201403), and the Beijing Municipal Science and Technology Achievement Transformation and Industrialization Projects funds (Z121100006112057).
References
- 1.Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin Psychol Rev 2012; 32: 704–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit Ther Res 2005; 29: 29–45. [Google Scholar]
- 3.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 2007; 116: 80–85. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behav Res Ther 2007; 45: 2664–2678. [DOI] [PubMed] [Google Scholar]
- 5.Duque A, Vazquez C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry 2015; 46: 107–114. [DOI] [PubMed] [Google Scholar]
- 6.Ellis AJ, Beevers CG, Wells TT. Attention allocation and incidental recognition of emotional information in dysphoria. Cognit Ther Res 2011; 35: 425–433. [Google Scholar]
- 7.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991; 100: 316–336. [DOI] [PubMed] [Google Scholar]
- 8.Kellough JL, Beevers CG, Ellis AJ, et al. Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav Res Ther 2008; 46: 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez A, Vazquez C. Looking at the eyes of happiness: positive emotions mediate the influence of life satisfaction on attention to happy faces. J Pos Psychol 2014; 9: 435–448. [Google Scholar]
- 10.Shane MS, Peterson JB. An evaluation of early and late stage environmental processing of positive and negative information in dysphoria. Cogn Emot 2007; 21: 789–815. [Google Scholar]
- 11.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 2008; 165: 969–977. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund S, Rude SS. Evidence of latent depressive schemas in formerly depressed individuals. J Abnorm Psychol 1995; 104: 517–525. [DOI] [PubMed] [Google Scholar]
- 13.Brodaty H, Luscombe G, Anstey KJ, et al. Neuropsychological performance and dementia in depressed patients after 25-year follow-up: a controlled study. Psychol Med 2003; 33: 1263–1275. [DOI] [PubMed] [Google Scholar]
- 14.Clark L, Kempton MJ, Scarna A, et al. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry 2005; 57: 183–187. [DOI] [PubMed] [Google Scholar]
- 15.Hammar A, Ardal G. Cognitive functioning in major depression–a summary. Front Hum Neurosci 2009; 3: 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preiss M, Kucerova H, Lukavsky J, et al. Cognitive deficits in the euthymic phase of unipolar depression. Psychiat Res 2009; 169: 235–239. [DOI] [PubMed] [Google Scholar]
- 17.Lange C, Adli M, Zschucke E, et al. Affective set-shifting deficits in patients with major depression in remission. J Psychiatr Res 2012; 46: 1623–1626. [DOI] [PubMed] [Google Scholar]
- 18.Reischie FM, Neu P. Comordity of mild cognitive disorder and depression–a neuropsychological analysis. Eur Arch Psychiatry Clin Neurosci 2000; 250: 186–193. [DOI] [PubMed] [Google Scholar]
- 19.Koetsier GC, Volkers AC, Tulen J, et al. CPT performance in major depressive disorder before and after treatment with imipramine or fluvoxamine. J Psychiatr Res 2002; 36: 391–397. [DOI] [PubMed] [Google Scholar]
- 20.Neu P, Bajbouj M, Schilling A, et al. Cognitive function over the treatment course of depression in middle-aged patients: correlation with brain MRI signal hyperintensities. J Psychiatr Res 2005; 39: 129–135. [DOI] [PubMed] [Google Scholar]
- 21.Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: an event related potentials study. Biol Psychol 2009; 81: 169–176. [DOI] [PubMed] [Google Scholar]
- 22.Goulden N, McKie S, Thomas EJ, et al. Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol Psychiatry 2012; 72: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 2006; 19: 34–39. [DOI] [PubMed] [Google Scholar]
- 24.Isaac L, Vrijsen JN, Rinck M, et al. Shorter gaze duration for happy faces in current but not remitted depression: evidence from eye movements. Psychiatry Res 2014; 218: 79–86. [DOI] [PubMed] [Google Scholar]
- 25.Sears CR, Newman KR, Ference JD, et al. Attention to emotional images in previously depressed individuals: an eye-tracking study. Cogn Ther Res 2011; 35: 517–528. [Google Scholar]
- 26.Gotlib IH, Cane DB. Construct accessibility and clinical depression: a longitudinal investigation. J Abnorm Psychol 1987; 96: 199–204. [DOI] [PubMed] [Google Scholar]
- 27.Segal ZV, Gemar M, Truchon C, et al. A priming methodology for studying self-representation in major depressive disorder. J Abnorm Psychol 1995; 104: 205–213. [DOI] [PubMed] [Google Scholar]
- 28.Spruyt A, Hermans D, De Houwer J, et al. On the nature of the affective priming effect: affective priming of naming responses. Soc Cognition 2002; 20: 227–256. [Google Scholar]
- 29.Karparova SP, Kersting A, Suslow T. Disengagement of attention from facial emotion in unipolar depression. Psychiatry Clin Neurosci 2005; 59: 723–729. [DOI] [PubMed] [Google Scholar]
- 30.Koster EH, Leyman L, De Raedt R, et al. Cueing of visual attention by emotional facial expressions: The influence of individual differences in anxiety and depression. Personality and Individual Differences 2006; 41: 329–339. [Google Scholar]
- 31.Romero N, Sanchez A, Vazquez C. Memory biases in remitted depression: The role of negative cognitions at explicit and automatic processing levels. J Behav Ther Exp Psychiatry 2014; 45: 128–135. [DOI] [PubMed] [Google Scholar]
- 32.Weierich MR, Treat TA, Hollingworth A. Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion 2008; 22: 985–1018. [Google Scholar]
- 33.Yovel I, Mineka S. Emotion-congruent cognitive biases: the perspective of hierarchical models of emotional disorders. Personality and Individual Differences 2005; 38: 785–795. [Google Scholar]
- 34.Toh WL, Rossell SL, Castle DJ. Current visual scanpath research: a review of investigations into the psychotic, anxiety, and mood disorders. Compr Psychiatry 2011; 52: 567–579. [DOI] [PubMed] [Google Scholar]
- 35.National Institute of Mental Health. CGI (Clinical Global Impression) Scale—NIMH. Psychopharmacol Bull 1985; 21: 839–844. [Google Scholar]
- 36.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 38.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 39.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cogn Emot 2005; 19: 433–451. [DOI] [PubMed] [Google Scholar]
- 41.Eizenman M, Yu LH, Grupp L, et al. A naturalistic vision scanning approach to assess selective attention in major depressive disorder. Psychiatry Res 2003; 118: 117–128. [DOI] [PubMed] [Google Scholar]
- 42.Caseras X, Garner M, Bradley BP, et al. Biases in visual orienting to negative and positive scenes in dysphoria: an eye movement study. J Abnorm Psychol 2007; 116: 491–497. [DOI] [PubMed] [Google Scholar]
- 43.Ahokas A, Kaukoranta J, Wahlbeck K, et al. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17 beta-estradiol: a preliminary study. J Clin Psychiatry 2001; 62: 332–336. [DOI] [PubMed] [Google Scholar]
- 44.Douma SL, Husband C, O’Donnell ME, et al. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 2005; 28: 364–375. [DOI] [PubMed] [Google Scholar]
- 45.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav 2009; 97: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oquendo MA, Turret J, Grunebaum MF, et al. Sex differences in clinical predictors of depression: a prospective study. J Affect Disord 2013; 150: 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadlinger HA, Isaacowitz DM. Looking happy: the experimental manipulation of a positive visual attention bias. Emotion 2008; 8: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baert S, De Raedt R, Schacht R, et al. Attention bias training in depression: therapeutic effects depend on depression severity. J Behav Ther Exp Psychiatry 2010; 41: 265–274. [DOI] [PubMed] [Google Scholar]
- 49.Watkins ER, Moulds ML. Reduced concreteness of rumination in depression: A pilot study. Personality and Individual Differences 2007; 43: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]