Abstract
Objectives
Chronic gneck and shoulder pain (CNSP) is a common clinical symptom of cervical spondylotic radiculopathy. Several studies using resting-state functional magnetic resonance imaging (rs-fMRI) have reported that most chronic pain diseases are accompanied by structural and functional changes in the brain. However, few rs-fMRI studies have examined CNSP. The current study investigated cerebral structural and functional changes in CNSP patients.
Methods
In total, 25 CNSP patients and 20 healthy volunteers participated in the study. 3D-T1W and rs-fMRI images were acquired. Voxel-based morphometry analysis was applied to structural images, and regional homogeneity (ReHo) was extracted from rs-fMRI. Statistical analysis was performed on post-processing images and ReHo parameter maps.
Results
The results revealed no significant differences in brain structure between the two groups. In the patient group, ReHo values were significantly increased in the bilateral middle frontal gyrus and decreased in the left insula, superior frontal gyrus, middle cingulate gyrus, supplementary motor area, right postcentral gyrus, and superior parietal lobule.
Conclusions
This initial structural and rs-fMRI study of CNSP revealed characteristic features of spontaneous brain activity of CNSP patients. These findings may be helpful for increasing our understanding of the neuropathology of CNSP.
Keywords: Chronic neck and shoulder pain, resting-state functional magnetic resonance imaging, regional homogeneity, spontaneous brain activity, voxel-based morphometry, fasting
Introduction
Chronic neck and shoulder pain (CNSP) is a common clinical symptom of cervical spondylotic radiculopathy (CSR).1 Clinical evidence has demonstrated that patients with long-duration chronic pain may develop accumulating brain damage due to the repetitive occurrence of pain-related processes2 accompanied by dysfunction of sensory, cognitive, memory and affective processing.3–5 A number of studies have been conducted to investigate the underlying cerebral changes caused by chronic pain, including changes in neuroplasticity, neurochemistry, electrophysiology, brain structure and function.6–10 Most functional imaging studies observing major abnormalities in patients with chronic pain of various aetiologies reporting have reported changes in brain structure, aberrant spontaneous neural activity and disturbed functional connectivity related to pain.6,7,10–13
Voxel-based morphometry (VBM) studies of other chronic pain conditions have reported reduced grey matter in specific brain regions.6,7,12 Thus, the current study explored changes in brain structure using VBM methodology.
Resting-state functional magnetic resonance imaging (rs-fMRI) has been found to be an effective approach to evaluate spontaneous neural activity based on blood oxygen level-dependent contrast.14 In the last decade, a number of studies have used rs-fMRI to investigate changes in neural activity caused by different chronic pain sources, including migraine, somatoform pain, low back pain, visceral pain and fibromyalgia.15–19 Regional homogeneity (ReHo) is an important parameter that can be extracted from rs-fMRI data sets.20 ReHo was first proposed by Zang YF and colleagues, suggesting that ReHo exhibits superior performance compared with model-driven methods, due to its sensitivity in the detection of unpredicted and complex spontaneous hemodynamic responses in human brain function.21,22 In addition, ReHo has been used to investigate the regional activity characteristics of each voxel within the brain, which was not possible with earlier methods.2 Moreover, the high test-retest reliability of ReHo has been confirmed by carefully optimizing the data, preprocessing and computational implementation.23 These benefits have prompted the wide application of ReHo for discovering changes in brain function under pain conditions. Specific alterations of ReHo values have been identified for the various types of chronic pain.15,17,20 Wang et al.20 reported that patients with tension-type headache exhibited significantly lower ReHo values in the bilateral caudate nucleus, precuneus, putamen, left middle frontal gyrus (MFG) and superior frontal gyrus (SFG) and that these regions were involved in the integration and processing of pain. Another study of migraine without aura reported a significant decrease in the ReHo values in the right anterior cingulate cortex, prefrontal cortex, orbitofrontal cortex and supplementary motor area (SMA).15 In a study of experimentally-induced low back pain, Zhang et al.17 suggested that brain regions exhibiting abnormal ReHo values were potentially associated with pain processing. These results confirmed the diagnostic validity of ReHo for neuropsychiatric disorders in the field of chronic pain. Taken together, these findings indicate that ReHo can be considered a reliable and effective measure for investigating and characterizing spontaneous brain activity, providing valuable information for understanding the neural mechanisms of CNSP.
Although many rs-fMRI studies have investigated the relationships between changes in ReHo values and different types of chronic pain, few have focused on CNSP, which is a subtype of clinical chronic pain. Based on previous evidence, we hypothesized that CNSP patients would exhibit changes in brain structure and ReHo. We tested this hypothesis by exploring structural changes using VBM, and investigating the characteristics of spontaneous brain activity of patients with CNSP using ReHo.
Patients and methods
Participants
In total, 25 patients with CNSP caused by CSR and 20 sex-, age- and education-matched healthy volunteers with no neurological or pain disorders were included in this study.
The inclusion criteria for the CNSP group were as follows: (1) CNSP persisted for more than 3 months and involvement of the nerve root was found with MRI or CT (several patients with severe pain and a high VAS scoreexhibited weakness and difficulty moving their upper limbs); (2) positive findings of pain-related symptoms in a physical examination, including tenderness, nerve root traction and foraminal compression; (3) no history of neurological or psychiatric disorders or cognitive dysfunction; (4) no accompanying pain in other parts of the body, or CNSP caused by other causes (e.g., frozen shoulder or muscle strain); and (5) no other treatment was administered (i.e., drugs or acupuncture) that could potentially affect neurological function.
All included subjects provided written informed consent prior to the acquisition of MR images, and the study was approved by the Medical Research Ethics Committee of Yichang Central People’s Hospital (Yichang, China).
Data acquisition
MRI data acquisition was performed using a 3.0-Tesla scanner (Philips Intera Achieva, Best, Netherlands). Headphones and foam pads were used to reduce noise interference and minimize head motion. Participants were instructed to lie still in a relaxed position with their eyes closed, while remaining awake and avoiding any specific thoughts. Functional images were acquired using an echo planar imaging sequence (TR = 2000 ms, TE = 35 ms, voxel size =3.75 × 3.75 × 4 mm3, FA = 80°, slice thickness = 4 mm, slices = 35, matrix size =64 × 64 × 64, FOV = 24 × 24 cm2; 230 frames were obtained, including the first 12 dummy scans).Three-dimensional T1-weighted structural images were collected using a fast spoiled-gradient echo sequence (TR = 11.1 ms, TE = 5 ms, FA = 20°, slice thickness = 1 mm, FOV = 24 × 24 cm2, matrix size = 256 × 256, voxel size = 1 × 1 ×1 mm3).
Data preprocessing
The structural and rs-fMRI images were preprocessed using Statistical Parametric Mapping (SPM8, http://www.restfmri.net/forum/SPM) and Data Processing Assistant for Resting-State fMRI (DPARSF, http://www.restfmri.net/forum/DPARSF), respectively.
For structural images, manual correction was used if realignments with head movements were more than 2 mm in translation and 2° in rotation. Brain tissue types were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) in native space, and modulation was performed. The resulting gray matter volume (GMV) images were normalized to Montreal Neurological Institute (MNI) standard space and smoothed with an isotropic Gaussian kernel. For rs-fMRI images, the first 20 time points (including the first 12 dummy scans) were removed; and then slice timing was done due to scan with an interval technique. Realignments with head movements were less than 2 mm in translation and 2° in rotation. Functional images were normalized to MNI standard space using the echo planar imaging (EPI) template, and resampled to 3 × 3 × 3 mm3. The linear trend of the signals was removed, and a filter with a frequency range of 0.01–0.08 Hz was applied.
ReHo analyses
ReHo was computed based on the KCC of the time series of the voxel and its nearest neighbours (26 voxels) using DPARSF, followed by a smoothing procedure with a Gaussian kernel with a full-width-half-maximum (FWHM) of 4 mm to minimize the noise and residual differences of the preprocessed images.
Statistical analysis
Independent two-sample t-tests were used to compare the continuous demographic variables, and chi-square tests were used to examine the proportions between groups with SPSS 13.0. One-sample t-tests were performed on the individual ReHo maps for each group using REST Statistical Analysis software to explore within-group ReHo characteristics. Two-sample t-tests were applied to examine differences in the ReHo values and GMV between the patient and control groups. Age, sex, and education levels were analysed as covariates.
Demographic and Clinical Characteristics
The demographic characteristics of all participants are shown in Table 1. There were no significant differences in demographic variables between the two groups (p > 0.05).
Table 1.
Demographic characteristics of CNSP patients and healthy controls.
| Patients (n = 25) | Controls (n = 20) | p-value | |
|---|---|---|---|
| Handedness (left / right) | 0 / 25 | 0 / 20 | – |
| Sex (male / female) | 13 / 12 | 10 / 10 | 0.540 |
| Age (mean ± SD, years) | 47.68 ± 10.99 | 42.50 ± 11.94 | 0.261 |
| Education (mean ± SD, years) | 12.36 ± 3.57 | 13.20 ± 3.76 | 0.732 |
| Pain intensity (VAS score) | 5.44 ± 2.20 | – | – |
Note: there were no significant differences in demographic variables between the two groups (p > 0.05)
Results
VBM results
Independent two-sample t-tests revealed no significant differences in GMV between the CNSP and control groups (p < 0.05).
Within-group ReHo maps
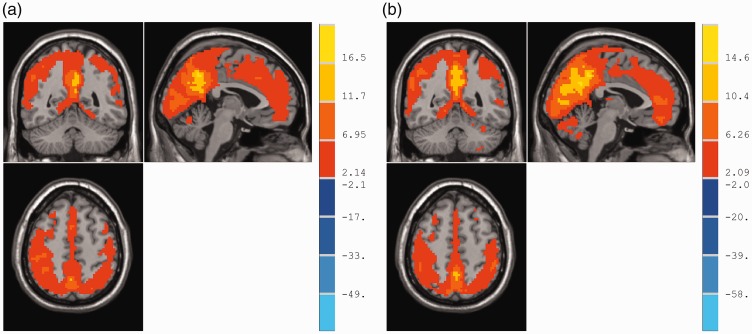
Figure 1 shows the ReHo maps of the CNSP and control groups. In both groups, standardized ReHo values in the posterior cingulate cortex, precuneus, inferior parietal lobule, and medial prefrontal cortex were significantly higher than the mean global (whole-brain) values. The ReHo patterns were similar to those of the human default mode network (DMN).
Figure 1.
One-sample t test of ReHo maps within-groups.
(a) and (b) represent the control and patient groups, respectively. In both groups, the standardized ReHo values in the posterior cingulate cortex, precuneus, inferior parietal lobule, and medial prefrontal cortex were significantly higher than the global mean values. The colour bar indicates the t-value of the brain regions exhibiting ReHo changes (p < 0.005, AlphaSim correction).
Group differences in ReHo
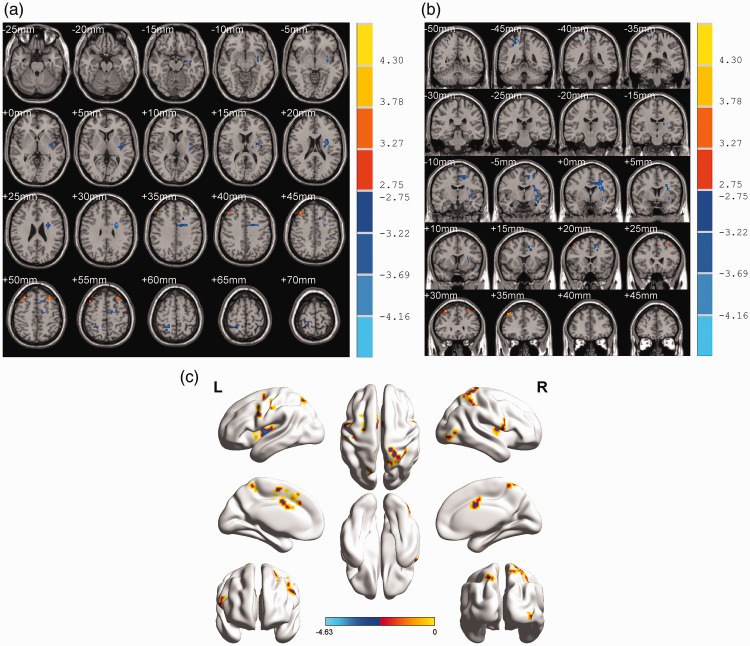
As seen in the ReHo maps in Figure 2, the analysis revealed significant differences in brain activity between the CNSP and control groups (p < 0.01; AlphaSim correction, cluster threshold ≥ 18). The results showed significantly decreased ReHo values in the left insula, SFG, middle cingulate gyrus (MCG), SMA and right postcentral gyrus (PCG) and superior parietal lobule (SPL). In contrast, significantly increased ReHo values were mainly found in the bilateral MFG (See Table 2 for a detailed list of all regions exhibiting increased ReHo values).
Figure 2.
Differences in ReHo values between-groups.
(a) and (b) show the axial and coronal views, respectively. Blue brain regions represent decreased ReHo values, and red regions indicate increased ReHo values in CNSP patients relative to the control group. The right colour bar represents the t value of brain regions with ReHo changes. (two-tailed two-sample t-test; p < 0.01, AlphaSim correction); (c) shows the activated areas mapped to the cerebral cortex.
Table 2.
Brain regions showing significant differences in ReHo values between patients and controls.
| Brain regions | Hemisphere | BA | Cluster size (voxels) | Peak MNI coordinates (x, y, z) | Peak intensity (t) |
|---|---|---|---|---|---|
| Increased values | |||||
| Middle Frontal Gyrus | R | 9 | 37 | 43, 34, 42 | 3.80064 |
| Middle Frontal Gyrus | L | 9 | 21 | −33, 29, 52 | 3.36197 |
| Decreased values | |||||
| Insula | L | 48 | 49 | −39, −5, 7 | − 3.84911 |
| Superior Parietal Lobule | R | 2 | 26 | 21, −47, 61 | − 3.44543 |
| Postcentral Gyrus | R | 2 | 27 | 20, −44, 61 | − 4.17862 |
| Superior Frontal Gyrus | L | 32 | 20 | −13, 21, 48 | − 3.54077 |
| Supplementary Motor Area | L | 6 | 10 | −12, −9, 53 | − 3.61352 |
| Middle Cingulate Gyrus | L | 24 | 26 | −2, 3, 37 | − 2.75453 |
Note: Comparisons were conducted with a significance criterion of p < 0.01, multiple comparisons corrected with AlphaSim, cluster size > 18. BA: Brodmann area; MNI: Montreal Neurological Institute, x, y, z, coordinates of primary peak locations in MNI space.
Discussion
The current study was a preliminary investigation of alterations in brain structure and function in patients with CNSP using VBM and ReHo analysis, respectively. We found that VBM of T1-weighted anatomical images revealed no significant structural changes in GMV in CNSP patients. However, the analysis demonstrated alterations of regional brain spontaneous activity in specific brain areas.
VBM analyses
A number of previous studies using VBM have reported that various types of long-term chronic pain can lead to a reduction of GMV in pain processing areas.11,12,24,25 In contrast to previous reports, the current findings revealed no decrease in GMV. To investigate this discrepancy, in future studies we will refine the CNSP patient groups according to clinical information, including patient’s age, pain duration, attack frequency, and pain intensity, and analyse changes in brain structure for each group. Several previous studies have reported that alterations in brain GMV caused by chronic pain can be reversible.26,27 Recent studies have suggested that chronic pain is more likely to cause network dysfunction, rather than structural abnormalities.28 Therefore, more research attention should be focused on brain functional changes in patients with chronic pain.
Within-group ReHo maps
To identify the reliability of the functional analysis using ReHo, one sample t-tests were performed within the patient and control groups. The results revealed that ReHo values in the posterior cingulate cortex, precuneus, inferior parietal lobule and medial prefrontal cortex were all significantly higher than the global mean values, for both the CNSP patient and control groups. In addition, the observed ReHo patterns were similar to those of the human DMN, confirming the reliability of the analysis method.29
ReHo differences between the CNSP and control groups
Compared with healthy controls, CNSP patients exhibited abnormal neural activity in several specific brain regions. The results showed that the regions with increased ReHo values were predominantly located at the bilateral MFG, and that those with decreased ReHo values were located in the left insula, SFG, MCG, SMA and right PCG, and SPL. These results are largely consistent with those of previous studies of different types of chronic pain.2,15,17,20,30 The abnormal activity identified in the current study was predominantly concentrated in the left hemisphere, possibly related to the clinical observation of left-hemispheric predominance in right-handed patients. Tanja et al.31 reported left-hemisphere dominance of the early sensory component of pain perception using brain electrical source analysis. The MCG is part of the limbic lobe, which is associated with affective processing during the experience of pain.32 According to the James-Lange theory, the insula and limbic lobe are critical for emotional processing and pain memory, a role previously ascribed to the limbic system.33 A number of neuroimaging studies have revealed that the insula is a functionally comprehensive, heterogeneous and multisensory integrated brain region that participates in the processing of pain perception and emotion, as well as the sensory discrimination of pain perception, acting as the primary cortex for interoception.33,34,35 The insula and cingulate gyrus are key regions involved in the “pain matrix”, which is considered to be involved in the perception of pain intensity and emotional, homeostatic, sensorimotor and cognitive functioning.36,37. A recent study suggested that the insula plays an important role in the triggering of the pain matrix network and the resulting emergence of the subjective pain experience.38 Previous studies have suggested that the resting state anterior insula-anterior cingulate cortex/mid-cingulate cortex (ACC/MCC) system integrates interoceptive emotional information to form a subjective representation of the body, and there is evidence that the entire insula-MCC system is likely to be involved in environmental monitoring, response selection and skeletomotor body orientation.39 Thus, the decreased ReHo values in the MCG and insula observed in the current study suggest that the abnormalities may be related to functional impairments in the long-term pain-related affective response. The frontal lobe is involved in a range of comprehensive and complex functions, including integrating and regulating affective and memory information, personality, and cognition.40 The SFG and MFG play a role in modulating the cortical and subcortical nociceptive pathways.41 Thus, the ReHo values in bilateral MFG are likely to be significantly increased when people are in a state of chronic pain. Many previous studies of various types of pain have reported ReHo changes in the frontal lobe, but have typically focused on the prefrontal lobe, which is related to affective processing.42,43 Further studies are needed to explore the role of the MFG in pain processing in more depth. The decrease in ReHo values we observed in PCG is consistent with the results of a previous study.17 The PCG comprises the primary somatosensory cortex (S1), which is involved in the somatotopic changes associated with pain intensity, and the discrimination of the sensory components of pain perception (e.g., location, intensity and quality).17,44,45 The SPL and inferior parietal lobule also belong to S1. The main function of the parietal lobe is to integrate various types of sensory information, which is similar to the role of the PCG.44 A number of previous reports have proposed that the SMA is crucial for linking cognition to action, and is primarily involved in controlling movements (since neurons in the SMA project directly to the spinal cord), pain anticipation and affective processing.46–48 In patients with CNSP, decreased ReHo values in the SMA may be related to deficits in pain modulation and affective response inhibition.15 In the current study, several patients with severe pain and a high VAS score exhibited weakness and difficulty moving their upper limbs, which may also be associated with decreased ReHo values in the SMA. Based on the current findings and the results of previous studies, we speculate that patients with different types of chronic pain may share a common neurological and pathophysiological basis, and that long-term chronic pain is not only involves pain, but also accompanied with the dysfunction of sensory, cognitive and affection.
The MCG, which exhibited decreased ReHo values, is part of the anterior DMN, the insula participates in the composition of the cingulate-insular network (CIN), and the PCG and SMA are a part of the sensorimotor network (SMN).49 Abnormal brain activity in these regions may have an impact on pain-related intrinsic connectivity networks, including the DMN, CIN and SMN in CNSP during the resting state. No significant changes in ReHo values were found in the ACC or PFC, in contrast with several previous reports.2,12,13,15,17 The current findings suggest that brain regions exhibiting changes in ReHo values may be related to the location of pain and its various components (e.g., pain duration, attack frequency and pain intensity). The discrepancy between the current results and previous reports may be related to these factors.
Further considerations
The current findings suggest that regional abnormalities of selective brain regions are related to pain perception, cognition and affective processing. These results may have implications for the clinical treatment of CNSP, raising the possibility that the subjective pain perception of patients with CNSP might be decreased by reducing brain activation in targeted areas associated with the pain processing network.50,51 Although preliminary findings are promising, more research is needed before rs-fMRI can be used routinely in the clinic. In addition future studies with larger sample sizes are needed to confirm the current findings. Specifically, further work is needed to compare the influence of various components of pain on brain function, including pain duration, attack frequency and pain intensity. Further exploration of altered functional connectivity in these brain regions is necessary.
Conclusions
In conclusion, the current study used ReHo to reveal abnormal neuronal activity in multiple regions involved in the integration and processing of pain signals in the resting-state in CNSP patients, including the bilateral MFG, left insula, SFG, MCG, SMA, and right PCG and SPL. This preliminary study suggests that these brain regions may be suitable targets for chronic pain treatment, and provide a foundation for other research to extend treatment strategies for CNSP patients.
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Thoomes EJ, Scholten-Peeters GG, de Boer AJ, et al. Lack of uniform diagnostic criteria for cervical radiculopathy in conservative intervention studies: a systematic review. Eur Spine J 2012; 21: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L, Liu J, Dong X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain 2013; 14: 85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denkinger MD, Lukas A, Nikolaus T, et al. Multisite pain, pain frequency and pain severity are associated with depression in older adults: results from the ActiFE Ulm study. Age Ageing 2014; 43: 510–514. [DOI] [PubMed] [Google Scholar]
- 4.Linton SJ. A transdiagnostic approach to pain and emotion. J Appl Biobehav Res 2013; 18: 82–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Homs J. Nocioceptive pain, neuropathic pain and pain memory. Neurologia 2009; 24: 419–422. [in Spanish, English Abstract]. [PubMed] [Google Scholar]
- 6.May A. Chronic pain may change the structure of the brain. Pain 2008; 137: 7–15. [DOI] [PubMed] [Google Scholar]
- 7.Seifert F, Maihöfner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol 2011; 24: 515–523. [DOI] [PubMed] [Google Scholar]
- 8.Fayed N, Andres E, Rojas G, et al. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 2012; 126: 115–125. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism 2010; 59(Suppl 1): S9–S15. [DOI] [PubMed] [Google Scholar]
- 10.Otti A, Guendel H, Henningsen P, et al. Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci 2013; 38: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vartiainen N, Forss N. Imaging of brain changes in chronic pain. Duodecim 2014; 130: 1507–1514. [in Finnish, English Abstract]. [PubMed] [Google Scholar]
- 12.Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed 2013; 26: 58–64. [DOI] [PubMed] [Google Scholar]
- 13.Malinen S, Vartiainen N, Hlushchuk Y, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci USA 2010; 107: 6493–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology 2014; 272: 29–49. [DOI] [PubMed] [Google Scholar]
- 15.Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed 2012; 25: 806–812. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino A, Okamoto Y, Kunisato Y, et al. Distinctive spontaneous regional neural activity in patients with somatoform pain disorder: A preliminary resting-state fMRI study. Psychiatry Res 2014; 221: 246–248. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SS, Wu W, Liu ZP, et al. Altered regional homogeneity in experimentally induced low back pain: a resting-state fMRI study. J Neuroeng Rehabil 2014; 11: 115–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong JY, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting Brain. J Neurosci 2013; 33: 11994–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napadow V, Harris RE. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of ‘centralized’ pain? Arthritis Res Ther 2014; 16: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Du H, Chen N, et al. Regional homogeneity abnormalities in patients with tension-type headache: a resting-state fMRI study. Neurosci Bull 2014; 30: 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Du H, Chen N, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 22.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage 2010; 49: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo XN, Xu T, Jiang L, et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage 2013; 65: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013; 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 25.Ivo R, Nicklas A, Dargel J, et al. Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 2013; 22: 1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009; 29: 13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 2013; 8: 518–534. [DOI] [PubMed] [Google Scholar]
- 28.Naegel S, Holle D, Desmarattes N, et al. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin 2014; 6: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Jiao Y, Chen YC, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 2014; 63: 749–760. [DOI] [PubMed] [Google Scholar]
- 30.An L, Cao QJ, Sui MQ, et al. Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neurosci Bull 2013; 29: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlereth T, Baumgärtner U, Magerl W, et al. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. NeuroImage 2003; 20: 441–454. [DOI] [PubMed] [Google Scholar]
- 32.Zubieta JK, Ketter TA, Bueller JA, et al. Regulation of human affective responses by anterior cingulated and limbic mu-opioid neurotransmission. Arch Gen Psychiatry 2003; 60: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 33.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev 2014; 24: 77–87. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Liu J, Zhang F, et al. Effects of long-term acupuncture treatment on resting state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. Plos One 2014; 9: e99538–e99538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 2013; 34: 109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 2009; 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favilla S, Huber A, Pagnoni G, et al. Ranking brain areas encoding the perceived level of pain from fMRI data. Neuroimage 2014; 90: 153–162. [DOI] [PubMed] [Google Scholar]
- 38.Isnard J, Magnin M, Jung J, et al. Does the insula tell our brain that we are in pain? Pain 2011; 152: 946–951. [DOI] [PubMed] [Google Scholar]
- 39.Abe N, Suzuki M, Mori E, et al. Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions. J Cogn Neurosci 2007; 19: 287–295. [DOI] [PubMed] [Google Scholar]
- 40.Michael J, Zigmond, Joseph T, Coyle, Lewis P. Rowland. Neurobiology of Brain Disorders. In: Pressman P, Howard JR. (eds). Disorders of frontal lobe function, 1st ed USA: Academic Press, 2015, pp. 542–557. [Google Scholar]
- 41.Yang FC, Chou KH, Fu JL, et al. Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain 2013; 154: 801–807. [DOI] [PubMed] [Google Scholar]
- 42.Absinta M, Rocca MA, Colombo B, et al. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 2012; 32: 109–115. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 2008; 28: 598–604. [DOI] [PubMed] [Google Scholar]
- 44.Kanda M, Nagamine T, Ikeda A, et al. Primary somatosensory cortex is actively involved in pain processing in human. Brain Res 2000; 853: 282–289. [DOI] [PubMed] [Google Scholar]
- 45.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis. Neurophysiol Clin 2000; 30: 263–288. [DOI] [PubMed] [Google Scholar]
- 46.Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci 2006; 26: 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy M, Piché M, Chen JI, et al. Cerebral and spinal modulation of pain by emotions. Proc. Natl. Acad. Sci 2009; 106: 20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geha PY, Baliki MN, Harden RN, et al. The brain in chronic CRPS pain: abnormal gray–white matter interactions in emotional and autonomic regions. Neuron 2008; 60: 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otti A, Guendel H, Henningsen P, et al. Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci 2013; 38: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emmert K, Breimhorst M, Bauermann T, et al. Comparison of anterior cingulate vs. insular cortex as targets for real-time fMRI regulation during pain stimulation. Front Behav Neurosci 2014; 8: 350–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouwense SA, de Vries M, Schreuder LT, et al. Systematic mechanism-orientated approach to chronic pancreatitis pain. World J Gastroenterol 2015; 21: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]