Abstract
Objective
Prostate cancer is a malignant tumour that poses a serious risk to human health. Epidemiological studies suggest that it may be associated with vitamin D receptor gene (VDR) polymorphisms. Previous work investigated potential risks between Taq I (rs731236) and Bsm I (rs1544410) VDR polymorphisms with prostate cancer in humans; however, results are inconsistent.
Methods
We conducted a meta-analysis to retrieve genetic association analyses of rs731236 and rs1544410 polymorphisms with prostate cancer from studies published between 2006–2016. Pooled odds ratios with 95% confidence intervals were used to assess genetic associations, and heterogeneity was assessed by Q and I2statistics.
Results
Our findings suggest a significant association between rs731236 and prostate cancer risk in Asians and African Americans, but rs1544410 was not associated with prostate cancer under three genetic models.
Conclusion
Future studies including larger sample sizes and the analysis of gene functions are needed to help develop prostate cancer treatment.
Keywords: VDR, polymorphisms, Taq I, Bsm I, meta-analysis, prostate cancer
Introduction
Prostate cancer originates from epithelial cells and is a serious threat to human health. Its incidence in China was reported to be 9.92/10 million in 2012, representing the sixth most common male malignant tumour. Similar incidences were also seen in the United States, where 192,000 new cases of prostate cancer were reported in 2009 according to the American Cancer Society.1 In recent years, numerous medical studies have made important progress in the field. Clinical studies showed that the incidence of prostate cancer increases with age, with a high incidence of disease concentrated in individuals 70–80 years of age. However, patients with familial hereditary prostate cancer are usually less than 50 years old.2 An increased disease incidence is also related to frequent sexual activity, a high-fat diet,3 race, and regional location.
Molecular biology and epidemiological studies results suggest that the pathogenesis of prostate cancer may be associated with single nucleotide polymorphisms (SNPs) in several genes.4–8 For example, polymorphisms of the vitamin D receptor gene (VDR) are closely associated with prostate cancer. VDR is located on human chromosome 12 and encodes the nuclear hormone receptor for vitamin D3.9,10 VDR is a ligand-dependent nuclear transcription factor, which plays an important role in maintaining calcium metabolism, and regulating cell proliferation and differentiation.11 Several SNPs have been identified in VDR that appear to influence the risk of cancer and other disease,12,13 including bone mineral density, hyperparathyroidism, and osteomalasia.14–16 In normal and malignant prostate cells, VDR expression mediates the biological actions of 1,25(OH)2D,17–19 and polymorphisms in different regions of VDR cause different effects. The Bsm I (rs1544410) restriction site is in intron 8 of VDR; this polymorphism does not affect the amino acid sequence of VDR, but many studies have suggested that it is closely related to prostate cancer risk.20–23 The Taq I (rs731236) polymorphism is a synonymous mutation located in VDR exon 9, which is also associated with prostate cancer risk.20,24–29
Several studies have investigated the potential risk of Taq I (rs731236) and Bsm I (rs1544410) polymorphisms on prostate cancer worldwide. However, the results are inconsistent.30–32 Therefore, we conducted a new meta-analysis to assess the effect of these two SNPs on the risk of prostate cancer.
Materials and methods
Search strategy and data extraction
We carried out a search of the literature to retrieve association analyses of Taq I (rs731236) and Bsm I (rs1544410) polymorphisms with prostate cancer published between 2006–2016. We searched PubMed, Springer, and ScienceDirect databases using the search terms ‘Taq I (or rs731236)’, ‘Bsm I (or rs1544410)’, ‘prostate cancer’, and ‘association analysis’. For data extraction, we paid attention to the publication time, country of publication, population information, genetic models used, case and control sample size, and polymorphism genotype and allele frequencies.
Statistical analysis and meta-analysis
We detected allele frequencies by Hardy–Weinberg equilibrium (HWE) using the χ2 test. Ideally, allele frequencies were stable and unchanged (P > 0.05). Heterogeneity was tested for using Q and I2 statistics, with P < 0.05 indicating significant difference. In the absence of heterogeneity, the fixed-effects model was used to calculate the odds ratio (OR) of each study; otherwise the random-effects model was used. The strength of association between Taq I (rs731236), Bsm I (rs1544410), and prostate cancer was accessed by calculating pooled ORs and 95% confidence intervals (CIs) under additive, dominant, and recessive genetic models. Publication bias was tested by Begg’s test and Egger’s linear regression. STATA software (version 12.0) was used for statistical analysis.
Results
Data statistics
A total of eight case–control studies about the Taq I (rs731236) polymorphism and the relationship between prostate cancer were identified.30–37 These included a total of 1,720 prostate cancer patients (502 Asians, 829 Caucasians, and 389 African Americans) and 1,729 controls (730 Asians, 866 Caucasians, and 133 African Americans). A total of six case–control studies about the Bsm I (rs1544410) polymorphism and the relationship between prostate cancer were identified.30,32–35,37 These included a total of 1,555 prostate cancer patients (350 Asians, 816 Caucasians, and 389 African Americans) and 1,376 controls (369 Asians, 870 Caucasians, and 137 African Americans). In these studies, the Bsm I (rs1544410) allele frequency was in line with the HWE (χ2 test, P > 0.05) (Table 1).
Table 1.
Sample information and VDR polymorphism (Taq I and Bsm I) genotyping data in the current meta-analysis.
| Ethnicity (country) | Author | Year of publication | rs731236 (Taq I) |
rs1544410 (Bsm I) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case/control genotype |
Hardy– Weinberg P-value | Case/control genotype |
Hardy– Weinberg P-value | |||||||
| CC | CT | TT | GG | AG | AA | |||||
| Asians (Lebanon) | Ezzi et al. | 2014 | 23/26 | 38/48 | 7/5 | 0.006 | 18/9 | 43/41 | 7/29 | NS |
| Asians (China) | Bai et al. | 2009 | 0/0 | 10/9 | 112/121 | NS | 0/1 | 8/21 | 114/108 | NS |
| Asians (China) | Hu et al. | 2014 | 2/1 | 10/22 | 96/219 | NS | – | – | – | – |
| Asians (Pakistan) | Yousaf et al. | 2014 | 4/32 | 13/11 | 27/76 | 1.01E-17 | – | – | – | – |
| Asians (India) | Manchanda et al. | 2010 | 16/30 | 52/60 | 92/70 | 0.011 | 42/56 | 102/79 | 16/25 | NS |
| Caucasians (America) | Nunes et al. | 2016 | 10/23 | 62/75 | 60/71 | NS | 14/28 | 63/70 | 55/71 | NS |
| Caucasians | Holt et al. | 2009 | 106/108 | 349/328 | 242/261 | NS | 239/255 | 339/331 | 106/115 | NS |
| African Americans | Holt et al. | 2009 | 11/7 | 45/27 | 58/29 | NS | 57/27 | 47/26 | 7/13 | NS |
| African Americans | Jingwi et al. | 2015 | 19/10 | 99/33 | 157/27 | NS | 22/11 | 117/33 | 139/27 | NS |
NS, No statistically significant differences (P ≥ 0.05)
Meta-analysis and publication bias
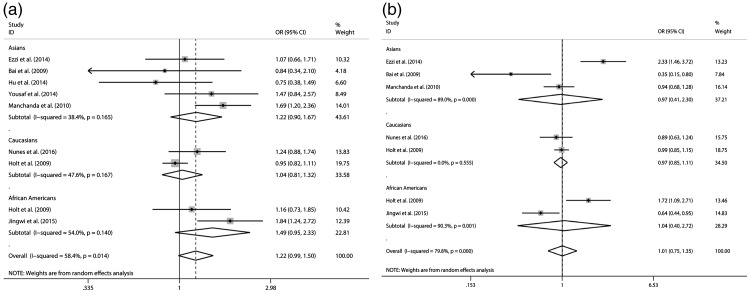
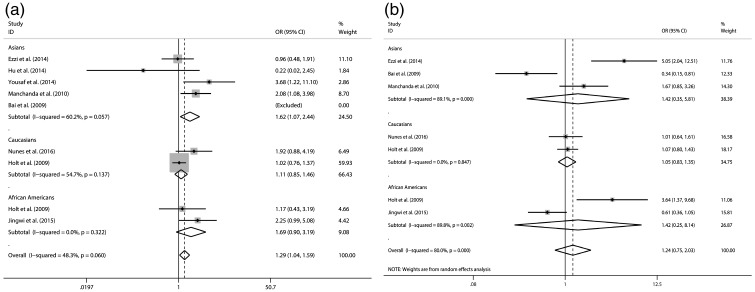
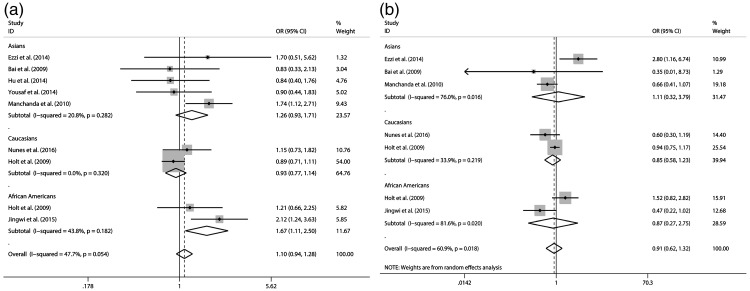
The results of the associations between Taq I (rs731236) and Bsm I (rs1544410) polymorphisms with prostate cancer and heterogeneity are shown in Table 2 and Figures 1–3. Our meta-analysis suggested that Taq I (rs731236) is associated with prostate cancer in the Asian population (dominant model: OR = 1.618, 95% CI 1.071–2.445, P = 0.022) and African American population (recessive model: OR = 1.668, 95% CI 1.115–2.496, P = 0.013) under the dominant model and recessive model, respectively. However, Bsm I (rs1544410) was not associated with prostate cancer under any of the three genetic models (additive model, OR = 1.005, 95% CI 0.746–1.353, not significant (NS); dominant model, OR = 1.237, 95% CI 0.753–2.031, NS; recessive model, OR = 0.906, 95% CI 0.623–1.316, NS).
Table 2.
Summary of ORs and 95% CIs under different genetic models and heterogeneity estimates.
| SNP | Genetic model | Population | Pooled odds ratio [95% confidence interval] P-value | Heterogeneity |
Begg’s test P-value | Egger’s test P-value | |
|---|---|---|---|---|---|---|---|
| I 2 | Q-test (P-value) | ||||||
| rs731236 (TaqI) | Additive (T/C) | Asians | 1.224 [0.899–1.666] NS | 38.40% | NS | NS | NS |
| Caucasians | 1.035 [0.812–1.319] NS | 47.60% | NS | NS | – | ||
| African Americans | 1.487 [0.948–2.330] NS | 54.00% | NS | NS | – | ||
| Total | 1.217 [0.988–1.499] NS | 58.40% | 0.014 | – | – | ||
| Dominant (CT + TT/CC) | Asians | 1.618 [1.071–2.445] 0.022 | 60.20% | 0.057 | NS | NS | |
| Caucasians | 1.110 [0.847–1.456] NS | 54.70% | NS | NS | – | ||
| African Americans | 1.694 [0.898–3.195] NS | 0.00% | NS | NS | – | ||
| Total | 1.288 [1.040–1.594] 0.020 | 48.30% | NS | – | – | ||
| Recessive (TT/CC + CT) | Asians | 1.259 [0.929–1.708] NS | 20.80% | NS | NS | NS | |
| Caucasians | 0.932 [0.765–1.135] NS | 0.00% | NS | NS | – | ||
| African Americans | 1.668 [1.115–2.496] 0.013 | 43.80% | NS | NS | – | ||
| Total | 1.095 [0.940–1.276] NS | 47.70% | NS | – | – | ||
| rs1544410 (BsmI) | Additive (A/G) | Asians | 0.969 [0.408–2.301] NS | 89.00% | 0 | NS | NS |
| Caucasians | 0.971 [0.845–1.115] NS | 0.00% | NS | NS | – | ||
| African Americans | 1.043 [0.400–2.722] NS | 90.30% | 0.001 | NS | – | ||
| Total | 1.005 [0.746–1.353] NS | 79.80% | 0 | – | – | ||
| Dominant (AA + AG/GG) | Asians | 1.420 [0.347–5.814] NS | 89.10% | 0 | NS | NS | |
| Caucasians | 1.054 [0.826–1.346] NS | 0.00% | NS | NS | – | ||
| African Americans | 1.424 [0.249–8.139] NS | 89.80% | 0.002 | NS | – | ||
| Total | 1.237 [0.753–2.031] NS | 80.00% | 0 | – | – | ||
| Recessive (AA/AG + GG) | Asians | 1.109 [0.324–3.794] NS | 76.00% | 0.016 | NS | NS | |
| Caucasians | 0.846 [0.582–1.230] NS | 33.90% | NS | NS | – | ||
| African Americans | 0.867 [0.273–2.750] NS | 81.60% | 0.02 | NS | – | ||
| Total | 0.906 [0.623–1.316] NS | 60.90% | 0.018 | – | – | ||
NS, no statistically significant differences (P ≥ 0.05)
Figure 1.
Forest plot of odds ratios for prostate cancer (additive model) a: Taq I (rs731236); b: Bsm I (rs1544410).
Figure 2.
Forest plot of odds ratios for prostate cancer (dominant model) a: Taq I (rs731236); b: Bsm I (rs1544410).
Figure 3.
Forest plot of odds ratios for prostate cancer (recessive model) a: Taq I (rs731236); b: Bsm I (rs1544410).
We used Begg’s test and Egger’s linear regression to estimate the publication bias. As shown in Table 2, the results provided statistical evidence of no publication bias (P > 0.05) in case–control studies of Asians, Caucasians, and African Americans.
Discussion
Several previous studies have reported an association of the Taq I (rs731236) and Bsm I (rs1544410) polymorphisms with prostate cancer.20–29 However, other investigations reached the opposite conclusion.30–32 In the present study, we conducted a meta-analysis of recently published genetic association analyses. The results suggested that Bsm I (rs1544410) was not associated with prostate cancer under the additive, dominant, or recessive genetic models. These negative association results could be explained by our method of identifying studies from the literature, or could reflect the fact that we did not analyse other prostate cancer risk factors such as atmospheric pollution, autoimmune diseases, and dietary factors. Moreover, the observed heterogeneity may also explain why no association was detected between Bsm I (rs1544410) and prostate cancer risk.
We did reveal a significant association between the Taq I (rs731236) polymorphism and prostate cancer risk in both Asian and African American populations (Table 2 and Figures 2–3). In 1994, Morrison et al.38 reported that the Taq I (rs731236) polymorphism affects VDR transcriptional activity and mRNA stability, thus altering the abundance of VDR protein, and in turn affecting vitamin D levels. Low vitamin D levels have been shown to increase the risk of prostate cancer,39 which agrees with our meta-analysis findings and previous epidemiological studies and gene function research.
By extension, our results show that genetic association analysis between susceptibility loci and disease involving small sample sizes does not provide solid evidence. Increasing the sample size would avoid the false-positive results obtained from local samples. Larger investigations should therefore be conducted together with molecular function studies of susceptibility genes and loci. This will ultimately provide an important theoretical basis for the development of prostate cancer clinical treatment.
Contributors
All authors have reviewed the final version of this manuscript and approved its submission for publication.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–249. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg GD, Carter BS, Beaty TH, et al. Family history and the risk of prostate cancer. Prostate 1990; 17: 337–347. [DOI] [PubMed] [Google Scholar]
- 3.Dimitropoulou P, Lophatananon A, Easton D, et al. Sexual activity and prostate cancer risk in men diagnosed at a younger age. BJU Int 2009; 103: 178–185. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Freije D, Carpten JD, et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 1996; 274: 1371–1374. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Meyers D, Freije D, et al. Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet 1998; 20: 175–179. [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Walker AH, Zeigler-Johnson C, et al. Association of HPC2/ELAC2 genotypes and prostate cancer. Am J Hum Genet 2000; 67: 1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpten J, Nupponen N, Isaacs S, et al. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet 2002; 30: 181–184. [DOI] [PubMed] [Google Scholar]
- 8.Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet 2004; 13(Spec No 1): R103–R121. [DOI] [PubMed] [Google Scholar]
- 9.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 2008; 4: 404–412. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol 2006; 92: 60–64. [DOI] [PubMed] [Google Scholar]
- 11.Basit S. Vitamin D in health and disease: a literature review. Br J Biomed Sci 2013; 70: 161–172. [DOI] [PubMed] [Google Scholar]
- 12.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007; 7: 684–700. [DOI] [PubMed] [Google Scholar]
- 13.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 1989; 320: 980–991. [DOI] [PubMed] [Google Scholar]
- 14.Carling T, Kindmark A, Hellman P, et al. Vitamin D receptor alleles b, a, and T: risk factors for sporadic primary hyperparathyroidism (HPT) but not HPT of uremia or MEN 1. Biochem Biophy Res Commun 1997; 231: 329–332. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MF, Ramachandran A, Ogunkolade BW, et al. Allelic variation in the vitamin D receptor influences susceptibility to IDDM in Indian Asians. Diabetologia 1997; 40: 971–975. [DOI] [PubMed] [Google Scholar]
- 16.Uitterlinden AG, Burger H, Huang Q, et al. Vitamin D receptor genotype is associated with radiographic osteoarthritis at the knee. J Clin Invest 1997; 100: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GJ, Stapleton GE, Ferrara JA, et al. The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha, 25-dihydroxyvitamin D3. Cancer Res 1992; 52: 515–520. [PubMed] [Google Scholar]
- 18.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 1993; 132: 1952–1960. [DOI] [PubMed] [Google Scholar]
- 19.Kivineva M, Blauer M, Syvala H, et al. Localization of 1, 25-dihydroxyvitamin D3 receptor (VDR) expression in human prostate. J Steroid Biochem Mol Biol 1998; 66: 121–127. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Matsui H, Ohtake N, et al. Vitamin D receptor gene polymorphism in familial prostate cancer in a Japanese population. Int J Urol 2003; 10: 261–266. [DOI] [PubMed] [Google Scholar]
- 21.Habuchi T, Suzuki T, Sasaki R, et al. Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res 2000; 60: 305–308. [PubMed] [Google Scholar]
- 22.Ingles SA, Coetzee GA, Ross RK, et al. Association of prostate cancer with vitamin D receptor haplotypes in African-Americans. Cancer Res 1998; 58: 1620–1623. [PubMed] [Google Scholar]
- 23.Chokkalingam AP, McGlynn KA, Gao YT, et al. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res 2001; 61: 4333–4336. [PubMed] [Google Scholar]
- 24.Tayeb MT, Clark C, Haites NE, et al. CYP3A4 and VDR gene polymorphisms and the risk of prostate cancer in men with benign prostate hyperplasia. Br J Cancer 2003; 88: 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luscombe CJ, French ME, Liu S, et al. Prostate cancer risk: associations with ultraviolet radiation, tyrosinase and melanocortin-1 receptor genotypes. Br J Cancer 2001; 85: 1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamasaki T, Inatomi H, Katoh T, et al. Clinical and pathological significance of vitamin D receptor gene polymorphism for prostate cancer which is associated with a higher mortality in Japanese. Endocr J 2001; 48: 543–549. [DOI] [PubMed] [Google Scholar]
- 27.Gsur A, Madersbacher S, Haidinger G, et al. Vitamin D receptor gene polymorphism and prostate cancer risk. Prostate 2002; 51: 30–34. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros R, Morais A, Vasconcelos A, et al. The role of vitamin D receptor gene polymorphisms in the susceptibility to prostate cancer of a southern European population. J Hum Genet 2002; 47: 413–418. [DOI] [PubMed] [Google Scholar]
- 29.Blazer DG, 3rd, Umbach DM, Bostick RM, et al. Vitamin D receptor polymorphisms and prostate cancer. Mol Carcinog 2000; 27: 18–23. [DOI] [PubMed] [Google Scholar]
- 30.Bai Y, Yu Y, Yu B, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet 2009; 10: 125–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousaf N, Afzal S, Hayat T, et al. Association of vitamin D receptor gene polymorphisms with prostate cancer risk in the Pakistani population. Asian Pac J Cancer Prev 2014; 15: 10009–10013. [DOI] [PubMed] [Google Scholar]
- 32.Holt SK, Kwon EM, Peters U, et al. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol, Biomarkers Prev 2009; 18: 1929–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes SB, de Matos Oliveira F, Neves AF, et al. Association of vitamin D receptor variants with clinical parameters in prostate cancer. Springerplus 2016; 5: 364–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35: 1549–1558. [PMC free article] [PubMed] [Google Scholar]
- 35.El Ezzi AA, Zaidan WR, El-Saidi MA, et al. Association of benign prostate hyperplasia with polymorphisms in VDR, CYP17, and SRD5A2 genes among Lebanese men. Asian Pac J Cancer prev 2014; 15: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, Qiu Z, Zhang L, Cui F. Kallikrein 3 and vitamin D receptor polymorphisms: potentials environmental risk factors for prostate cancer. Diagn Pathol 2014; 9: 84–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manchanda PK, Konwar R, Nayak VL, et al. Association of genetic variants of the vitamin D receptor (VDR) gene (Fok-I, Taq-I and Bsm-I) with susceptibility of benign prostatic hyperplasia in a North Indian population. Asian Pac J Cancer Prev 2010; 11: 1005–1008. [PubMed] [Google Scholar]
- 38.Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature 1994; 367: 284–287. [DOI] [PubMed] [Google Scholar]
- 39.Corder EH, Guess HA, Hulka BS, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol, Biomarkers Prev 1993; 2: 467–472. [PubMed] [Google Scholar]